Abstract
Blackleg disease caused by the pathogen Leptosphaeria maculans is the most devastating disease of canola (Brassica napus, oilseed rape). This disease occurs in all canola-growing regions of the world except China. It has been suggested that L. maculans contaminated seed and admixture (associated plant debris) could lead to a blackleg epidemic in China and as a consequence, restrictions on importations into China were placed on canola seed from Australia and Canada in 2009. We show that canola pods infected by L. maculans can lead to seed contamination, and resultant seedling infection, which then leads to cankering in adult plants. The fungus can sexually reproduce over summer on stubble derived from these plants. Airborne sexual spores are then released in the following year– thus completing the life cycle of the fungus from a contaminated seed and providing a potential source for an epidemic, particularly in countries such as China where canola cultivars do not have high levels of resistance to L. maculans. Furthermore, sexual fruiting bodies can also be produced on admixture. The presence of blackleg lesions on canola pods correlated with seed contamination by the blackleg fungus. Viability of L. maculans is reduced on contaminated seed over a twelve month period. Surveying blackleg disease in field trials in Australia showed that the presence and degree of stem cankers did not correlate with the level of pod infection. This suggests that pod lesions are likely to arise as a result of new infection events, rather than the pathogen moving from pre-existing infections (stem cankers) onto the pods. Furthermore, pod infections are likely to be a result of seasonal conditions rather than specific to regions where canola is cropped at a high intensity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blackleg disease (phoma stem canker) is caused by the fungus Leptosphaeria maculans and is the most serious disease of Brassica napus (canola, oilseed rape) worldwide, causing > US$900 M in losses per growing season worldwide (Fitt et al. 2008). This disease can be minimised through management strategies such as avoidance of last year’s crop debris (stubble), rotation with other crops and fungicide applications as well as breeding for resistance (Marcroft et al. 2004; Aubertot et al. 2006; Van de Wouw et al. 2015). Resistance to L. maculans is governed by both minor (qualitative) and major (quantitative) resistance (Delourme et al. 2006). Minor gene resistance, expressed in the stem of the adult plant, is poorly understood but is regarded as isolate-non-specific and due to the contribution of numerous genes each with a small additive effect (Delourme et al. 2006; Stuthmann et al. 2007). Major gene resistance is conferred by single major genes, which are usually dominant, and interact in a gene-for-gene manner with L. maculans whereby for each resistance gene in the host there is a corresponding avirulence gene in the pathogen (Balesdent et al. 2005). Seventeen resistance genes (Rlm1-Rlm11, RlmS, LepR1-LepR4, BLMR1, BLMR2) have been reported (Yu et al. 2005; Delourme et al. 2006; Yu et al. 2008; Van de Wouw et al. 2009; Long et al. 2011; Balesdent et al. 2013) and two of these, Rlm2 and LepR3, have been cloned (Larkan et al. 2013; Larkan et al. 2015). Of the corresponding avirulence genes, ten have been mapped and seven (AvrLm1, AvrLm2, AvrLm3, AvrLm4-7, AvrLm6, AvrLmJ1 and AvrLm11) have been cloned (Balesdent et al. 2005; Gout et al. 2006; Fudal et al. 2007; Parlange et al. 2009; Ghanbarnia et al. 2012; Balesdent et al. 2013; Plissonneau et al. 2015; Van de Wouw et al. 2014; Ghanbarnia et al. 2015). Recently it has been shown that this major gene resistance is expressed in seed pods as well as in cotyledons and true leaves (Elliott et al. 2015).
Leptosphaeria maculans is reported in all countries where canola is grown except China (West et al. 2000; Fitt et al. 2008; Liu et al. 2014; Zhang et al. 2014). China is the major canola producing country in the world, generating approximately 30 % of the world’s total production from approximately 8 m hectares sown to canola annually (Fitt et al. 2008; Liu et al. 2014). Chinese cultivars have been screened in Australia and have extremely low levels of resistance to L. maculans (Li et al. 2008). In recent times, L. maculans has spread into Canada (1975–1998), Poland (1994–2007) and Mexico (2001). Fitt et al. (2008) modelled the likelihood of an epidemic in China and suggested that China take precautions to prevent this occurring. The main disease threat was considered to be the import of L. maculans-contaminated canola seed and associated admixture, which includes small pieces of crop debris that cannot be easily separated from the seed - for instance, sections of the pod sheath and branches etc. Zhang et al. (2014) has proposed that it is unlikely that L. maculans could be efficiently transmitted from contaminated seed to cause disease on seedlings and that admixture was likely to be a bigger risk.
In 2009, China restricted the import of canola seed from countries where L. maculans was present (Zhang et al. 2014). By 2010 and 2012 an agreement was made with Canada and Australia, respectively, to import canola seeds into Chinese ports located in areas where very little canola is grown. However, for Australia, restrictions have remained in place for some export ports due to the high intensity of canola cropping in the surrounding region and high levels of seed infection as determined by the Administration of Quality Supervision, Inspection and Quarantine (AQSIQ).
The aims of the research described in this paper were to determine the risk of blackleg epidemics from contaminated seed and admixture arising from infected seed pods.
Material and methods
Collection of blackleg infected and clean pods and admixture of B. napus
A field site at Mt Hope, South Australia was identified as having high levels of blackleg pod infection following several periods of high rainfall during pod set (August through to October) in 2013. Pods were collected at plant maturity from the B. napus cultivar ATR- Stingray. This cultivar was chosen as it has no effective major-gene resistance to Australian isolates of L. maculans but good minor-gene resistance and is grown commercially across many regions of Australia (Marcroft et al. 2012a). Pods were categorised as either infected if blackleg lesions were visible (Fig. 1a) (termed infected pods) or clean if no lesions were visible (termed clean pods). Three hundred infected and 300 clean pods were picked randomly from the field site and stored in brown paper bags at room temperature under low humidity until further use (up to 12 months). Seeds were left within the pods until they were used in experiments.
Leptosphaeria maculans infected Brassica napus pods, admixture and the resulting contaminated seed. (a) B. napus pods infected with L. maculans at time of harvest. (b) Sample of admixture (crop debris) collected during the harvest of seeds. Mature pseudothecia are visible on a piece of pod sheath (arrow). (c) Seeds plated out to detect contamination from L. maculans. The square box indicates seeds that were in contact with the lesion on the pod. Hyphal growth is visible around the contaminated seed. (d) L. maculans pycnidia on contaminated seed
Admixture was also collected from the same site. After harvest of the site by a mechanical harvester, a seed sample (including admixture) was collected. Seed and admixture were separated by aspiration and the admixture was then put in a string bag and placed in a paddock (field) on bare earth. These conditions enable development of sexual fruiting bodies (pseudothecia) of L. maculans. In June of the following year (2014) the admixture was inspected for the presence of pseudothecia (Fig. 1b). Pieces of the admixture were attached to the inside of the lid of a petri dish using Vaseline®. The pieces of admixture were then moistened to trigger discharge of ascospores, which were then collected and cultured on 10 % Campbell’s V8 juice agar.
Relationship between pod infection and seed contamination
Ten infected and ten clean pods were carefully opened in a laminar flow hood and using sterile tweezers, each seed placed in the same order that they appeared in the pod, onto an agar plate containing ¼ strength potato dextrose medium (Fig. 1c). A separate agar plate was used for each pod. For infected pods, the positions of lesions were marked on the plates so that seeds that were in contact with the lesion could be identified. Plates were incubated at 22 °C with 12 h photoperiod for 3 days to allow fungal growth from any contaminated seed. After 3 days, fungal growth was visible around contaminated seed and asexual fruiting bodies (pycnidia) were present on some seed (Fig. 1c and d). To confirm that colonies were indeed L. maculans, the Internal Transcribed Spacer region of ribosomal DNA was amplified by PCR as described previously (Van de Wouw et al. 2008) and sequenced.
Since canola seed can be stored for up to 12 months prior to export from Australia, we determined whether the viability of L. maculans on seed would change over this time period. At the time of harvest and again at 1, 3, 6, 9 and 12 months post-harvest, seeds were removed from pods and examined for L. maculans contamination as described above. The seed remained in the pods and were stored in paper bags at room temperature until assessment.
Effect of seed contamination by blackleg on seed germination, seedling infection, seedling cankering and seedling death
Directly after harvest, 10 infected and 10 clean pods were split open in a laminar flow hood and the total number of seeds counted. All seeds from each pod were then sown into punnets (small rectangular plastic containers) containing sterile potting mix. One punnet was used per pod. Punnets were placed in a glasshouse (20 °C with natural daylight) to allow seeds to germinate. Twelve days after sowing, the numbers of germinated seedlings in each punnet were counted and percentage of seed germination determined. Seedlings were grown to the 3rd leaf stage and the presence of leaf lesions, seedling cankers and mortality rate were recorded.
Survival and completion of fungal life cycle from blackleg contaminated seed
Four plants from each punnet (originating from the same pod) described above were transplanted into 20 cm diameter pots and grown to maturity in a shade house in Horsham, Victoria. The first four seedlings from each punnet were selected, regardless of whether blackleg infection was detected or not. At maturity these plants were cut at the crown and the cross-sectional area was visually assessed for disease severity (percentage blackening—0, 10, 20 to 100 %) (Marcroft et al. 2012b). This experiment was undertaken in the shade house at a time of the year when canola was not sown in the surrounding area and at a location remote from canola crops to reduce any infection from wind-borne ascospores in the natural environment.
After assessment, the stems of all the mature plants were placed in a paddock (field) on bare earth, thus enabling development of pseudothecia of L. maculans. In June of the following year the stems were inspected for the presence of pseudothecia. Sections of the stems that appeared to have pseudothecia were excised and attached to the inside of the lid of a petri dish using Vaseline®. The stems were then moistened to trigger discharge of ascospores, which were then collected and cultured on 10 % Campbell’s V8 juice agar. To confirm that the resultant cultures were indeed L. maculans, the ITS region was amplified using PCR and sequenced, as described above.
Survey of pod lesions and disease severity across Eastern Australia
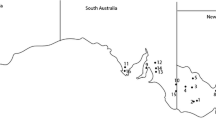
Brassica napus cultivar ATR-Stingray was sown in 21 field sites across eastern Australia (Fig. 2, Table 1). At maturity, plants from each site were assessed for both disease severity at the crown, and pod infection. Twenty consecutive plants from a single row within a plot were removed. To determine the percentage of pod infection, the number of pods on six of the plants was counted and the average number of pods per plant was determined. The number of pods with a lesion was then counted on all 20 plants. This information was used to calculate the mean percentage of pods with lesions per plant. The same 20 plants were assessed for disease severity at the crown, as described above.
Location of field sites where B. napus cultivar ATR- Stingray was assessed for L. maculans infected pods. Numbers refer to locations listed in Table 1
Results
Infected pods result in contaminated seeds and admixture
Directly after harvest, seeds from ten infected and ten clean pods were removed from the pod casing and placed on water agar in the same order as in the pod. From infected pods, 47 % of seeds were contaminated (Fig. 3). Furthermore, where seeds were in contact with the lesion in an infected pod, contamination by L. maculans was always detected. No contaminated seeds were detected in any of the clean pods at this time.
Seed contamination was assessed at 1, 3, 6, 9 and 12 months post-harvest. By 9 months, the percentage of contamination was 22 % suggesting that viability of L. maculans had reduced over time (Fig. 3). At 3 months and 9 months, three seeds out of 246, and three seeds out of 224, respectively, from clean pods showed contamination. Taken together, these data suggest that visual assessment of pods at harvest can be used as an indication of contaminated seed.
Admixture comprising pod sheaths and stems collected during harvest of seeds had pseudothecia (Fig. 1b). Ascospores were released when the pseudothecia were moistened.
Contaminated seeds can lead to infected seedlings, cankered mature plants and sexual fruiting bodies on stubble
Directly after harvest, seeds were sown to determine germination rates and ability for contaminated seed to lead to infected seedlings. Seeds sown from infected pods had a lower germination percentage (75.6 %) than seeds from clean pods (98.2 %). Furthermore, of the 203 seedlings that germinated from infected pods, eight seedlings (3.9 %) died by the 3rd leaf stage from blackleg infection and a further four plants (1.9 %) displayed blackleg leaf lesions. No blackleg symptoms were visible on any seedlings grown from seed from the clean pods (221 seeds in total).
Four seedlings grown from seed of each pod were grown to maturity and assessed for disease severity as well as plant mortality. Plants grown from the seed originating from infected pods had an average stem canker disease severity score of 26 %, with seven of the 40 plants having scores > 90 %. Furthermore, five of the 40 plants (12.5 %) grown from seed originating from infected pods died. In contrast, plants grown from the seed originating from clean pods had a very low average disease severity score (0.5 %) with two of the 40 plants having a disease severity score of 10 %. No plants died.
Stems of all forty plants were placed on bare earth over the summer. Two stems from plants originating from infected seed produced sexual fruiting bodies and ascospores were captured when stems were moistened.
Taken together, these data show that contaminated seed can result in infected seedlings, stem cankers in mature plants and sexual reproduction on stubble. Thus L. maculans can complete its life cycle from contaminated seed and on admixture.
Pod infection is not specific to crops in particular regions of south eastern Australia
A three-year survey of levels of pod infection across 21 field sites in south eastern Australia (Table 1) sown to cultivar ATR-Stingray was conducted. In 2013, the percentage of pods infected with blackleg ranged from 0 % on plants at four sites to 41 % at Mt Hope, South Australia. In 2014, the percentage of pods infected with blackleg was much lower, ranging from 0 % at 18 sites to 9 % at Mt Hope. In 2015, the percentage of pod infection was low in NSW and Vic (ranging from 0–4 %) but higher in SA (ranging from 0 to 23 %). Plants at 15 sites had blackleg pod infections in 2013, but no pod infections in 2014. In both 2014 and 2015, no pod infection was detected in any Victorian sites. The only site that had pod infection every year was Mt Hope in SA.
Plants at Mt Hope had 41 % pod infection in 2013 and this decreased to 9 % in 2014 while plants at Lockhart and Kaniva had 11 % and 15 %, respectively, in 2013 and no infection in 2014. These changes in pod infection are possibly due to environmental factors such as rainfall events during pod set (August-October). Mt Hope, Lockhart and Kaniva had 130, 109 and 141 mm of rain, respectively, during pod set in 2013; whereas in 2014, only 31, 54 and 47 mm of rain, respectively (Australian Bureau of Meteorology).
Pod infection is not correlated with disease severity
As well as pod infection, stem canker disease severity of B. napus cultivar ATR- Stingray was assessed at the sites described above in 2013 (Table 1). Disease severity scores ranged from 0 to 55 % across all sites. The percentage of blackleg infected pods was plotted against disease severity and the linear regression calculated (Fig. 4). A R2 value of 0.0215 was obtained suggesting there was no correlation between the presence of pod lesions and stem canker disease severity.
Discussion
Leptosphaeria maculans has spread into all canola growing regions except China (Fitt et al. 2008). An epidemic in China may occur if contaminated seeds and/or admixture fall from trucks during movement of seed from the ports through agricultural areas where canola is grown. Currently trade restrictions are in place preventing import of canola seeds into China from Australian ports including Wallaroo and Port Adelaide, South Australia and Albany, Western Australia, due to high intensity of canola cropping in those surrounding areas and the high levels of seed infection as determined by AQSIQ. Our data collected in three years—2013, where rainfall was high during pod set, and 2014 and 2015 where rainfall was low, suggest that pod infection and subsequent seed contamination may depend on seasonal rainfall, rather than intensity of canola cropping or levels of canker disease severity. Our data suggest that restrictions on certain Australian ports may not be an effective measure for minimising export of contaminated seed.
We have shown that contaminated seed can lead to infected seedlings when grown in glasshouses. This is consistent with findings in Western Australia and Canada where contaminated seed was sown into fields and the resultant seedlings developed blackleg lesions and resultant mature plants had stem cankers (Wood and Barbetti 1977; Chigogora and Hall 1995). These experiments had been carried out to investigate the impact of contaminated seed leading to new infections in neighbouring fields. In the current study we have been interested in whether contaminated seed can lead to an epidemic in a country or geographic region where L. maculans has not previously been reported. For this to occur, the fungus must undergo the sexual cycle. Our data show that cankered plants grown from contaminated seed can lead to the production of sexual fruiting bodies and therefore L. maculans can complete its life cycle. Furthermore, admixture can also lead to the production of sexual fruiting bodies. As mentioned previously, Fitt et al. (2008) modelled the probability and spread of a blackleg epidemic in China with the assumption that contaminated seed and/or admixture could lead to the release of ascospores. Our data support these assumptions.
Previously Chigogora and Hall (1995) showed a correlation between the severity of stem cankers and pod infection. This is inconsistent with our findings. We assessed both stem canker severity and pod infection on individual plants while Chigogora and Hall (1995) looked at entire plots, which could explain the difference in results. Our data suggest that pod infection is likely to result from spores released as a result of rainfall during the podding growth stage, rather than from growth up the plant from the stem canker into the pods. It is not known whether the spores causing this infection are ascospores or pycnidiospores.
Whether L. maculans is growing on the surface of the seed only or whether it can infect the seed as well is unknown. In the current study, six seeds from 470 (1.3 %) collected from clean pods were contaminated with L. maculans. This suggests that the fungus might have entered the seed pod but not caused a lesion. Our data show that the viability of L. maculans decreases dramatically over a 9 month time-frame following harvest. This is relevant as seed is often stored prior to export. Therefore, decreases in the viability of L. maculans on contaminated seed decreases the risk of an epidemic after export. In the current study, seeds were maintained in the pod and therefore protected from the environment. If the seeds were not maintained within the pod, the viability may have decreased even further.
References
Aubertot, J. N., West, J. S., Bousset-Vaslin, L., Salam, M. U., Barbetti, M. J., & Diggle, A. J. (2006). Improved resistance management for durable disease control: a case study of phoma stem canker of oilseed rape (Brassica napus). European Journal of Plant Pathology, 114, 91–106.
Balesdent, M. H., Barbetti, M. J., Li, H., Sivasithamparam, K., Gout, L., & Rouxel, T. (2005). Analysis of Leptosphaeria maculans race structure in a worldwide collection of isolates. Phytopathology, 95, 1061–1071.
Balesdent, M. H., Fudal, I., Ollivier, B., Bally, P., Grandaubert, J., Eber, F., et al. (2013). The dispensable chromosome of Leptosphaeria maculans shelters an effector gene conferring avirulence towards Brassica rapa. New Phytologist, 198, 887–898.
Chigogora, J. L., & Hall, R. (1995). Relationships among measures of blackleg in winter oilseed rape infection of harvested seed by Leptosphaeria maculans. Canadian Journal of Plant Pathology, 17, 25–30.
Delourme, R., Chevre, A. M., Brun, H., Rouxel, T., Balesdent, M. H., Dias, J. S., et al. (2006). Major gene and polygenic resistance to Leptosphaeria maculans in oilseed rape (Brassica napus). European Journal of Plant Pathology, 114, 41–52.
Elliott, V. L., Marcroft, S. J., Howlett, B. J., & Van de Wouw, A. P. (2015). Gene-for-gene resistance is expressed in cotyledons, leaves and pods in the Leptosphaeria maculans-Brassica napus pathosystem but not during stem colonisation. Plant Breeding, Manuscript accepted.
Fitt, B. D. L., Hu, B. C., Li, Z. Q., Liu, S. Y., Lange, R. M., Kharbanda, P. D., et al. (2008). Strategies to prevent spread of Leptosphaeria maculans (phoma stem canker) onto oilseed rape crops in China; costs and benefits. Plant Pathology, 57, 652–664.
Fudal, I., Ross, S., Gout, L., Blaise, F., Kuhn, M. L., Eckert, M. R., et al. (2007). Heterochromatin-like regions as ecological niches for avirulence genes in the Leptosphaeria maculans genome: map-based cloning of AvrLm6. Molecular Plant-Microbe Interactions, 20, 459–470.
Ghanbarnia, K., Fudal, I., Larkan, N. J., Links, M. G., Balesdent, M., Profotova, B., et al. (2015). Rapid identification of the Leptosphaeria maculans avirulence gene AvrLm2 using an intraspecific comparative genomics approach. Molecular Plant Pathology.
Ghanbarnia, K., Lydiate, D. J., Rimmer, S. R., Li, G., Kutcher, R., Larkan, N. J., et al. (2012). Genetic mapping of the Leptosphaeria maculans avirulence gene corresponding to the LepR1 resistance gene of Brassica napus. Theoretical and Applied Genetics, 124, 505–513.
Gout, L., Fudal, I., Kuhn, M. L., Blaise, F., Eckert, M., Cattolico, L., et al. (2006). Lost in the middle of nowhere: the AvrLm1 avirulence gene of the Dothideomycete Leptosphaeria maculans. Molecular Microbiology, 60, 67–80.
Larkan, N. J., Lydiate, D. J., Parkin, I. A., Nelson, M. N., Epp, D. J., Cowling, W. A., et al. (2013). The Brassica napus blackleg resistance gene LepR3 encodes a receptor-like protein triggered by the Leptosphaeria maculans effector AVRLM1. New Phytologist, 197, 595–605.
Larkan, N. J., Ma, L., & Borhan, H. (2015). The Brassica napus receptor-like protein RLM2 is encoded by a second allele of the LepR3/Rlm2 blackleg resistance locus. Plant Biotchnology Journal.
Li, C. X., Wratten, N., Salisbury, P., Burton, W., Potter, T. D., Walton, G., et al. (2008). Response of Brassica napus and B. juncea germplasm from Australia, China and India to Australian populations of Leptosphaeria maculans. Australasian Plant Pathology, 37, 162–170.
Liu, Z., Akinwunmi, O., Latunde-Dada, A. O., Hall, A. M., & Fitt, B. D. L. (2014). Phoma stem canker disease on oilseed rape (Brassica napus) in China is caused by Leptosphaeria biglobosa ‘brassicae’. European Journal of Plant Pathology, 140, 841–857.
Long, Y., Wang, Z., Sun, Z., Fernando, D. W. G., McVetty, P. B. E., & Li, G. (2011). Identification of two blackleg resistance genes and fine mapping of one of these two genes in a Brassica napus cultivar ‘Surpass400’. Theoretical and Applied Genetics, 122, 1223–1231.
Marcroft, S. J., Elliott, V. L., Cozijnsen, A. J., Salisbury, P. A., Howlett, B. J., & Van de Wouw, A. P. (2012a). Identifying resistance genes to Leptosphaeria maculans in Australian Brassica napus cultivars based on reactions to isolates with known avirulence genotypes. Crop and Pasture Science, 63, 338–350.
Marcroft, S. J., Sprague, S. J., Pymer, S. J., Salisbury, P. A., & Howlett, B. J. (2004). Crop isolation, not extended rotation length, reduces blackleg (Leptosphaeria maculans) severity of canola (Brassica napus) in south-eastern Australia. Australian Journal of Experimental Agriculture, 44, 601–606.
Marcroft, S. J., Van de Wouw, A. P., Salisbury, P. A., Potter, T. D., & Howlett, B. J. (2012b). Rotation of canola (Brassica napus) cultivars with different complements of blackleg resistance genes decreases disease severity. Plant Pathology, 61, 934–944.
Parlange, F., Daverdin, G., Fudal, I., Kuhn, M. L., Balesdent, M. H., Blaise, F., et al. (2009). Leptosphaeria maculans avirulence gene AvrLm4-7 confers a dual recognition specificity by the Rlm4 and Rlm7 resistance genes of oilseed rape, and circumvents Rlm4-mediated recognition through a single amino acid change. Molecular Microbiology, 71, 851–863.
Plissonneau, C., Daverdin, G., Ollivier, B., Blaise, F., Degrave, A., Fudal, I., Rouxel T., & Balesdent, B. H. (2015). A game of hide and seek between avirulence genes AvrLm4-7 and AvrLm3 in Leptosphaeria maculans. New Phytologist. doi:10.1111/nph.13736.
Stuthmann, D. D., Leonard, J. J., & Miller-Garvin, J. (2007). Breeding crops for durable resistance to disease. Advances in Agronomy, 95, 319–367.
Van de Wouw, A. P., Lowe, R. G. T., Elliott, C. E., Dubois, D. J., & Howlett, B. J. (2014). An avirulence gene, AvrLmJ1, from the blackleg fungus, Leptosphaeria maculans, confers avirulence to Brassica juncea cultivars. Molecular Plant Pathology, 15, 523–530.
Van de Wouw, A. P., Marcroft, S. J., Barbetti, M. J., Hua, L., Salisbury, P. A., Gout, L., et al. (2009). Dual control of avirulence in Leptosphaeria maculans towards a Brassica napus cultivar with ‘sylvestris-derived’ resistance suggests involvement of two resistance genes. Plant Pathology, 58, 305–313.
Van de Wouw, A. P., Marcroft, S. J., & Howlett, B. J. (2015). Blackleg disease of canola in Australia. Crop and Pasture Science, Manuscript accepted.
Van de Wouw, A. P., Thomas, V. L., A.J., C., Marcroft, S. J., Salisbury, P. A., & Howlett, B. J. (2008). Identification of Leptosphaeria biglobosa ‘canadensis’ on Brassica juncea stubble from northern New South Wales. Australasian Plant Disease Notes, 3, 124–128.
West, J. S., Evans, N., Liu, S., Hu, B. C., & Peng, L. (2000). Leptosphaeria maculans causing stem canker of oilseed rape in China. Plant Pathology, 49, 800.
Wood, P. M., & Barbetti, M. J. (1977). The role of seed infection in te spread of blackleg of rape in Western Australia. Australian Journal of Experimental Agriculture and Animal Husbandry, 17, 1040–1044.
Yu, F., Lydiate, D. J., & Rimmer, S. R. (2005). Identification of two novel genes for blackleg resistance in Brassica napus. Theoretical and Applied Genetics, 110, 969–979.
Yu, F., Lydiate, D. J., & Rimmer, S. R. (2008). Identification and mapping of a third blackleg resistance locus in Brassica napus derived from B. rapa subsp. sylvestris. Genome, 51, 64–72.
Zhang, X., White, R. P., Demir, E., Jedryczka, M., Lange, R. M., Islam, M., et al. (2014). Leptosphaeria spp., phoma stem canker and potential spread of L. maculans on oilseed rape crops in China. Plant Pathology, 63, 598–612.
Acknowledgments
We thank the Grains Research and Development Corporation for funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
Angela P. Van de Wouw and Vicki L. Elliott contributed equally to this work.
Rights and permissions
About this article
Cite this article
Van de Wouw, A.P., Elliott, V.L., Ware, A. et al. Infection of canola pods by Leptosphaeria maculans and subsequent seed contamination. Eur J Plant Pathol 145, 687–695 (2016). https://doi.org/10.1007/s10658-015-0827-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-015-0827-0