Abstract
The concentration and bioaccessibility of potentially toxic metals, including As, Cd, Cr, Cu, Mn, Ni, Pb and Zn, were determined in surface soil samples from a mining community (Kenyasi) and a non-mining community (Sunyani) in Ghana, to investigate the contribution of mining activities to the environmental burden of potentially toxic metals. The study found significant differences in metal concentrations (p < 0.05) in As, Cd, Cu, Mn, Ni, and Zn, but no significant difference (p > 0.05) in Pb and Cr between the two communities. The study found a moderate correlation between pH and metal concentrations in the mining community and a moderate positive correlation with As, Cd, Cr, Cu, Ni, and Zn in the non-mining community. The distribution pattern revealed elevated levels of toxic metals in the southeastern corridor of the mining community, which is close to a gold mine. Most heavy metals were concentrated in the commercial community’s southern zone, with more residents and private elementary schools. Metal bioaccessibility was variable, and except for Cu and Zn, the mean bioaccessibility was less than 50% for a given metal. Contamination factor, geoaccumulation index, and soil enrichment factor suggested very high contamination of Cd, and a considerable to moderate contamination of As, Ni, Zn, and Cu at both the mining and non-mining communities. The above observations and the pollution and risk indices employed in this study confirmed that the mining community was more polluted (PLI = 2.145) than the non-mining community (PLI = 1.372). The total metal hazard (HI) exceeded thresholds by three and four times at non-mining and mining sites. Regular monitoring is necessary, especially in the mining community, to prevent soil metal accumulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals pose a significant threat to humans and biota once their concentrations rise above their natural background concentrations (Kinuthia et al., 2020). The biological effects of metals vary. For instance, copper, iron, zinc, and manganese play biochemical and cellular functions and are required by the human body. In contrast, arsenic, cadmium, mercury, and lead are harmful even at minute concentrations and have no known beneficial biochemical functions. Many natural and anthropogenic factors may contribute to toxic metal contamination of the environment. The natural processes include forest fires, atmospheric deposition, and surface mineralization, while human inputs comprise mining, vehicular and incinerator emissions, industrial discharges, and metal-containing agricultural fertilizers and pesticides (Mihankhah et al., 2020). Human exposure to metal-borne soil particles is primarily through inhalation, dermal contact, and/or ingestion (Darko et al., 2017a, 2017b). However, at many heavy metal contamination sites, the incidental soil ingestion pathway is the most significant and always the most dangerous route of exposure, especially for children.
Mining is a prime source of metal pollution in the environment. However, mining contributes substantially to a nation’s growth and development and cannot be stopped completely. The mining industry in Ghana, for instance, accounts for 5% of the nation’s gross domestic product (GDP) with gold mining accounting for 90% of total mineral exports (Karn et al., 2021). Therefore, the primary aim of Ghana’s mining and mineral industry and development is tailored toward gold. Ghana is the largest gold producer in Africa, producing about 80.5 tons annually (Wasserman et al., 2003). The detrimental environmental effects caused by mining are widely known globally (Cordy et al., 2013). Whether large-scale or small-scale, mining activities have the potential to pollute the surface soil of surrounding communities. The negative effects of gold mining in Ghana, such as human insecurity, environmental and socioeconomic impacts, and related governance challenges, have previously been reported (Ros–Tonen et al., 2021).
Metal exposure can be estimated using pollution and hazard indices derived from total metal concentrations. It is commonly assumed that all the measured metal concentration is bioavailable to humans after exposure, but this is not the case. The total concentrations overestimate exposure because the soil's physicochemical properties can alter metal mobility, causing a decrease in bioavailability (Chen et al., 2020).
The in-vivo bioavailability tests have been used to estimate the percentage of available metal contaminants that can absorb into the human digestive tract (Basta & Juhasz, 2014). However, due to high cost of in-vivo studies, time constraints, and ethical concerns related to the in-vivo bioavailability studies, the in-vitro digestion protocols that mimic the human digestive system have been developed (Darko et al., 2019). The in-vitro methods measure bioaccessibility, which represents the portion of the soluble contaminant in a synthetic digestive medium which could be available for absorption. Incorporating bioaccessibility results into risk analysis improves the overall accuracy because it gives a good representation of the amount of the contaminant that goes into the bloodstream (Dodd et al., 2017).
The current study investigated the potential sources of toxic metals and their distribution patterns in Kenyasi, a Ghanaian town known for mining. Results from sampled soils from Sunyani, a town devoid of mining but known for commercial activities, are used as a comparison. Hazard indices are also used to measure contamination levels, and an in-vitro bioaccessibility assay is employed to estimate the potential risk to human health from incidental ingestion of soil-borne metal contaminants.
Materials and methods
Study area
The Sunyani municipality (latitude 7.34°; longitude −2.33°) covers approximately 506.7 km2 and is the capital of Ghana's Bono Region. The Sunyani municipality is in Ghana's wet semi-equatorial climate zone. Monthly temperatures range from 23 to 33 °C, with the lowest in August and the highest in March and April. The average amount of rain is 88.99 cm (Ghana Statistical Service, 2014). The municipality experiences a double maxima rainfall pattern, with the main rainy season lasting from March to September and the minor rainy season lasting from October to December. During the rainy season, the average relative humidity rises to around 80% and falls to less than 70% during the dry season. The municipality is geologically underlain by the Precambrian formation, which is thought to be rich in mineral deposits (Ghana Statistical Service, 2014).
The municipality has no records of mining. The major occupation of the indigenes is farming, which is usually done at the outskirts of town. In contrast, Kenyasi (latitude 6.98° and longitude -2.39°), the capital of the Asutifi North District in the Ahafo Region, has been home to both large-scale and artisanal small-scale gold mining operations for many years (Kwaansa-Ansah et al., 2017). The area is made up of the Birimian rocks and Tarkwaian rocks, which form metavolcanic and meta-sedimentary units, respectively (Perrouty et al., 2012). Agriculture is the most common occupation in Kenyasi. Plantain and cassava are two food crops grown by residents of the area, primarily for subsistence, while cocoa is the main cash crop.
Soil sampling
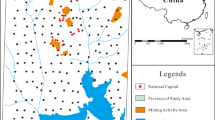
The study areas were mapped on a 0.5 × 0.5 km2 cluster, and soil samples were taken at coordinate intersections (Fig. 1). Where the original points were inaccessible due to interferences, such as tarred roads and houses, sampling points were moved 3 m to the left or right of the exact sampling point within the same cluster (Darko et al., 2017a, 2017b).
A clean, dry shovel was used to collect approximately 500 g of soil at a surface soil depth of 0–10 cm, which is presumed to be the layer with which humans are most likely to come into contact during daily routines and thus provides improved human risk assessment (Dodd et al., 2017). The digging implements were scrubbed with detergents and rinsed with deionized water after each sample point to minimize cross-contamination during sampling. Ninety soil samples were taken across the Sunyani municipality and 49 from Kenyasi. All soil samples were transported to the laboratory and air-dried in a fume hood at room temperature. The dried samples were thoroughly cleaned of debris, stones, and pebbles before being powdered and sieved to a sieved particle size of 250 m, representing the fraction of soil particle size that can stick to the skin, particularly the hands.
Soil physicochemical parameters (pH, electrical conductivity and total organic matter)
Electrical conductivity and soil pH were measured using a Oakton PCSTestr35 multiparameter probe (Quebec, Canada) from a 1:5 (soil/water) supernatant liquid (Amponsah et al., 2022). The total organic carbon in the samples was determined using the loss on ignition method (Darko et al., 2019). Briefly, a 1 g aliquot of soil was directly weighed into a crucible and heated at 105 °C for 2 h. After cooling to room temperature in a desiccator, the samples were placed in a pre-heated Thermolyne muffle furnace at 550 °C for 4 h. The samples were removed from the furnace, cooled to room temperature (overnight) in a desiccator, and re-weighed. Organic matter content was determined by calculating the difference between initial and final weights. The analyses were done in triplicates.
X-ray fluorescence analysis
Metal concentrations in the samples were initially screened using a Niton XL3t GOLDD + field portable X-ray fluorescence (FP-XRF) spectrometer (Rweyemamu et al., 2020), and the findings were later confirmed on the inductively coupled plasma-mass spectrometer (ICP-MS). Analysis of NIST 2709a standard reference materials (SRM), procedure blanks, and recovery tests were among the quality checks implemented during the XRF runs. Satisfactory recoveries were obtained for As (88 ± 7%), Cd (57 ± 5%), Cr (78 ± 6%), Cu (102 ± 14%), Mn (120 ± 16%), Ni (91 ± 12%), Pb (89 ± 8%), and Zn (107 ± 15%). The repeatability of the analysis—variability in the measurement system is caused by the measurement device—was assessed using 5 replicates of the reference material and was found to be 4–5%, indicating a high level of reliability (Huang et al., 2023).
Sample digestion and ICP-MS analysis
Following US EPA Method 3051A, 0.3 g aliquots of soil samples were microwave-digested (Anton Parr Multiwave GO Plus, Saint-Laurent, Canada) in 9 mL concentrated nitric acid and 3 mL concentrated hydrochloric acid (da Silva et al., 2014). The resulting solutions were analyzed with an Agilent 7800 ICP-MS (Agilent Technologies. Canada), following the US EPA Method 6020B protocols (Akanchise et al., 2020). The quality control protocols included the analysis of procedure blank, a standard reference material and a duplicate sample. The concentrations in the procedure blanks were < 0.1 mg/kg indicating minimal interferences from the reagents and equipment. The recoveries for the NIST 2711a SRM were As (91%), Cd (95%), Cr (77%) Cu (83%), Fe (85%), Ni (101%), Pb (92%) and Zn (82%) which were all within the control limits. The relative standard deviations of 5 replicate analyses of the SRM indicated good precision, being 5.3% for As, 2.9% for Cd, 0.1% for Cr, 6.9% for Cu, 3.3% for Fe, 0.2% for Mn, 2.3% for Ni, 1.8% for Pb, and 2.3% for Zn.
In-vitro bioaccessibility assay
The procedure for the in-vitro bioaccessibility assay was based on the US EPA standard operating procedure (US EPA, 2017). In summary, a 1 g aliquot of sieved sample was extracted for about 1 h by end-to-end rotation in 100 mL of 30 g/L glycine buffered to pH 1.5. The extract was filtered through a 0.45 m cellulose acetate syringe filter, and the filtrate was assayed for metals using an ICP-MS (Chen et al., 2020). The elemental bioaccessibility for each sample was expressed as the percentage extract concentration relative to the total metal concentration. Bioaccessibility was not determined for metals whose concentrations were below the method’s detection limits.
Extend of contamination
The level of metal contamination was assessed using the contamination factor, geoaccumulation index, enrichment factor, and potential ecological risk index (Qing et al., 2015).
Contamination factor
The contamination factor (CF), ratio of the sampled metal concentration to background concentration (Eq. 1), was used to determine the level of contamination (Dung et al., 2013).
The reference samples were collected from the UNER Botanical Gardens in Sunyani because it is pristine and has not been impacted by human activities such as mining. Soil contamination was classified as uncontaminated (CF < 1), moderate (CF = 1–3), significant (CF = 3–6), or highly contaminated (CF > 6), (Asamoah et al., 2021).
Geoaccumulation index
Equation 2 was used to calculate the geoaccumulation index (Igeo), a measure of contamination by each metal.
Mc and Bn denote the metal’s mean concentration, and geochemical baseline values, respectively, while the constant, 1.5, accounts for lithogenic and anthropogenic inputs (Asare et al., 2019).
Enrichment factor
The enrichment factor (EF) was used to differentiate between anthropogenic and natural sources of metal pollution (Eq. 3). Fe was used for the normalization because it has a relatively high concentration in the natural environment.
Mc and Fec are the sampled metal and iron concentrations, respectively, whereas Mr and Fer are the metal and iron concentrations in shale (Li et al., 2021). An EF greater than one indicates anthropogenic metal enrichment, while an EF less than one indicates no metal enrichment.
Risk index and potential ecological risk
To assess the ecological risk each metal presents, the potential ecological risk coefficient (\({E}_{r}f\)) or risk indices (RI) were computed (Eq. 4).
\({T}_{r}f\) is the toxic response factor (metal toxicity), while CF is the contamination factor. The \({T}_{r}f\) used are: As = 10, Cd = 30, Cr = 2, Cu = Mn = Pb = 5, Ni = 6, and Zn = 1 (Hakanson, 1980). The ecological risks classified as: low risk (\({E}_{r}f\) < 50), moderate risk (\({E}_{r}f\) = 50–100), considerable risk (\({E}_{r}f\) = 100–300), high risk (\({E}_{r}f\) = 300–500), very high risk (\({E}_{r}f\) ≥ 500).
The potential ecological risk index (PERI), the weighted average of the individual risk indices (Eq. 5), was used to assess the extent of metal pollution.
where n is the number of metals studied (8 in this study). The literature-based categorization (Swarnalatha et al., 2015) used were low risk (PERI < 80), moderate risk (PERI = 80–100), significant risk (PERI = 100–150), high risk (PERI = 150–300), and very high risk (PERI > 300).
Fractions of the daily reference dose of metals
The fractions of daily reference dose (ßτ), representing the percentage of the daily dose that individuals may ingest due to incidental soil ingestion per gram of soil, were computed using Eq. 6. The factor 1.6 represents the modeled soil ingestion rate, derived from a mechanistic approach that considers hand-to-mouth activity and using Canadian receptor characteristics (CEPA, 1999; Wilson et al., 2013).
Human health risk assessment
The chemical daily intake (CDI in mg kg−1 day−1) due to incidental ingestion of the metals were computed using Eq. 7, the modeled soil ingestion rate (1.6 mg/kg/day) (CEPA, 1999; Wilson et al., 2013), assumed adult body weight of 70 kg (WHO, 2021), life expectancy of 64 years (World Bank, 2020), and assuming life-long exposure.
The hazard quotient (HQ) for non-cancer risk was calculated by dividing the products of the CDI and adjusted for relative bioavailability by the respective tolerable daily intake (in mg/kg/d) as shown in Eq. 8.
The relative bioavailability (RBA) of arsenic and lead was calculated using validated bioaccessibility-to-bioavailability conversions (US-EPA, 2002), as shown in Eqs. 9 and 10, respectively.
Due to the lack of in-vivo/in-vitro equations for the remaining metals, the RBA of the other metals' bioaccessibility values were used directly. The tolerable daily intake values (in mg/kg/day) used were 0.0003 for As, 0.0005 for Cd, 0.003 for Cr, 0.01 for Cu, 0.02 for Ni, 0.011 for Mn, 0.0036 for Pb and 0.3 for Zn (Health Canada, 2008).
The overall hazard, also called hazard index (HI) was obtained as the sum of the individual hazard quotients (HQ) for each metal, as shown by Eq. 11.
Statistical analyses
Three basic statistical methods – correlation, regression analysis, and two independent samples t-tests were used to analyze the data. The two independent samples t-tests (Eq. 6), was used to determine the variations in the measured parameters and to test whether the population means of the sites differed. Because our dataset is continuous, Pearson's correlation analysis was used to assess the strength of the relationship between physicochemical parameters (EC, pH), and metal concentrations. A two-tailed test at 95% confidence interval was used to compare whether the means at the different sites were significantly different.
Results and discussion
Physicochemical parameters
Table 1 shows the soil pH, total organic carbon, and electrical conductivities for the mining and the non-mining sites. The pH values for the mining community ranged between 4.80 and 9.20, with approximately 53% of the samples recording pH values less than 7, denoting the acidic nature of the soil samples. The remaining samples (47%) were slightly neutral to strongly basic. This trend contrast with the non-mining community which recorded pH ranging from 5.59 to 10.25, with approximately 30% of the samples being acidic with pH < 7. Out of the remaining 70% non-acidic soils, 12% had pH greater than 8.4, indicating that they are strongly alkaline. The group mean in relation to pH are statistically significantly different with a p value of 0.028 (p < 0.05). The soil pH affects the rate of migration and retention of heavy metal in soils. At elevated soil pH, heavy metals have more excellent retention and lower solubility probably due to their ability to form complexes at low pH (Sintorini et al., 2021).
The mining site exhibited a range of total organic carbon (TOC) values from 2.20 to 8.90%, averaging 3.28%. Conversely, the non-mining site displayed TOC values ranging from 4.98 to 6.35%, with an average of 5.91%. The TOC content at the non-mining site was significantly higher (p = 0.001) compared to the mining site. The elevated TOC levels at the non-mining site could be attributed to diverse land use patterns, vegetation cover, or organic contributions from the surrounding environments (Leul et al., 2023). The mining activities, soil disturbance, vegetation removal, and contamination can all contribute to a low TOC content in a mining area (Cetin et al., 2023). This has implications for metal availability in soils because it reduces organic matter's ability to bind metals (Kan et al., 2022). This can increase metal mobility, leaching, and runoff, impacting water quality. Furthermore, low TOC can impact nutrient cycling, soil structure, and plant growth. The effect of low TOC on metal availability is affected by local condition, hence soil conservation practices, vegetation restoration, organic amendments, and site-specific studies are recommended to address these implications.
The electrical conductivity of each sample indicates the concentration of ions present. Higher electrical conductivity in soil is associated with a high number of hydrogen ions in the soil, which may reduce metal mobility in the soil (Yuan et al., 2021). The electrical conductivity of soil samples in the mining community ranged from 120.49 to 802.00 µS/cm with an average value of 159.45 ± 44.87 µS/cm, while it ranged from 120.06 to 1239.00 µS/cm with an average value of 238.78 ±91.45 µS/cm in the non-mining community. The EC values differed significantly with a p value of 0.012 (p < 0.05). Both communities’ mean values are far lower than the CCME soil quality guideline of 2000 µS/cm (CCME, 2007), indicating low levels of inorganic ions in the surface soils (Akoto et al., 2017).
ICP-MS and FP-XRF data comparison
There was a strong linear correlation between the FP-XRF the ICP-MS datasets. The correlation coefficients for As, Cd, Mn, Ni, Pb, and Zn were 0.98, 0.96, 0.45, 0.59, 0.99, and 0.98, respectively. The slopes of the regression lines for As, Cd, Pb, and Zn were greater than 0.90, indicating excellent comparability between the FP-XRF and ICP-MS data and hence no need for data corrections, whereas the r2 values for Cr, Cu, Mn, and Ni ranged between 0.27 and 0.59.
Heavy metal levels across commercial and mining communities
Because Ghana currently lacks soil quality guidelines, the study's findings were compared to values from the control site, Canadian soil quality guidelines, and Clarke values. Table 2 presents the statistical summary of the analysis. The average arsenic concentrations were significantly different (p < 0.05) between the two communities. The mean arsenic content recorded in the mining community was 7.31 ± 4.48 mg/kg, greater than the 4.31 ± 2.25 recorded in the non-mining community. This suggests that anthropogenic activities particularly mining, have caused an increase in the levels of As in the Kenyasi township. At both communities, the mean arsenic level was lower than the CCME soil quality guideline of 12 mg/kg, but exceeded the Clarke value of 1.5 mg/kg (Burchfiel, 1983) and was far higher than the control site value of 0.02 mg/kg. This implies that commercial activities in the Sunyani township have also increased arsenic levels. This necessitates a regular monitoring scheme to mitigate the potential impacts on humans, plants, and other living organisms in these two communities. Arsenic concentrations in surface soils at peri-urban areas in Kumasi (Konwuruk et al., 2021) is higher than the average in the current study, most likely due to higher anthropogenic activities in Kumasi than in Sunyani and Kenyasi, Kumasi being busier and larger.
Cadmium concentrations differed significantly (p < 0.05) between the two communities, and were generally higher in the mining community (Kenyasi), ranging from ≤ 4.88 to 37.75 mg/kg with a mean value of 17.32 ± 8.86 mg/kg. Despite being lower than the mining community’s mean level and the CCME guideline, the cadmium concentrations in the non-mining community (7.04 ± 2.77 mg/kg) were 5 times higher than the Clarke value and 70 times the control site. This non-essential metal may have entered the environment via uncontrolled disposal of municipal wastes, sewage sludge, and nickel–cadmium batteries. These are prevalent activities found in Sunyani and may be probable factors for the elevated Cd levels. The concentrations of Cd found in the non-mining community (Sunyani) was much higher than in Kumasi (Darko et al., 2017a, 2017b)—a bigger and more populated city in Ghana.
Chromium concentration in the commercial hub, Sunyani (71.24 ± 46.19 mg/kg) was higher than in the mining community (55.48 ± 49.14 mg/kg). This may be due to the industrial use of chromium in steel production, petroleum products, metal plating, vehicular emissions, wood processing, burning of municipal wastes, and fertilizer which predominates in Sunyani than in Kenyasi. Elevated levels of Cr have been linked to allergic dermatitis and hair loss, and inflammation of the nose lining by Cr inhalation (Guo et al., 2020). The p value of 0.062 which is greater than 0.05 shows that there was no significant difference among the two communities with respect to Cr concentrations.
Copper at the study communities were generally low and significantly different (p < 0.05), with the mining community (Kenyasi) recording a higher concentration, than the non-mining, commercial hub (Sunyani). The mean levels at both communities were lower than the CCME soil quality guideline but greater than the control site. High levels of copper in surface soils are usually linked to anthropogenic inputs such as agricultural and household pesticides. Copper concentrations in uncontaminated surface soils are typically less than 50 mg/kg (Glennon et al., 2014), indicating that the surface soils in both communities are not contaminated with copper.
The average concentration of zinc in the mining community (159.19 ± 33.83 mg/kg) was about three times greater than the average mean in the non-mining community (57.45 ± 17.74 mg/kg). The mean value of zinc recorded at the commercial hub is greater than recorded in a study conducted at Sunyani magazine (Asamoah et al., 2021). Zinc content at the study communities were lower than the CCME guideline but greater than the control site. Elevated levels of zinc have been associated with anthropogenic activities such as mining, fossil fuel combustion, industrial wastewater, and automobile emissions (Keshavarzi & Kumar, 2020). The mean concentration of Zn differed significantly (p < 0.05) among the two communities.
Levels of lead obtained in the two study communities were comparatively low and not significantly different (s > 0.05), recording a mean concentration of 6.09 ± 3.35 mg/kg and 5.61 ± 1.52 mg/kg in the mining and the non- mining communities, respectively. Lead accumulation in top soil has been linked to mining activities, as well as waste disposal and fossil fuel combustion (Assi et al., 2016). The mean concentrations at both communities were greater than the 1.16 mg/kg recorded at the control site. This is probably due to the anthropogenic inputs. For instance, historical use of leaded gasoline and industrial metallurgical processes may contribute to the concentration in the commercial hub. Lead is a highly bioavailable metal with low mobility but high toxicity, especially in children, as well as a long retention time in topsoil (Azeh Engwa et al., 2019).
Figures 2 and 3 display the spatial distribution of the metals across the mining and the non-mining communities, respectively. Generally, the southeastern corridor of the mining town recorded higher metal concentrations of Cd, Cr, Cu, Ni, and Pb. Southeastern area of the mining community is closer to the main road used by miners in their activities. Furthermore, there are ongoing building projects by the miners around this zone of the town and may explain the high metal levels recorded (Karn et al., 2021). Around this same zone is an auto mechanic shop. Toxic metals may be released into the environment as a result of auto-electrical and mechanical operations such as soldering, and welding (Nna Orji et al., 2018). As, Zn, and Mn were more concentrated around the middle belt where there are more residents.
In the non-mining community, elevated levels of As, Cr, Mn, Ni and Pb were concentrated at the southern zone. The southern part of Sunyani is called Abesim where there are more residential buildings than any suburb of Sunyani. In this same area are some farming activities and some of the soil samples were collected from the farms. Also, many primary (basic) schools are in this part of the town. Automobile emissions, uncontrolled disposal of wastes from homes, and agricultural activities may have all contributed to the rise toxic metal levels (Kinuthia et al., 2020). Cadmium was highly concentrated along the middle, northeastern and southwestern parts of the town.
Toxic metal contamination
The contamination factor (CF), geoaccumulation index (I-geo), and enrichment factor (EF), were employed in this study to ascertain whether the sources of toxic metal contamination were anthropogenic (Dung et al., 2013). The CF values showed very high contamination of Cd, considerable contamination of As, moderate contamination of Ni, Zn, Cu, and low contamination of Cr, Mn, and Pb in the mining community. In the non-mining community, all the metals except As, Cd, and Cr, showed low contamination. However, Cd showed very high soil contamination in the area. The EF confirmed extremely high enrichment of Cd, significant enrichment of As, and moderate enrichment of the remaining toxic metals in the two study areas.
Furthermore, Igeo results showed extreme pollution of the surface soil with cadmium in the mining community and substantial pollution in the non-mining community. All, except arsenic in the mining area revealed background levels for the remaining toxic metals. The ecological risk indices were used to define the overall contamination level of the two study areas. All the toxic metals except Cd showed low risk at both study sites. The values of Cd revealed very high risks in both the mining and non-mining communities. Cadmium is known to have elevated levels in Ghanaian surface soils (Akanchise et al., 2020). The PLI values of 2.145 and 1.372 (PLI > 1) in the mining and the non-mining communities, respectively, show contamination of the surface soils at both communities with the mining site as more polluted.
Correlation analysis
Correlation analysis and the two independent samples t-test were used to measure the extent to which the physicochemical parameters (EC, pH, and TOC) and the metal concentrations are related at the mining and non-mining sites. Table 3 shows the correlation analysis of pH and EC, and all the metal concentrations for the mining site. There was a moderate correlation between pH and EC, and all the metal concentrations, even though they are statistically insignificant (p > 0.05). The striking correlation, however, is between pH and As. In contrast, a positive correlation was found between pH and Cd, and Cr, respectively.
There was a moderate positive correlation between pH and As, Cd, Cr, Cu, Ni, and Zn at the non-mining site. However, Cu and Pb were negatively correlated with pH. A moderate positive correlation was also observed between EC and some of the metals, including As, Cu, Ni and Pb. The observed moderate positive correlation suggests that an increase in EC is associated with higher concentrations of the metals. However, it is important to note that this correlation does not establish a causal relationship between EC and the observed metal concentrations. Various factors, including environmental conditions, geographical location, industrial activities, and other variables, could potentially influence both EC levels and the presence of these metals. Further investigation and analysis are necessary to elucidate the underlying mechanisms and potential drivers of this correlation. On the contrary, a moderate negative correlation was seen between EC and Cd, Cr, Mn, and Zn. Overall, positive, or negative correlations will increase or decrease the levels of EC.
In-vitro bioaccessibility
A physiologically based extraction test simulating the sequential digestion in the gastrointestinal tract was applied to the surface soil samples collected from both the mining and the non-mining community to estimate the bioaccessible fraction of the eight metals. Descriptive summary statistics, including mean and standard deviation, are presented in Table 4. The mean bioaccessible fraction for the metals were in the decreasing order Cu > Zn > Cd > Pb > Mn > Ni > As > Cr, ranging from 7.5% in As to 59.3% in Cu. The variation in the metal bioaccessibility could be as a result of elemental speciation and soil physicochemical properties (Turner & Ip, 2007). The bioaccessibility of Cu and Zn was relatively high compared to a study conducted in urban soils of Kumasi, Ghana (Darko et al., 2017a, 2017b). This may be due to the metals not accumulated in the surface soil for long to undergo processes such as surface precipitation and leaching making them more soluble and more bioaccessible (Dodd et al., 2017). The percentage bioaccessibility of Pb, Mn, Ni, As, and Cr (38.7 ± 15.7, 35.4 ± 13.9, 8.7 ± 5.4, 7.5 ± 4.9, and 5.1 ± 4.3, respectively) in the area was relatively low when compared to data for surface soils at Gbani—another mining community in Ghana (Darko et al., 2019).
Fractions of the daily reference dose of metals
The fractions of the daily reference dose (Table 4) provided by incidental metal ingestion in soil differ between mining and non-mining sites. In general, potentially toxic metals such as Cd, Ni, and Pb have higher fractions of daily reference dose in the mining site than in the non-mining site. This implies that inadvertent ingestion of soil from mining areas may contribute to a higher intake of these metals, raising health concerns due to increased exposure.
The high value of Ni fractions, 39.94% and 71.03% in unmined and mined soils, respectively, raise concerns and require special consideration. Nickel is a trace element that the human body requires in trace amounts. However, prolonged or excessive exposure to high levels of nickel can impair physiology and immune development in children, and, in extreme cases, have carcinogenic effects (Begum et al., 2022).
Comparing these fractions provides valuable insights into the potential risks associated with metal contamination in soil, especially in mining areas. It underscores the importance of effective environmental management practices and regulatory measures to mitigate the exposure of individuals to these harmful metals and ensure the well-being of communities living in or near such areas.
Hazard indices
The hazard index and hazard quotients for various metals in non-mining and mining environments are shown in Table 5. The HQ is a metric used to assess the potential risk to human health or the environment posed by a specific substance, such as a metal. A hazard quotient greater than one indicates that there is a potential risk associated with that substance's exposure.
The risks (in terms of HI and HQ) observed in non-mining areas were generally lower than those observed in mining areas. With the exception of Mn, none of the metals posed a health risk (HQ > 1) in non-mining areas. Otherwise, Cd and Mn, on the other hand, registered higher HQ than the threshold in the mining locations, indicating significant risk. Again, the combined hazard of all the metals (HI) exceeded the threshold by three-, and four-folds, respectively, at the non-mining and mining sites. Monitoring and managing these metals is critical in order to reduce their negative impact on human health and the environment.
Conclusion
This study successfully compared the distribution pattern and the in-vitro bioaccessibility of potential toxic metals in surface soils from a mining and a non-mining community in Ghana. Average concentrations of all the potentially toxic metals at the two communities exceeded the concentrations recorded at the conQ9trol site, indicating contamination of the surface soils in the study areas. The mining community had higher mean metal concentrations (As, Cd, Cu, Ni, Pb, and Zn), implying that mining and related activities contaminate the topsoil, particularly in the southeastern zone. Toxic metal concentrations were higher in the commercial town's southern part, where most residents live. This study's findings indicate that mining and related activities have had a negative impact on the community. The risk indices and bioaccessibility results show that the overall risks of ingesting these metal-laden soils are low. Future studies should focus on establishing a relationship between the bioaccessibility of the toxic metals in soil, air, and water around mining communities in Ghana. Regulatory bodies must also ensure constant monitoring to alleviate, if not eradicate soil pollution emanating from mining activities.
Data availability
Data are available upon request to the corresponding author.
References
Akanchise, T., Boakye, S., Borquaye, L. S., Dodd, M., & Darko, G. (2020). Distribution of heavy metals in soils from abandoned dump sites in Kumasi, Ghana. Scientific African, 10, e00614. https://doi.org/10.1016/j.sciaf.2020.e00614
Akoto, O., Bortey-Sam, N., Ikenaka, Y., Nakayama, S. M. M., Baidoo, E., Yohannes, Y. B., & Ishizuka, M. (2017). Contamination levels and sources of heavy metals and a metalloid in surface soils in the Kumasi Metropolis, Ghana. Journal of Health and Pollution, 7(15), 28–39. https://doi.org/10.5696/2156-9614-7.15.28
Amponsah, L. O., Dodd, M., & Darko, G. (2022). Gastric bioaccessibility and human health risks associated with soil metal exposure via ingestion at an E-waste recycling site in Kumasi, Ghana. Environmental Geochemistry and Health, 44(2), 497–509. https://doi.org/10.1007/s10653-020-00760-7
Asamoah, B. D., Asare, A., Okpati, S. W., & Aidoo, P. (2021). Heavy metal levels and their ecological risks in surface soils at Sunyani magazine in the bono region of Ghana. Scientific African, 13, e00937. https://doi.org/10.1016/j.sciaf.2021.e00937
Asare, A., Asamoah, B. D., & Sanful, P. O. (2019). Assessment of heavy metal contaminants using pollution indices in Ankobra River at Prestea Huni-Valley District, Ghana. Journal of Geoscience and Environment Protection, 07(09), 25–35. https://doi.org/10.4236/gep.2019.79003
Assi, M. A., Hezmee, M. N. M., Haron, A. W., Sabri, M. Y., & Rajion, M. A. (2016). The detrimental effects of lead on human and animal health. Veterinary World, 9(6), 660–671. https://doi.org/10.14202/vetworld.2016.660-671
Azeh Engwa, G., Udoka Ferdinand, P., Nweke Nwalo, F., & N. Unachukwu, M. (2019). Mechanism and Health Effects of Heavy Metal Toxicity in Humans. In Poisoning in the Modern World - New Tricks for an Old Dog? (p. 23). https://doi.org/10.5772/intechopen.82511
Basta, N. T., & Juhasz, A. (2014). Using in vivo bioavailability and/or in vitro gastrointestinal bioaccessibility testing to adjust human exposure to arsenic from soil ingestion. Reviews in Mineralogy and Geochemistry, 79(1), 451–472. https://doi.org/10.2138/rmg.2014.79.9
Begum, W., Rai, S., Banerjee, S., Bhattacharjee, S., Mondal, M. H., Bhattarai, A., & Saha, B. (2022). A comprehensive review on the sources, essentiality and toxicological profile of nickel. RSC Advances. https://doi.org/10.1039/d2ra00378c
Burchfiel, B. C. (1983). Continental crust. Scientific American. https://doi.org/10.1038/scientificamerican0983-130
CCME. (2007). Canadian Council of Ministers of the Environment (CCME). Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health. Soil Quality Index 1.0 Technical Report. Canadian Council of Ministers of the Environment (Vol. 1). Winnipeg. https://www.ec.gc.ca/ceqg-rcqe
CEPA. (1999). Canadian exposure factors used in human health risk assessments. Fact sheet series: Topics in risk assessment of substances under the Canadian Environmental Protection Act, 1999 (Vol. 1999).
Cetin, M., Isik Pekkan, O., Bilge Ozturk, G., Cabuk, S. N., Senyel Kurkcuoglu, M. A., & Cabuk, A. (2023). Determination of the impacts of mining activities on land cover and soil organic carbon: Altintepe gold mine case, Turkey. Water, Air, and Soil Pollution, 234(4), 1–17. https://doi.org/10.1007/s11270-023-06274-z
Chen, X., Singh, A., & Kitts, D. D. (2020). In-vitro bioaccessibility and bioavailability of heavy metals in mineral clay complex used in natural health products. Scientific Reports, 10(1), 8823. https://doi.org/10.1038/s41598-020-65449-4
Cordy, P., Veiga, M., Crawford, B., Garcia, O., Gonzalez, V., Moraga, D., et al. (2013). Characterization, mapping, and mitigation of mercury vapour emissions from artisanal mining gold shops. Environmental Research, 125, 82–91. https://doi.org/10.1016/j.envres.2012.10.015
da Silva, Y. J. A. B., do Nascimento, C. W. A., & Biondi, C. M. (2014). Comparison of USEPA digestion methods to heavy metals in soil samples. Environmental Monitoring and Assessment, 186(1), 47–53. https://doi.org/10.1007/s10661-013-3354-5
Darko, G., Boakye, K. O., Nkansah, M. A., Gyamfi, O., Ansah, E., Yevugah, L. L., et al. (2019). Human health risk and bioaccessibility of toxic metals in topsoils from Gbani mining community in Ghana. Journal of Health and Pollution, 9(22), 1–11. https://doi.org/10.5696/2156-9614-9.22.190602
Darko, G., Dodd, M., Nkansah, M. A., Aduse-Poku, Y., Ansah, E., Wemegah, D. D., & Borquaye, L. S. (2017a). Distribution and ecological risks of toxic metals in the topsoils in the Kumasi metropolis. Ghana. Cogent Environmental Science, 3(1), 1354965. https://doi.org/10.1080/23311843.2017.1354965
Darko, G., Dodd, M., Nkansah, M. A., Ansah, E., & Aduse-Poku, Y. (2017b). Distribution and bioaccessibility of metals in urban soils of Kumasi. Ghana. Environmental Monitoring and Assessment, 189(6), 260. https://doi.org/10.1007/s10661-017-5972-9
Dodd, M., Richardson, G. M., Wilson, R., Rencz, A., & Friske, P. (2017). Elemental concentrations and in vitro bioaccessibility in Canadian background soils. Environmental Geochemistry and Health, 39(4), 759–777. https://doi.org/10.1007/s10653-016-9846-9
Dung, T. T. T., Cappuyns, V., Swennen, R., & Phung, N. K. (2013). From geochemical background determination to pollution assessment of heavy metals in sediments and soils. Reviews in Environmental Science and Bio/technology, 12(4), 335–353. https://doi.org/10.1007/s11157-013-9315-1
Ghana Statistical Service. (2014). Sunyani municipality. https://doi.org/10.1016/j.jdeveco.2009.09.012
Glennon, M. M., Harris, P., Ottesen, R. T., Scanlon, R. P., & O’Connor, P. J. (2014). The Dublin SURGE Project: Geochemical baseline for heavy metals in topsoils and spatial correlation with historical industry in Dublin, Ireland. Environmental Geochemistry and Health, 36(2), 235–254. https://doi.org/10.1007/s10653-013-9561-8
Guo, W., Zhang, Z., Zheng, N., Luo, L., Xiao, H., & Xiao, H. (2020). Chemical characterization and source analysis of water-soluble inorganic ions in PM2.5 from a plateau city of Kunming at different seasons. Atmospheric Research. https://doi.org/10.1016/j.atmosres.2019.104687
Hakanson, L. (1980). An ecological risk index for aquatic pollution control.a sedimentological approach. Water Research, 14(8), 975–1001. https://doi.org/10.1016/0043-1354(80)90143-8
Huang, X., Chang, M., Han, L., Li, J., Li, S.-W., & Li, H.-B. (2023). Variation of lead bioaccessibility in soil reference materials: Intra- and inter-laboratory assessments. Chemosphere, 312, 137293. https://doi.org/10.1016/j.chemosphere.2022.137293
JECFA. (2009). Evaluation of certain food additives. WHO Technical Report 952. Sixty-ninth report of the Joint FAO/WHO Expert Committee on Food Additives. 17–26 June 2008. World Health Organization technical report series, (974). http://whqlibdoc.who.int/trs/WHO_TRS_952_eng.pdf
Kan, Z., Liu, W., Liu, W., Lal, R., Dang, Y. P., Zhao, X., & Zhang, H. (2022). Mechanisms of soil organic carbon stability and its response to no-till: A global synthesis and perspective. Global Change Biology, 28(3), 693–710. https://doi.org/10.1111/gcb.15968
Karn, R., Ojha, N., Abbas, S., & Bhugra, S. (2021). A review on heavy metal contamination at mining sites and remedial techniques. IOP Conference Series: Earth and Environmental Science, 796(1), 012013. https://doi.org/10.1088/1755-1315/796/1/012013
Keshavarzi, A., & Kumar, V. (2020). Spatial distribution and potential ecological risk assessment of heavy metals in agricultural soils of Northeastern Iran. Geology, Ecology, and Landscapes, 4(2), 87–103. https://doi.org/10.1080/24749508.2019.1587588
Kinuthia, G. K., Ngure, V., Beti, D., Lugalia, R., Wangila, A., & Kamau, L. (2020). Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: Community health implication. Scientific Reports, 10(1), 8434. https://doi.org/10.1038/s41598-020-65359-5
Konwuruk, N., Borquaye, L. S., Darko, G., & Dodd, M. (2021). Distribution, bioaccessibility and human health risks of toxic metals in peri-urban topsoils of the Kumasi Metropolis. Scientific African, 11, e00701. https://doi.org/10.1016/j.sciaf.2021.e00701
Kwaansa-Ansah, E. E., Amenorfe, L. P., Armah, E. K., & Opoku, F. (2017). Human health risk assessment of cyanide levels in water and tuber crops from Kenyasi, a mining community in the Brong Ahafo Region of Ghana. International Journal of Food Contamination, 4(1), 16. https://doi.org/10.1186/s40550-017-0061-y
Leul, Y., Assen, M., Damene, S., & Legass, A. (2023). Effects of land use types on soil quality dynamics in a tropical sub-humid ecosystem, western Ethiopia. Ecological Indicators, 147, 110024. https://doi.org/10.1016/j.ecolind.2023.110024
Li, Y., Zhou, H., Gao, B., & Xu, D. (2021). Improved enrichment factor model for correcting and predicting the evaluation of heavy metals in sediments. Science of the Total Environment, 755, 142437. https://doi.org/10.1016/j.scitotenv.2020.142437
Mihankhah, T., Saeedi, M., & Karbassi, A. (2020). A comparative study of elemental pollution and health risk assessment in urban dust of different land-uses in Tehran’s urban area. Chemosphere, 241, 124984. https://doi.org/10.1016/j.chemosphere.2019.124984
Nna Orji, C., Abdulrahman, F. W., & Rosemary Isu, N. (2018). Pollution status of heavy metals in spent oil-contaminated soil in Gwagwalada. Asian Journal of Applied Chemistry Research, 4(1), 1–10. https://doi.org/10.9734/ajacr/2018/v1i29623
Perrouty, S., Aillères, L., Jessell, M. W., Baratoux, L., Bourassa, Y., & Crawford, B. (2012). Revised Eburnean geodynamic evolution of the gold-rich southern Ashanti Belt, Ghana, with new field and geophysical evidence of pre-Tarkwaian deformations. Precambrian Research, 204–205, 12–39. https://doi.org/10.1016/j.precamres.2012.01.003
Qing, X., Yutong, Z., & Shenggao, L. (2015). Assessment of heavy metal pollution and human health risk in urban soils of steel industrial city (Anshan), Liaoning, Northeast China. Ecotoxicology and Environmental Safety, 120, 377–385. https://doi.org/10.1016/j.ecoenv.2015.06.019
Ros-Tonen, M. A. F., Aggrey, J. J., Somuah, D. P., & Derkyi, M. (2021). Human insecurities in gold mining: A systematic review of evidence from Ghana. The Extractive Industries and Society, 8(4), 100951. https://doi.org/10.1016/j.exis.2021.100951
Rweyemamu, A. R., Nkansah, M. A., Darko, G., & Dodd, M. (2020). Contamination and human health risk due to toxic metals in dust from transport stations in the Kumasi Metropolis. Ghana. Chemistry Africa, 3(3), 831–843. https://doi.org/10.1007/s42250-020-00174-z
Santé Canada. (2008). Federal contaminated site risk assessment in Canada. Part IV: Spreadsheet tool for human health preliminary quantitative risk assessment (PQRA).
Sintorini, M. M., Widyatmoko, H., Sinaga, E., & Aliyah, N. (2021). Effect of pH on metal mobility in the soil. In IOP Conference Series: Earth and Environmental Science (Vol. 737). https://doi.org/10.1088/1755-1315/737/1/012071
Swarnalatha, K., Letha, J., Ayoob, S., & Nair, A. G. (2015). Risk assessment of heavy metal contamination in sediments of a tropical lake. Environmental Monitoring and Assessment, 187(6), 1–14. https://doi.org/10.1007/s10661-015-4558-7
Turner, A., & Ip, K. H. (2007). Bioaccessibility of metals in dust from the indoor environment: Application of a physiologically based extraction test. Environmental Science and Technology, 41(22), 7851–7856. https://doi.org/10.1021/es071194m
Ullah, A. K. M. A., Maksud, M. A., Khan, S. R., Lutfa, L. N., & Quraishi, S. B. (2017). Dietary intake of heavy metals from eight highly consumed species of cultured fish and possible human health risk implications in Bangladesh. Toxicology Reports, 4, 574–579. https://doi.org/10.1016/j.toxrep.2017.10.002
US-EPA. (2002). Toxicological Profile for Arsenic. In ATSDR’s Toxicological Profiles (Vol. 139, p. 559). Atlanta, Georgia. https://doi.org/10.1201/9781420061888_ch33
US EPA. (2017). Standard Operating Procedure for an In Vitro Bioaccessibility Assay for Lead and Arsenic in Soil. Washinton. https://nepis.epa.gov/Exe/ZyNET.exe/P100GESL.TXT?ZyActionD=ZyDocument&Client=EPA&Index=2011+Thru+2015&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=. Accessed 19 November 2022.
Wasserman, J. C., Hacon, S., & Wasserman, M. A. (2003). Biogeochemistry of Mercury in the Amazonian Environment. AMBIO: A Journal of the Human Environment, 32(5), 336–342. https://doi.org/10.1579/0044-7447-32.5.336
WHO. (2021). Obesity and overweight. Geneva 27, Switzerland. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 31 May 2022.
Wilson, R., Jones-Otazo, H., Petrovic, S., Mitchell, I., Bonvalot, Y., Williams, D., & Richardson, G. M. (2013). Revisiting dust and soil ingestion rates based on hand-to-mouth transfer. Human and Ecological Risk Assessment, 19(1), 158–188. https://doi.org/10.1080/10807039.2012.685807
World Bank. (2020). Ghana: Country Environmental Analysis. Washington DC 20433. https://documents1.worldbank.org/curated/en/419871588578973802/pdf/Ghana-Country-Environmental-Analysis.pdf. Accessed 31 May 2022.
Yuan, L., Guo, P., Guo, S., Wang, J., & Huang, Y. (2021). Influence of electrical fields enhanced phytoremediation of multi-metal contaminated soil on soil parameters and plants uptake in different soil sections. Environmental Research, 198, 111290. https://doi.org/10.1016/j.envres.2021.111290
Acknowledgements
We are grateful to the Royal Roads University, Victoria, Canada, for funding Matt Dodd’s trips to Ghana and the Sheathe Project (www.sheathe.org) for lab space and instrumentation.
Funding
Authors did not receive any direct funding for this study.
Author information
Authors and Affiliations
Contributions
Matt Dodd and Godfred Darko conceptualized the study, while Benjamin Darko Asamoah and Lily Lisa Yevugah conducted the fieldwork and map work, respectively. Benjamin Darko Asamoah, Matt Dodd, and Godfred Darko worked in the lab on various aspects of the project, such as data and statistical analysis. Marian Asantewah Nkansah and Alexander Boateng also contributed to the team's data analysis efforts. Benjamin Darko Asamoah, Godfred Darko, and Marian Asantewah Nkansah collaborated on the drafting process, and all authors contributed to proofreading the final manuscript.
Corresponding author
Ethics declarations
Competing interest
All the authors declare that they have no known financial and personal relationships with other people or organizations that could inappropriately influence their work.
Ethical approval
All authors have read, understood, and have complied as applicable with the statement on "Ethical responsibilities of Authors".
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asamoah, B.D., Dodd, M., Yevugah, L.L. et al. Distribution and in-vitro bioaccessibility of potentially toxic metals in surface soils from a mining and a non-mining community in Ghana: implications for human health. Environ Geochem Health 45, 9875–9889 (2023). https://doi.org/10.1007/s10653-023-01776-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-023-01776-5