Abstract
Estuarine ecosystems are not only physically and chemically dynamic, but also harbor unique and often specialist floral and faunal species. Nonetheless, they are subject to an increasing threat from existing human and projected climatic activities. This study examines the heavy metal contents of the Ulhas rivere stuary in Maharashtra, India – a biodiversity-rich and critically important ecosystem for native and migrant species. The drainages (within the catchment area) which supply water to the estuary were investigated to ascertain heavy metal levels, possibly resulting from anthropogenic activities. Concentrations of selected environmentally important heavy metals such as chromium (Cr), lead (Pb), nickel (Ni), copper (Cu), cadmium (Cd), zinc (Zn) and mercury (Hg) were measured using standard techniques. The findings reveal pre-occupying pollution levels that constitute a significant threat to both terrestrial and aquatic species dependents on the estuary. The abundance of heavy metals in the estuary was in the order of Pb > Ni > Cr > Cu > Zn > Hg > Cd (Pb between 0.018 mg/l to0.039 mg/l; Cd 0.006 mg/l to 0.009 mg/l). The contamination of heavy metals (often toxic over the recommended levels) was found to be high at sampling sites under the direct influence of human habitats and industries. The finding of this study not only adds to the available information regarding the status of the estuary, but also highlights the critical need to implement targeted strategies towards management and protect this vital ecosystem.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals (HM henceforth) in various ecosystems have received extensive attention because they are foremost toxic, non-biodegradable, and easily accumulated and magnified independent organisms. The existence of HM in aquatic ecosystems (freshwater and marine) has led to an ever-growing concern over their adverse influence on floral and faunal species, and on the availability of potable water which can potentially impact the wellbeing of communities. The concentration of HM in aquatic ecosystems has increased considerably due to the input from largely untreated industrial waste, sewage runoff and agriculture discharges, particularly in developing economies (Prica et al. 2008; Yang et al. 2012; Gao et al. 2015). Associated aquatic bodies of marine ecosystems,(e.g. estuaries) are at the foremost of this adverse impact. This is not surprising as the highly dynamic nature of marine environment allows for very rapid assimilation of pollutants (of various kinds) by processes such as dilution, oxidation or sequestration into associated sediments (Fianko et al. 2007), and the limited capacity for such assimilation. The occurrence of elevated levels of HM can be a good indicator of anthropogenic pollution, rather than the natural enrichment of the sediment by geological weathering (Karen and Baird 2001).
The effect of HM on estuarine ecosystems can range from beneficial, to worrying, to dangerously toxic (leading to a high level of lethality among species), for example, zinc (Zn) and copper (Cu) are required for metabolic activity in living organisms, lie in the narrow window between their essentiality and toxicity (Fatoki et al. 2002). Others like cadmium (Cd) and lead (Pb) exhibit extreme toxicity even at trace levels (Merian 1991) thus, necessitating their regular monitoring. This is because through the natural process of bio magnification, minute quantities of HM become part of the various food chains, and their concentration becomes elevated to levels which can potentially prove to be toxic to both humans and other organisms (Webb 1975; Tsuehiya 1978). HM are stable and persistent environmental contaminants of coastal waters and its sediment, therefore, their level of toxicity can be measured in relation to the metallic elements present. It is important to note that since the effect of this HM is cumulative, potable water should contain none/trace amounts of them. Apart from serious health hazards, HM pollution has the potential to lead to adverse economic and social impacts on livelihoods and food security of communities (Rathod and Patil 2009). It should be noted that Zn, Cd, Pb, chromium (Cr), nickel (Ni), and mercury (Hg) are common pollutants widely distributed in aquatic environments. Their sources are mainly from weathering of minerals and soils, atmospheric deposition, some industrial and domestic effluents and urban storm water run-off. The detection and determination of these HM is therefore of considerable importance not only for establishing their potentially adverse influence on associated ecosystems (Deshpande et al. 2009), but also for monitoring and mitigating the pathways by which they reach to critical levels in the hydrosphere.
Coastal zones are interface ecosystem between terrestrial and oceanic environment, an estuary is one of them. An Estuary is a transitional zone between the river and sea it has specific ecological properties and biological composition. It offers an immense biological wealth characterized by the diversified, rich flora and fauna, play important roles in carbon sequestration, primary productivity and prevent coastal zone erosion. The rivers can carry many sediment particles, particulates matters, nutrients, trace elements, which can have deposited along the mouth of estuary and their present of significant quantities of HM which can be accumulated and biomagnifies in aquatic ecosystems, resulting in sublethal effects consequent destruction of habitat (Xu et al. 2004). It eventually leads to adverse effect on human also due to the consumption of contaminated sea food (Wang 2002). Toxic trace elements in the form of metallic compound or recalcitrant can be taken up by the burrowing fauna and flora by different physiological process and entering into the food chain as a result of their bioaccumulation and bio-concentration in the food web (Wu et al. 2005).
Study area, Ulhas River estuary is the most important estuary in the Arabian Sea region of India. It provides a living space for large and diverse group of organism like epiphytes, invertebrate, and fishes. Moreover, its support the mangrove ecosystem and act as good nursery ground for the larvae of many commercially important Ichthyofauna like crabs, prawns, and fishes. Various anthropogenic discharges from rapid urbanization, large scale industrialization and uncontrolled sprawling settlements around the Ulhas river bed and their surrounding aquatic regions, became more critical in the case of the estuary ecosystem due to its landlocked and relatively stagnant nature. The trace and toxic elements are brought into the estuary in the form of dissolved and particulate (Jha et al. 2000) fluxes from chemical weathering, industrial effluents (Patel et al. 1985) and the sewerage discharge from the surrounding city (Mirajkar et al. 1995, Bhosale 1991).
A few studies conducted on Hg concentrations in water, sediments, and fish of Ulhas river estuary in the past have confined either to only one location or a part of the estuary or creek (Zingde and Desai 1981). Mercury and Nickel clearly show evidence of continuous input in estuary (Jha et al. 1999). There is increased lead and mercury concentration in superficial sediment and bioaccumulation of ferrous (Fe), Zn, and Pb in polychates in Ulhas estuary (Zingde 1999). The Ulhas River estuary is one of the inward waters characteristic in its environmental conditions due to the shallow depth, tidal currents, mangrove vegetation, salinity gradient, diurnal temperature variation etc.
The river is shallow having sandy basin since the land runoff carries huge sediments from its catchment area and the nearby thane creek which is connected through a narrow and shallow channel are under considerable environmental stress due to the indiscriminate release of industrial effluent and domestic waste water that goes largely untreated. The transport and distribution of heavy metal as well as radionuclides in water, suspended particulate matter and sediment in the inshore areas of Mumbai (Patel et al. 1985). A standing stock of about 77 kg excess mercury present in water and 14ton excess in sediment over the natural background, indication of its bioaccumulation in zooplankton and benthos in thane creek (Zingde and Desai 1981). Sahu and Bhosale (1991) were found the concentration of Hg <2 μg g−1 in the sediment of Ulhas estuary and high organic carbon content (26.2%) observed from Kolshet to Wagbil at the edge of Ulhas estuary by Nikam et al. 2009. Continuously increasing suspended solid and nutrients (PO4-P, NO3-N and SiO3-Si) are indicating the hypoxic condition of the Ulhas estuary (Mishra et al. 2007). The deterioration can be attributed to pollution by growing effluent load and excessive sand dredging activities. High industrial effluent discharges from Ambarnath-Ulhas Nagar industrial belt (Rathod 2005 and Sahu and Mukharji 1983) in an estuary and due to these decreasing in current velocity and depth of estuary (Nikam et al. 2009). Those are clearly shows that increasing evidence of pollution due to anthropogenic discharges from the surrounding areas and therefore, this study assesses the levels of dissolved HM – Cd, Pb, Ni, Cr, Cu, Zn and Hg in the Ulhas river estuary, a biodiversity rich ecosystem in Maharashtra, India; and investigates the potential pollution sources along the estuary, and from parts of the catchment.
Materials and methods
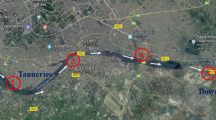
The study was conducted in the Ulhas river estuary (19°29′ to 19°18′N, 72°88′ to 72°49′E) near the city of Mumbai in the State of Maharashtra, India (Fig. 1). The Ulhas river originates at Budhemal Lake near Rajmachi Fort on the western slope of the Sahyadri range in Maharashtra. It is the largest river on the Konkan coast (a rugged section of India’s western coastline), and meets the Arabian Sea at Vasai Fort in Thane, Maharashtra. The entire catchment area of Ulhas river is underlain by basaltic rock type with the shallow alluvium formation as narrow stretches along the river (GWIGMDM 2013). Maharashtra has a high population and supports large industrial areas within and adjacent to its legislative boundary. Despite the best attempts of the municipal authorities, a large amount of improperly treated wastewater and industrial affluent is released in the catchment area.
The sampling stations (S1 – S5) were spread from Ghodbandar (S1; 19°16’N, 72°59′E) – the very narrow mouth of the river with a sandy bed, to Dongri Chouki (S5; 19°18.801’N, 72°47.242′E) – the broad mouth of the estuary with muddy banks and mangrove vegetation (Fig. 1). The geographical locations of the sampling stations (S1 – S5) were recorded using a Garmin ETrex handheld Global Positioning System (GPS).
Sampling was conducted on a monthly basis during September – February (annual autumn to winter months). In-situ water quality parameters, (i.e. temperature (°C), pH, salinity (‰), and conductivity (mS/cm)) were estimated on all sampling stations (S1 – S5) using standard portable instruments. Water samples (500 mL each) were collected in triplicates from each of the stations. Each sample set was preserved by bringing down the pH to a level > 2 using concentrated nitric acid (HNO3), and 250 ml of this portion was filtered through Whatman No. 41 (0.45 μm pore size) filter paper for analysis of dissolved HM and preserved. These portions were pre-concentrated 50x on a water bath, and subjected to HNO3 digestion using the microwave-assisted technique (Anton Parr, USA). Digested samples were diluted to 50 ml each, and subjected to analysis of the HM (Cu, Cd, Ni, Cr, Zn and Pb) by atomic absorption spectrophotometer (Analyst 800, Perkin Elmer, USA) using flame atomization. The cold vapor method (AAnalyst 800, Perkin Elmer with FIAS) was used to measure the concentration of Hg (APHA 2005).
The efficiency of the analytical method and instrumentation used was verified by the analysis of the reference materials obtained from the National Research Council of Canada for estuarine water (SLEW-3), and lobster hepatopancreas (TORT-2) in five replicates. HM concentrations and recoveries of the standard reference materials used are presented in Table 1. All the statistical analyses were carried out using Microsoft Excel 2010.
Results and discussion
Since pH and temperature (°C) affect the solubility and toxicity of HM in aquatic ecosystems, their range was used to assess the toxicity of HM in the estuary. For example, HM such as Cd, Pb, and Zn are most likely to have increased detrimental environmental effects as a result of a lowered pH (Fatoki et al. 2002). The average pH values of water samples in the estuary varied between 7.52 and 7.74, while temperature (°C) ranged between 21.33 °C and 24.31 °C. These values of pH and temperature are within the World Health Organization (WHO) recommended values of water used for domestic purposes. Since the estuarine system was largely influenced by the influx of freshwater and intrusion of seawater (during and post monsoon), the salinity range was between 10 and 35 ‰, and the conductivity was between 4.7 to 8.9 mS/cm.
Elevated levels of HM, (i.e. Pb, Cr, Ni, Cu, Zn, Cd, and Hg) were detected in the estuary. The abundance of these HM was in the order Pb > Ni > Cr > Cu > Zn > Hg > Cd. The HM concentrations in the freshwater reaches were generally lower than the estuarine region. The HM concentration was related to point and nonpoint source inflows from drainages discharging their effluents directly into the estuary (Fig. 1). The spatial HM concentrations in the estuary did not show any significant trend (except for Pb and Hg), indicating that the source might be diffused (Fig. 3).
Lead (Pb)
The temporal average value of Pb concentration in the estuary ranged from 0.0282 mg/l to 0.330 mg/l, with the highest value recorded during the month of November (Fig. 2a). The spatial average Pb concentration was recorded to be highest at the sampling sites S4 and S5 (Fig. 3b), possibly due to the effect of Pb leaching chemicals from fishing harbor and several jetties, effluent discharges, highway runoff, seepage from waste disposal sites, geology of the catchment, and the deposition of suspended material carried from the catchment area. Nutritionally/physiologically Pb is not an essential nutrient for humans or other organisms. Organometallic forms of Pb are more lipophilic (tending to combine with or dissolve in lipids or fats), and can easily penetrate biological membranes. As a result, alkyl lead species may be bio accumulated in food chains (Khan et al. 2014). Adverse chronic effects, (e.g. possible neurological damage in fetuses and children) may occur at 0.05 mg/l to 0.10or higher mg/l of Pb (DWAF 1996). These levels exceeded in the estuary, and the direct use of water without safe treatment should be avoided at all costs by dependent communities.
Mercury (Hg)
Although elemental Hg is relatively innocuous and non-toxic, it can be converted to organomercurials (an organic compound containing Hg), which are particularly toxic and retained in the cells of plants and other organisms (Favretto et al. 1997). In the present investigation, it was observed that the average Hg concentration was in the range of 0.0019 to 0.022 mg/l respectively, with the highest value recorded during the months of October and December (Fig. 2c). Sampling sites S1 and S2 recorded higher values of Hg concentration (Fig. 3g), and this could be due to the narrow mouth of the estuary which reduces water flow and help the elevate Hg concentration in addition to that effluent discharges from medical waste incineration plant at Kalyan and Maharashtra Industrial Development Corporation (MIDC) belt Dombivali. These levels are significantly high according to the domestic water standards and maximum tolerance limits for industrial effluents discharged of the Bureau of Indian standard (BIS) 1991.
Cadmium (Cd)
The average concentration of Cd in the estuary varied between 0.0066 mg/l and 0.0124 mg/l (Fig. 3c) with the highest value recorded during the months of November and January (Fig. 2b). Sampling site S1 in the freshwater region recorded the least level of Cd. The human health based guidelines established by the WHO and the United States Environmental Protection Agency (EPA) recommends 0.003 mg/l of Cd for drinking water (USEPA 1996; WHO 1996). The values obtained exceeded these guidelines at all the sampling sites (S1 – S5). In view of the fact that the major use of the water obtained from the catchment is for domestic purposes, the high levels of Cd recorded is of great concern. Cd is extremely toxic, and has the potential to cause adverse health effects such as renal diseases and cancer (Friberg et al. 1986; Fatoki et al. 2002). The probable sources of Cd in the estuary could be due to the geology of the catchment soils, and runoffs from agricultural soils where phosphate-based fertilizers are often used (Cd is a common impurity in such fertilizers). Other probable sources could include leachates (liquid that drains from a landfill) from disused Ni-Cd based batteries and Cd-plated items disposed at refuse dumps.
Zinc (Zn)
The average levels of Zn in the estuarine waters ranged between 0.0124 mg/l and 0.0292 mg/l with the highest value recorded during the months of November and February (Fig. 2b). The average concentration level of Zn recorded from sites S1 and S2 were high compared to the stations situated at the mouth of the river, possibly due to absorbance by suspended particles in the upper area of the estuary, and deposition on the sea bed (Florence and Batley 1977). The WHO recommended value for Zn in water for domestic supply is 3 mg/l (WHO 1996) hence, Zn should not be a problem in water used for domestic purposes. However, Zn (at the current levels) could be a problem for the aquatic ecosystem. Possible sources of Zn here include cans in garbage mounds, and rusty and unwanted galvanized scraps disposed along the catchment area.
Chromium (Cr)
The average levels of Cr recorded varied between 0.0275 mg/l and 0.0884 mg/l (Fig. 3a) which happen to be above the 0.01 mg/l limit recommended by WHO for domestic water use (WHO 1996), and can lead to severe health effects. The highest value was recorded during the month of February (Fig. 2a). The possible sources of Cr here could be the wastes of the industries involved in the production of ferrochrome, electroplating, pigment production and tanning; dumping of solid wastes, and municipal wastes.
Nickel (Ni)
In the present investigation, it was observed that Ni concentration was in the range of 0.085 to 0.113 mg/l (Fig. 3d), which is below the maximum tolerance limit for industrial effluent discharges (BIS 1991). The highest value was recorded during the months of September, November and January (Fig. 2a). Ni is a nutritionally essential trace HM for several animal species, micro-organisms and plants therefore, its deficiency or toxicity symptoms can occur when, too little or too much Ni is taken up. High levels of Ni develop chronic bronchitis and changes to the structure of lungs (Scott-Fordsmand 1997). Ni and Ni-compounds have many industrial and commercial uses, and they could be possibly responsible for its emission into the ecosystem.
Copper (Cu)
The average levels of Cu recorded varied between 0.0163 mg/l and 0.0426 mg/l (Fig. 3f), which happens to be slightly below the 0.05 mg/l limit recommended for domestic water (BIS 1991), with the highest value recorded during the month of February (see Fig. 2). Cu at low concentrations is essential to maintain cellular functions and metabolic enzymes (Monteiro et al. 2009) however, at higher concentrations free cupric ions are highly toxic in aquatic environments (Nor 1987), with the potential to damage intracellular proteins and cause apoptotic (programmed) cell death (Monteiro et al. 2009). The use of Cu as an antifouling coating in ships could be a source of this HM here.
There was monthly/seasonal variation in the HM concentration within the sampling period. The dry season registered elevated levels of the HM in comparison to the wet season. The HM concentration increased gradually from the post monsoon season (representing the dry season) (see Fig. 2) for Cr, Cu, and Zn. The dilution effect of the rainy season due to storm run-off into receiving rivers, and excessive evaporation of the surface water may be responsible for the observed trend.
The polluted water from Ulhas river coming to the estuary which is studied from selected sampling station and its analysis suggest that river water is the main cause for present estuarine condition. The continuous increase in HM concentration of the estuarine waters results in bioaccumulation of harmful metals which indirectly effect on the Human population through food chain.
Conclusion
The results of the study have indicated gross pollution of the Ulhas river estuary, especially with regard to HM. This potentially poses a health risk for several communities in the catchment who rely on the estuary primarily for their domestic use and dependent livelihood. The study concludes that higher mercury concentration may be due to lesser dilution of metal by reduced water flow and also from the effluent discharge from the medical waste incineration plant. Alkyl lead species is the most bio accumulated form in food chain, the main factors affecting the lead concentration in estuary is the geology of the catchment and the deposition of suspended material carried from the catchment area. Seepage from the waste disposal site particularly Ni-Cd battery disposal, tannery effluent disposal and drainage from the agricultural field fertilized with phosphate fertilizer where heavy metal particularly Cd is the integral impurities. An elevated level of HM is a good indication of anthropogenic pollution due to sewage and effluent discharges into the estuary, and warrants urgent strategies for sustainable management of estuary and to protect this vital ecosystem.
References
APHA (2005) Standard methods for the examination of water and wastewater, 21st Ed. American Public Health Association, Washington, DC
Bhosale U (1991) Heavy metals pollution around the Island City of Bombay, India. Chem Geol 90:285–305
Bureau of Indian Standard (BIS) (1991) Water Quality Assessment Authority, Government of India. http://wqaa.gov.in
Department of Water Affairs and Forestry (1996) Water quality guidelines, domestic use, vol. 1, 2nd edn. DWAF, Pretoria
Deshpande A, Bhendigeri S, Shirsekar T, Dhaware D, Khandekar RN (2009) Analysis of heavy metals in marine fish from Mumbai docks. Environ Monit Assess 159:493–500
Fatoki OS, Lujiza N, Ogunfowokon AO (2002) Trace metal pollution in Umtata River. Water SA 28(2):183–189
Favretto L, Campisi B, Reisenhofer E, Adami G (1997) Sewage pollution. Anal Chim Acta 344(3):251–259
Fianko JR, Osae S, Adomako D, Adotey DK, Serfor-Armah Y (2007) Assessment of heavy metal pollution of the Iture estuary in the central region of Ghana. Environ Monit Assess 131(1):467–473
Florence TM, and GE Batley (1977) Determination of the chemical forms of trace metals in natural waters, with special reference to copper, lead, cadmium and zinc. Talanta 24:151–158
Friberg L, Elinder CG, Kjellstroem T, Nordberg GF (Eds.) (1986) Cadmium and health: a toxicological and epidemiological appraisal. Effects and response Boca Raton: CRC Press Vol.11
Gao X, Zhou F, Chen CTA, Xing Q (2015) Trace metals in the suspended particulate matter of the Yellow River (Huanghe) estuary: concentrations, potential mobility, contamination assessment and the fluxes into the Bohai Sea. Cont Shelf Res 104:25–36
Ground Water Information Greater Mumbai District Maharashtra (GWIGMDM) (2013) Ministry of water resources. Governmentof India pp:7–10
Jha SK, Krishnamurthi TM, Pandit GG, Nambai KSV (1999) History of accumulation of mercury and nickel in Thane Creek, Mumbai, using 210 Pb dating technique. Sci Total Environ 236:91–99
Jha S, Chavan B, Pandit B, Negi B, Sadasivan S (2000) Behaviour and fluxes of trace and toxic elements in creek sediment near Mumbai, India. Environ Monit Assess 76:249–262
Karen B, Baird D (2001) Survey of heavy metals in the sediments of the Swartkops River estuary, Port Elizabeth South Africa. Water SA 27(4):461–466
Khan MB, Hofer A, Pavoni B (2014) Harmful elements in estuarine and coastal systems. PHEs, Environment and Human Health. https://doi.org/10.1007/978-94-017-8965-3_2
Merian E, (Ed.) (1991) Metals and their compounds in the environment. Occurrence, analysis and biological relevance. Weinheim (u. a.): VCH
Mirajkar P, Moily R, Kulkarni VV, Bhosale VM, Krishnamoorthy TM (1995) Preliminary studies on the distribution of heavy metals in Thane Creek ecosystem in relation to industrial effluent discharges, in proceedings of 4th National Symposium on environment, February 1995, Madras, India 7–10
Mishra V, Quadros G, Athalye RP (2007) Hydrological study of Ulhas river estuary, Maharashtra, India. J Aqua Biol 22 (1):97–104
Monteiro SM, dos Santos NM, Calejo M, Fontainhas-Fernandes A, Sousa M (2009) Copper toxicity in gills of the teleost fish, Oreochromis niloticus: effects in apoptosis induction and cell proliferation. Aquat Toxicol 94(3):219–228
Nikam V, Kumar A, Lalla K, Gupta K (2009) Conservation of wetland and mangroves in thane creek and Ulhas River Estury, India. J Environ Sci Eng 51(3):157–162
Nor YM (1987) Ecotoxicity of copper to aquatic biota a review. Environ Res 43:274–282
Patel B, Bangera VS, Patel S, Balani MC (1985) Heavy metals in the Bombay harbour area. Marine Pollution Bull 16:22–28
Prica M, Dalmacija B, Roncevic S, Krcmar D, Becelic M (2008) A comparison of sediment quality results with acid volatile sulfide (AVS) and simultaneously extracted metals (SEM) ratio in Vojvodina (Serbia) sediments. Sci Total Environ 389:235–244
Rathod M (2005) Effect of pollution on mudskipper fishery of Ulhas River estuary with a special reference to the biology of Boleopthalmus dussumieri (Cuv. & Val.) project report university Mumbai
Rathod SD, Patil NN (2009) Assessment of some hydrological parameters of ulhas river estuary, in the vicinity of thane city, Maharashtra state. J Aqua Biol 24(2):103–108
Sahu KC, Bhosale U (1991) Heavy metal pollution around the island city of Bombay, India. Part I: Quantification of heavy metal pollution of aquatic sediments and recognition of environmental discriminants. Chem Geol 90:263–283
Sahu K, Mukharji S (1983) Monitoring water and sediment of Ulhas river of north – east of Bombay. MAHASAGAR 16(2):135–142
Scott-Fordsmand JJ (1997) Toxicity of nickel to soil organisms in Denmark. Rev Environ Contam Toxicol 148(1):1–34
Tsuehiya K (1978) Cadmium studies in Japan (Review). BioMedical Press, North Holland
USEPA (1996) Drinking water regulations and health advisories. EPA 822-R-96-001
Wang WX (2002) Interactions of trace metals and different marine food chains. Mar Ecol Prog Ser 243:295–309
Webb M (1975) Metallothionein and toxicity of cd: proc. of NATO Sci. Conf. On Ecotoxicol. Res. Effects of heavy metals and organohalogen compounds.NY: plenum Press.Pp 177–186
WHO (1996) Health criteria and other supporting information. 2ndedd. Guidelines for drinking water, vol. 2, Geneva
Wu J, Meng XX, Li K (2005) Phytoremediation of soils contaminated by lead. Soils 37(3):258–264
Xu Y, Liu X, Ma A (2004) Current research on toxicity effect and molecular mechanism of heavy metals on fish. Marine Sciences 28(10):67–70
Yang YQ, Chen FR, Zhang L, Liu JS, Wu SJ, Kang M (2012) Comprehensive assessment of heavy metal contamination in sediment of the Pearl River estuary and adjacent shelf. Mar Pollut Bull 64:1947–1955
Zingde MD (1999) Marine pollution––what are we heading for? In: Somayajulu BLK (ed) Ocean science trends and future directions. Indian National Science Academy, New Delhi, pp 229–246
Zingde MD, Desai BN (1981) Mercury in Thane Creek, Bombay harbour. Mar Pollut Bull 12:237–241
Acknowledgments
Authors are thankful to the Director, Central Institute of Fisheries Education, Mumbai, for providing facilities to conduct the present research work. The first author acknowledges the financial support from the Indian council of Agricultural Research, New Delhi, India, in the form of research fellowship for this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
M., R.S., Shree, B.V., K., R.K. et al. Examining the heavy metal contents of an estuarine ecosystem: case study from Maharashtra, India. J Coast Conserv 23, 977–984 (2019). https://doi.org/10.1007/s11852-019-00702-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11852-019-00702-1