Abstract
Over 1000 people make a living by processing electronic and electrical waste (E-waste) and scrap metals for the recovery of valuable metals and integrated circuits at Dagomba Line, Kumasi, Ghana. The processing includes activities such as dismantling, open burning and open dumping of E-waste which can potentially release toxic metals into the environment and thus impact the health of recyclers and nearby residents. This study investigated the distribution of toxic metals (As, Cd, Cr, Cu, Hg, Ni, Pb, Sb and Zn) in surface soils at the E-waste recycling sites and determined the associated human health risk via ingestion incorporating bioaccessibility measurements. Metal concentrations in the activity sites were highly elevated, significantly higher than those in the surrounding area and exceeded international soil quality guidelines such as the Canadian soil quality guidelines for residential land use and the Dutch Intervention Value. Bioaccessibility was high for Pb (70.8%), Cd (64.1%), Cu (62.3%) and Ni (53.6%) which could be credited to the existence of oxidized species as a result of the E-waste burning. Non-carcinogenic effects were unacceptably high (hazard indices > 1) at 14 out of 31 sites, and the cancer risk for arsenic for adult workers was greater than 1 × 10−5 at five of the sampling sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Potentially toxic metals and metalloids such as antimony, arsenic, cadmium, chromium, copper, lead, mercury, nickel and zinc occur naturally in the environment in trace amounts through natural weathering of rocks and volcanic releases (Tchounwou et al. 2012). However, contributions from anthropogenic activities such as mining, the combustion of fossil fuels and the release of sewage have been of growing concern (De Pledge et al. 2009). The widespread applications of heavy metals in various areas—medicine, agriculture, domestic, electronic and industrial processes—have made their ubiquitous presence unsurprising (Kim et al. 2015). For simplicity, metals and metalloids are collectively referred to as metals in this paper. Discarded electrical and electronic equipment such as TVs, computers, laptops, phones and refrigerators (E-waste) contains trace metals that are used in different components. Worldwide 44.7 million tonnes of E-waste were generated in 2016 out of which only 20% were appropriately recycled (Balde et al. 2017). The remaining amount is largely unaccounted for and is either traded as second-hand goods, informally recycled or disposed of in landfills. Informal recycling methods involve mechanical disassembly followed by acid leaching or open burning to recover valuable metals such as Ag, Al, Au, Cu, Fe and Pd (Balde et al. 2017). The informal recycling of E-waste has been shown to introduce various metals (Ag, Cd, Co, Cr, Cu, Hg, Ni, Sb and Zn) into the surrounding soils (Ackah 2019; Adesokan et al. 2016; Cao et al. 2020; Han et al. 2019; Li et al. 2011; Moeckel et al. 2020; Ouabo et al. 2019; Singh et al. 2018).
Exposure of humans to metals in contaminated soil occurs via ingestion (intended or accidental), indirect ingestion, (e.g., consuming foods and drinks with particles of the contaminated soils), dermal contact or inhalation. Unfortunately, metals are non-biodegradable and have long biological half-lives and some tend to accumulate in the food chain leading to significant environmental and human health impacts. Adverse human health effects induced by these metals include developmental abnormalities, diabetes, cardiovascular diseases, neurologic and neurobehavioral disorders, hearing loss, hematologic and immunologic disorders and various types of cancer (Tchounwou 2008).
Fate and transport processes may impact contaminant concentrations in soil or sediment. Studies on tropical weathered soils have revealed that their physicochemical properties vary significantly from soils of similar texture developed under temperate climates. This is due to their mode of formation (Gidigasu 1971). In tropical soils, concentrations of metals are strongly affected by geochemical features of the primary rock, as well as the nature of weathering processes. Ferralitic weathering, which is dominant in the moist tropics, results in the oxidation and strong leaching of major and trace metals. The leached metals are transported down the soil profile by precipitation. Environmental constraints such as organic matter content, Fe–Mn co-precipitation and formation of insoluble precipitates regulate the extent of trace element redistribution (Olade 1987). These processes affect the bioavailability of metals in the soil.
Bioavailability is a dynamic process comprising three steps: environmental availability, environmental bioavailability and toxicological bioavailability. According to Kim and colleagues (Kim et al. 2015), the available quantity of the total content of metal in the soil determines the environmental availability; the fraction of dissolved metal species which can be taken up by plants and other organisms in the soil refers to environmental bioavailability; and toxicological bioavailability is the biological effects of heavy metal accumulation within plants and other organisms. This last step depends on several factors such as metabolism, detoxification and translocation. Heavy metals concentrations and bioavailability are influenced by factors such as pH, redox potential, temperature and salinity (De Pledge et al. 2009; Mohammed et al. 2011). The importance of metal speciation associated with changes in redox potential, surface complexes, concentrations of major ions, pH, organic ligand concentrations, etc., cannot be overemphasized. Human activities resulting in the dumping of acidifying substances into the environment, therefore, not only increase the concentrations of metals but also increase their bioavailability.
For human health risk assessment, the bioavailability of metals in contaminated soils is best estimated using in vivo animal studies, but because of logistical and ethical constraints accompanying studies on animals, various in vitro extraction protocols have been developed as a surrogate for in vivo bioavailability testing. In these in vitro tests, the fraction of the soil-bound metal that dissolves in a simulated gastrointestinal fluid is determined based on the assumption that solubilization is an important first step in metal bioavailability. The portion of the metal that is released into the simulated fluid is referred to as the bioaccessible fraction (Basta and Juhasz 2014; Cao et al. 2020). Incorporation of bioaccessibility data in the human health risk assessment provides a more realistic estimate of exposure to metals in soils (Basta and Juhasz 2014; Cao et al. 2020).
Dagomba Line is a suburb of Kumasi, the second largest city in Ghana. It is well noted for the informal recycling of E-waste—an activity that results in the pollution of soil (Kyere 2016), dust particles (SM Atiemo et al. 2012), water and sediment (Danquah et al. 2011) with various toxic heavy metals. E-waste management is a key problem many African countries are facing due to limited financial resources, lack of awareness and environmental legislation. Manual dismantling, open burning, dumping and landfilling are the predominant disposal methods used in Africa, which have potentially adverse consequences for human health and the environment. Due to the large import of electronic and electric equipment into Ghana each year and the fact that a large percentage are second-hand (Oteng-Ababio 2010), the economic incentive of indulging in the informal recycling of E-waste provides high motivation for the poor in the city (Kyere 2016; Prakash and Manhart 2010), to the extent that their desire to survive overrides the need to protect the environment.
Based on the potential for the anthropogenic pollution of soil with metals due to informal E-waste recycling, this study investigated the distribution of potentially toxic metals (As, Cd, Cr, Cu, Hg, Ni, Pb, Sb and Zn) in surface soils in areas where recycling activities are conducted at Dagomba Line, Kumasi, Ghana. Although ingestion, inhalation and dermal contact are all potentially important pathways for exposure to soil metal contaminants at the artisanal E-waste recycling sites, this study is limited to the human health risk associated with oral ingestion incorporating gastric bioaccessibility.
Methodology
Study Area: Dagomba Line in the Kumasi Metropolis
The Kumasi Metropolis lies between latitudes 6.35–6.40° and longitude 1.30–1.37° and has a population of 1.73 million inhabitants (Cobbinah and Erdiaw-Kwasie 2016). The climate in Kumasi is tropical, and the temperature is relatively constant all year round. It has an average annual rainfall of 1448 mm (Climate-data.org 2018). Electronic and electrical equipment (E-waste) recycling activities commenced in the Kumasi Metropolis in Akwatia Line, and Aboabo around 2006 and spread over to Dagomba Line and Oforikrom by 2007. A study in Ghana (Atiemo et al. 2016) showed that over 1000 people work on E-waste and general scrap processing in Dagomba Line. A recent walk across the study area revealed several workshops where groups of young men were seen dismantling electronic waste for valuable metals such as copper, aluminum and integrated circuits, while others came in carrying piles of E-waste on their heads or in trucks. Open burning of waste to recover metals was also noted.
Sample collection
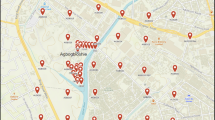
Soil sampling was conducted in two phases. Phase I focused on the main areas of E-waste recycling. Thirty-one soil samples were collected along a systematic sampling grid set at 100 m intervals (Fig. 1). In Phase II, a 600 m × 600 m gridded map of an extended area around the study site was created with intersections of the fishnet allocated as sampling points. A global positioning system application (GPS Essentials) was then used to access sampling points. Fifteen (15) samples were collected. In both phases, a pickaxe/shovel was used to loosen the soil at the designated points up to a depth of 10 cm. A plastic hand trowel was used to scoop the sample, homogenized in a tray and placed into a Ziploc bag.
Sample Preparation and Analysis
Soil samples were air-dried at ambient temperatures and sieved using 250-µm pore sized polyethylene sieves.
Soil pH and electrical conductivity were measured using a multi-parameter probe (Oakton PCSTestr35). For this analysis, 40 mL of distilled water was added to 20 g of the sieved soil, and the suspension was mixed and made to settle down for 30 min, after which the readings for electrical conductivity and pH of the supernatant were taken.
An X-ray Fluorescence Analyzer (Niton XL3t GOLDD +) was used to analyze the sieved soil for total metals based on US EPA Method 6200. For confirmatory analysis, 8 of the soil were analyzed using an inductively coupled plasma-mass spectrometry, (ICP-MS) after some modification to the aqua regia digestion process. For the digestion, 1.0 g of the sample was added to 10 ml of a 1:1:1 HCl–HNO3–H2O mixture, which was digested for an hour at 95 °C in a heating block. The digestate was centrifuged at 5000 g for 20 min following which an aliquot was pipetted and topped up to the volume with 0.5% HCl and analyzed using an Agilent 7800 ICP-MS based on US EPA Method 6020B (Darko et al. 2017).
Analysis of total mercury was carried out using the Lumex Mercury Analyzer RA915M with the Pyro-915 + attachment for solids. This is a real-time mercury detector that takes measurements based on internal calibration. For quality control, every sample was analyzed three times and the average concentration was recorded.
Bioaccessibility
The in vitro bioaccessibility assay (IVBA) was carried out according to US EPA standard operating protocols (Darko et al. 2017; U.S. EPA 2007). Aliquots (1 g) of the sieved soil samples were extracted for an hour through end-to-end rotation in a 100 mL portion of 30 g/L glycine adjusted to a pH of 1.5 with concentrated HCl. The extract was filtered and analyzed by ICP-MS as per the methodology used for the total metal analysis. To calculate the percent elemental bioaccessibility, the concentration in the IVBA extract was divided by the corresponding total elemental concentration. Samples with extract concentrations less than the limits of detection were not assessed for bioaccessibility. Quality control/quality assurances of data were ensured using certified reference material (NIST 2711), control and duplicate samples as well as procedure and reagent blanks. All laboratory procedures were also carefully followed. This would ensure that sample containers, filters and vessels introduce minimal contamination and that the experiment can be reproduced.
Statistical Analysis and Data Evaluation
Statistical analyses were performed using R software. Descriptive statistics such as the average, median, standard deviation, 25th, 75th and 95th percentiles were obtained. A test for normality indicated that metals concentrations were log-normally distributed, and hence, the nonparametric Wilcoxon Mann–Whitney test was used for comparisons between concentrations of different metals. An alpha level of 0.05 was used for all statistical tests.
There are currently no environmental soil quality guidelines for Ghana, and as such, the Dutch Target and Intervention Values (VROM 2000) and the Canadian Council of Ministers of the Environment (CCME) soil quality guidelines (CCME 2007) were used to assess the level of contamination as a first step. Since Dagomba Line is comprised of mixed residential, commercial and industrial land uses, the more delicate residential land use guidelines were selected, while the Dutch Intervention Values which represent serious soil pollution were used.
The pollution load index (PLI) (Gupta 2020; Tomlinson et al. 1980) was used to assess the degree of metal pollution. The PLI was computed using concentration factors (CFs) of each metal in the soil. The CF is the ratio of the concentration of each metal in the soil (Cmeasured) to the baseline or background value or the concentration in unpolluted soil (Cbackground).
In this study, background concentrations were estimated from the average metal total contents in unaffected soils from an extended area around the study site (Phase II sampling). For each sampling site, PLI was calculated as the nth root of the product of the n CF:
This index offers a simple, comparative assessment of the level of heavy metal pollution. Values of PLI = 1 indicate metal loads close to the background level; values above 1 indicate pollution is moderate (up to PLI = 3) and extreme levels (PLI > 5).
Human Health Risk Assessment (HHRA)
Humans usually come into contact with metal contaminants in soils via ingestion, inhalation and dermal contact. The ingestion pathway is usually of utmost importance out of the three provided contaminant concentrations are high as compared to the other sources. As such only, the risk posed via the oral ingestion route was calculated for adult workers. This was estimated using the hazard index (HI) and carcinogenic risk (CR) method. The general exposure equations used in this study are based on recommendations provided by the US EPA (U.S. EPA IRIS 2011) and Cao et al. (Cao et al. 2020). Equation 3 was used to calculate the mean daily intake of metal from soil ingestion (Cao et al. 2020; US EPA 2015) based on the model parameters in Table 1:
To obtain the hazard quotient (HQ) for non-cancer risk (Eq. 4), the ratio of CDI to the reference dose (RfD) (mg kg−1 d−1) was adjusted for relative bioavailability (RBA).
For As and Pb, the RBA was determined from the bioaccessibility values using RBA/IVBA regression equations. The relationship for As is expressed by Juhasz et al. (2014) as:
For Pb, the relationship based on US EPA (2007) is:
Due to the lack of corresponding regression equations for the remaining metals, the bioaccessibility value was used as a surrogate for the RBA (Cao et al. 2020).
The RfD values used were As: 0.0003 mg kg−1 d−1; Cd: 0.0003 mg kg−1 d−1; Cr: 0.003 mg kg−1 d−1; Cu: 0.01 mg kg−1 d−1; Hg 0.0003 mg kg−1 d−1; Ni; 0.02 mg kg−1 d−1; Pb: 0.0035 mg kg−1 d−1; Sb: 0.0005 mg kg−1 d−1; and Zn: 0.3 mg kg−1 d−1 (Cao et al. 2020; US EPA 2015). The HQ for the individual metals was summed to obtain the overall hazard index (HI). In this study, the HI (Eq. 7) represents the sum of the hazard quotients for As, Cd, Cr, Cu, Hg, Ni, Pb, Sb and Zn. HI > 1 implies elevated probability of non-carcinogenic effects occurring (Cao et al. 2020).
Carcinogenic risk (CR) was determined for arsenic only using Eq. 8:
where is the cancer slope factor (1.5 mg kg−1 d−1 for arsenic) (US EPA 2015).
Results and discussion
Comparison between XRF and ICP-MS data
Analysis of soil samples for total metals was conducted by XRF based on US EPA Method 6200. For comparability analysis, 15% of the samples were analyzed by ICP-MS and the data obtained were evaluated using linear regression analysis and correlation analysis. The y-intercept, the coefficient of determination (r)2 and the slope of the line for As, Cr, Cu, Ni, Pb, Sb, Zn are given in Table 2. Cadmium was not included in the analyses since the concentrations in most of the samples were below detection. There was a strong linear correlation between the XRF and ICP-MS data with r2 values between 0.834 and 0.999 (p < 0.001) indicating good comparability of the XRF to the confirmatory ICP-MS data. The gradients of the regression lines for As, Cu, Ni, Pb and Sb also suggested that not much data modification would be required to match the ICP-MS data to the XRF data, whereas Cr and Zn would require some adjustments.
Statistical summary of pH and metals data
The statistical summary for pH and the metals concentrations are given in Table 3. Out of the 31 samples from the E-waste recycling area, 87% were within the pH range of 7.0–8.5, with a mean value of 7.73. Only one sample was slightly acidic (pH = 6.65). The solubility and retention properties of metals in soil are highly dependent on soil pH; the higher the pH, the greater the retention and the lower the solubility of most metals (Bołzan and Cyraniak 2017). Electrical conductivity was variable and was found to be relatively higher at sampling locations where recycling and scrap metal activities dominated.
Metal concentrations showed a high degree of dispersion with standard deviations that were larger than the interquartile ranges. This was attributed to extreme values (outliers) that were not excluded from the data sets since they could represent hotspots. Metal concentrations in samples collected from the E-waste sites were highly elevated with the mean and median concentrations of As, Cr, Cu, Ni, Pb, Sb and Zn exceeding the Canadian soil quality guidelines for residential/parkland use (CCME R/P). Although the mean concentrations of these elements exceeded the Dutch Intervention Value (Dutch IV), the median concentrations were lower than the Dutch IV. The maximum concentrations for all the elements, except for Hg, exceeded both guidelines; Hg exceeded only the CCME R/P. This suggests “serious soil contamination” (VROM 2000).
Except for Cr, metal concentrations in the samples from the surrounding area (Phase 2) were substantially lower compared to the Dagomba Line E-waste sites. Figure 2 shows boxplots of As, Cd, Cu, Hg Ni, Pb, Sb and Zn from both the Dagomba Line E-waste recycling area and the surrounding. Concentrations of Cu, Zn, Sb, Ni and Pb in the recycling area exceeded the levels in the surrounding area by one order of magnitude, while Hg was two orders of magnitude higher. A Mann–Whitney U test indicated that the differences in As, Cu, Hg, Pb, Ni, Sn and Zn concentrations between the E-waste sites and the surroundings were statistically significant (As, p = 0.039; Cu p 0.001; Hg p < 0.001; Ni p = 0.011; Pb p < 0.001; Sb p < 0.001 and Zn p < 0.001), whereas there were no differences between Cd and Cr concentrations for the two areas (p = 0.169 and 0.788, respectively).
Metal concentrations in the Dagomba Line samples were within the range detected in other E-waste processing sites (Table 4).
Principal component analysis and correlation between metals
The relationship between the metals was explored using correlation analysis and principal component analysis. The correlation analyses showed strong associations between Cd, Ni, Pb, Sb and Zn with r2 between 0.578 and 940 (Table 5) suggesting these metals were probably from the same origin (recycling of electronic and electrical waste).
The principal component analysis was performed on the soil concentration data to uncover underlying patterns in the datasets that are otherwise not apparent. The different categories included in the study are the soil concentrations from the main recycling area (DL_soil) and the soil concentrations from the vicinity (DL_surr). Results from the analysis are shown in Figs. 3 and 4. Figure 3 shows that the two categories are significantly different from each other. The 95% ellipses drawn around each category indicate that with the addition of new points to each category, the mean of the resulting dataset would fall anywhere within the ellipses. Thus, this can be used to predict the outcomes of several scenarios without additional sampling campaigns.
The graph of variables, on the other hand, shows the main components (or dimensions) that are relevant in the analysis, i.e., Component 1 and Component 2, which have eigenvalues greater than 1, together explain 79.72% of the total variation in the datasets. Figure 4 also tells us the contribution of each variable to the formation of the axis according to the color palette “Ctr.” Hence, variables colored red contribute more to the formation of the axis than those colored blue, and so on. Moreover, it shows metals which are clustered together, e.g., Pb, Cu, Zn, belong to the same cluster, as do Cd, Ni, Cr and Hg. Most importantly, however, Fig. 4 tells us that all variables (metals) are strongly loaded positively unto the first component, meaning that they have the same source. While the recycling activity releases all the metals into the soil, Cu, Pb, Zn, As and Hg may also have alternate sources as indicated by their negative loadings to the second dimension.
Pollution load index
The pollution load index (PLI) was calculated for the Dagomba Line soils using the concentrations for samples from the KNUST Botanical Gardens—located about 6 km away from Dagomba Line—as background. The resulting data depicted in Fig. 5 indicated that there is extreme pollution at almost all points sampled within the main recycling area.
Bioaccessibility and human health risk assessment
The statistical summary of metal bioaccessibility is presented in Table 6. Bioaccessibility was variable among the metals with mean values in the order Pb > Cd > Cu > Ni > Sb > As > Cr. The relatively higher bioaccessibility of Pb, Cd, Cu and Ni may be due to the presence of oxidized species as a result of the E-waste burning; metal oxides are more bioaccessible (Ruby 2004). Pb bioaccessibility ranged from 49.0 to 90.2% which are higher than the range of 6.7 to 56% reported for the gastric phase bioaccessibility for E-waste burning sites in Agbogbloshie, Accra, Ghana (Cao et al. 2020). However, they were within the range of values (22.8 to 95.9%) for surface soil samples collected from commercial areas of Kumasi (Darko et al. 2017). Bioaccessibility of As varied from 4.5 to 21% and within the range of 2.1 to 39.8% reported for Agbogbloshie (Cao et al. 2020), whereas Sb bioaccessibility was lower for this study. Incorporation of bioaccessibility data into the risk calculation resulted in decreases in the individual metal hazard quotients as shown by the mean values in Fig. 6.
The hazard indices which represent the sum of the hazard quotients for As, Cd, Cr, Cu, Hg, Ni, Pb, Sb and Zn, incorporating bioaccessibility data for each sampling location, are summarized in Fig. 7. Unacceptable levels of non-carcinogenic effects (HI > 1) were recorded for 14 out of the 31 sites with Pb contributing the most to the HI followed by Cu. The clusters of high HI values are stretches/lanes consisting of a series of dismantling/open burning workshops. This observation is consistent with data from human health risk assessment at other E-waste recycling sites which showed Pb and Cu as the leading contributors to human health risk to the adult workers (Cao et al. 2020).
The cancer risk for As for adult workers at Dagomba was greater than 1 × 10−5 at five of the sampling sites which was considered unacceptable based on Canadian guidelines (Health Canada 2012) that the level of risk for cancer that was considered acceptable was one excess cancer death per 100,000 people exposed.
Conclusion
Gastric bioaccessibility and human health risk associated with the ingestion of potentially toxic metals including antimony, arsenic, cadmium, chromium, copper, lead, mercury, nickel and zinc found in soils at an E-waste recycling site in Kumasi, Ghana, were determined. The soil at the E-waste recycling site is mostly neutral and was found to be highly polluted with toxic metals at concentrations that exceeded international environmental soil quality guidelines. Metal concentration in the surroundings was significantly lower and less polluted than the sites where the E-waste recycling activities occur. Bioaccessibility—the fraction of the metal likely to dissolve in the gastric juice and become available for uptake into the bloodstream upon ingestion—was very high for Cd, Cu, Ni and Pb. Even though the inclusion of bioaccessibility data into the risk calculation resulted in decreases in the individual metal hazards, unacceptable levels of non-carcinogenic effects were noted at 14 out of the 31 sites investigated. The cancer risk for arsenic for adult workers was also high at five of the sampling sites. This study is limited to the human health risk associated with oral ingestion incorporating gastric bioaccessibility. It is recognized that inhalation and dermal contact are all potentially important pathways for exposure as such follow-up investigations are being conducted including air sampling to determine the risk associated with inhalation.
References
Ackah, M. (2019). Soil elemental concentrations, geoaccumulation index, non-carcinogenic and carcinogenic risks in functional areas of an informal e-waste recycling area in Accra, Ghana. Chemosphere, 235, 908–917. https://doi.org/10.1016/j.chemosphere.2019.07.014.
Adesokan, M. D., Adie, G. U., & Osibanjo, O. (2016). Soil Pollution by Toxic Metals near E-waste Recycling Operations in Ibadan, Nigeria. Journal of Health and Pollution, 6(11), 26–33. https://doi.org/10.5696/2156-9614-6-11.26.
Atiemo, S, Faabeluon, L., Manhart, A., Nyaaba, L., & Schleicher, T. (2016). Baseline Assessment on E-waste Management in Ghana. http://sustainable-recycling.org/sustainable-recycling/wp-content/uploads/2016/07/Sampson_2016_SRI-Ghana.pdf.
Atiemo, S. M., Ofosu, F., Aboh, I. J. K., & Kuranchie-Mensah, H. (2012). Assessing the heavy metals contamination of surface dust from waste electrical and electronic equipment (e-waste) recycling site in accra, Ghana. Research Journal of Environmental and Earth Sciences, 4(5), 605–611.
Balde, C. P., Forti, V., Gray, V., Kuehr, R., & Stegmann, P. (2017). The global e-waste monitor 2017. Tokyo: United Nations University. https://doi.org/10.1016/j.proci.2014.05.148.
Basta, N. T., & Juhasz, A. (2014). Using in vivo bioavailability and/or in vitro gastrointestinal bioaccessibility testing to adjust human exposure to arsenic from soil ingestion. Reviews in Mineralogy and Geochemistry, 79(1), 451–472. https://doi.org/10.2138/rmg.2014.79.9.
Bołzan, B. D., & Cyraniak, E. (2017). Evaluating the behavior of metals in soil environment. World News of Natural Sciences, 9, 19–34.
Cao, P., Fujimori, T., Juhasz, A., Takaoka, M., & Oshita, K. (2020). Bioaccessibility and human health risk assessment of metal(loid)s in soil from an e-waste open burning site in Agbogbloshie, Accra, Ghana. Chemosphere. https://doi.org/10.1016/j.chemosphere.2019.124909.
CCME. (2007). Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health CCME SOIL QUALITY INDEX 1.0 Technical Report. Canadian Council of Ministers of the Environment, Technical(CCME Soil Quality Index 1.0), pp. 1–10.
Cobbinah, P. B., & Erdiaw-Kwasie, M. O. (2016). Urbanization in Ghana (pp. 82–104). https://doi.org/10.4018/978-1-5225-0187-9.ch005.
Danquah, L., Abass, K., & Nikoi, A. A. (2011). Anthropogenic pollution of inland waters: the case of the Aboabo river in Kumasi. Ghana. Journal of Sustainable Development. https://doi.org/10.5539/jsd.v4n6p103.
Darko, G., Dodd, M., Nkansah, M. A., Ansah, E., & Aduse-Poku, Y. (2017). Distribution and bioaccessibility of metals in urban soils of Kumasi, Ghana. Environmental Monitoring and Assessment, 189(6), 260. https://doi.org/10.1007/s10661-017-5972-9.
De Pledge, M. H., Weeks, J. M., & Bjerregaard, P. (2009). Heavy Metals. In Handbook of Ecotoxicology (Vol. 2–2, pp. 543–569). https://doi.org/10.1002/9781444313512.ch27.
Gidigasu, M. D. (1971). The importance of soil genesis in the engineering classification of Ghana soils. Engineering Geology, 5(2), 117–161. https://doi.org/10.1016/0013-7952(71)90015-9.
Gupta, P. K. (2020). Pollution load on indian soil-water systems and associated health hazards: a review. Journal of Environmental Engineering, 146(5), 03120004. https://doi.org/10.1061/(asce)ee.1943-7870.0001693.
Han, Y., Tang, Z., Sun, J., Xing, X., Zhang, M., & Cheng, J. (2019). Heavy metals in soil contaminated through e-waste processing activities in a recycling area: Implications for risk management. Process Safety and Environmental Protection, 125, 189–196. https://doi.org/10.1016/j.psep.2019.03.020.
Health Canada. (2012). Part I: Guidance on Human Health Preliminary Quantitative Risk Assessment (PQRA), Version 2.0.
Juhasz, A. L., Herde, P., Herde, C., Boland, J., & Smith, E. (2014). Validation of the predictive capabilities of the sbrc-G in vitro assay for estimating arsenic relative bioavailability in contaminated soils. Environmental Science and Technology, 48(21), 12962–12969. https://doi.org/10.1021/es503695g.
Kim, R.-Y., Yoon, J.-K., Kim, T.-S., Yang, J. E., Owens, G., & Kim, K.-R. (2015). Bioavailability of heavy metals in soils: definitions and practical implementation—A critical review. Environmental Geochemistry and Health, 37(6), 1041–1061. https://doi.org/10.1007/s10653-015-9695-y.
Kyere, N. V. (2016). Environmental and Health Impacts of Informal E ‐ waste Recycling in Agbogbloshie, Accra, Ghana: Recommendations for Sustainable Management, 138
Li, J., Duan, H., & Shi, P. (2011). Heavy metal contamination of surface soil in electronic waste dismantling area: Site investigation and source-apportionment analysis. Waste Management and Research, 29(7), 727–738. https://doi.org/10.1177/0734242X10397580.
Moeckel, C., Breivik, K., Nøst, T. H., Sankoh, A., Jones, K. C., & Sweetman, A. (2020). Soil pollution at a major West African E-waste recycling site: Contamination pathways and implications for potential mitigation strategies. Environment International, 137(6), 105563. https://doi.org/10.1016/j.envint.2020.105563.
Mohammed, A. S., Kapri, A., & Goel, R. (2011). Heavy metal pollution: Source, impact, and remedies. In M. Khan, A. Zaidi, R. Goel, & J. Musarrat (Eds.), Biomanagement of Metal-Contaminated Soils Environmental Pollution (Vol. 20, pp. 1–28). Dordrecht: Springer. https://doi.org/10.1007/978-94-007-1914-9_1.
Olade, M. A. (1987). Dispersion of cadmium, lead and zinc in soils and sediments of a humid tropical ecosystem in Nigeria. In T. C. Hutchinson & K. M. Meema (Eds.), Lead, Mercury, Cadmium and Arsenic in the Environment (pp. 303–313). London: Wiley.
Oteng-Ababio, M. (2010). E-waste: An emerging challenge to solid waste management in Ghana. International Development Planning Review, 32(2), 191–206. https://doi.org/10.3828/idpr.2010.02.
Ouabo, R. E., Ogundiran, M. B., Sangodoyin, A. Y., & Babalola, B. A. (2019). Ecological risk and human health implications of heavy metals contamination of surface soil in e-waste recycling sites in Douala, Cameroun. Journal of Health and Pollution., 1, 1. https://doi.org/10.5696/2156-9614-9.21.190310.
Prakash, S., & Manhart, A. (2010). Socio-economic assessment and feasibility study on sustainable e-waste management in Ghana. Öko-Institut e.V.—Institute of Applied Ecology, 49, 1–118.
Ruby, M. V. (2004). Bioavailability of Soil-borne chemicals: Abiotic assessment tools. Human and Ecological Risk Assessment: An International Journal, 10(4), 647–656. https://doi.org/10.1080/10807030490484291.
Singh, A., Dwivedi, S. P., & Tripathi, A. (2018). Study of the toxicity of metal contamination in soil samples collected from abandoned e-waste burning sites in Moradabad, India. Nature, Environment and Pollution Technology, 17(3), 973–979.
Tchounwou, P. B. (2008). Arsenic toxicity and carcinogenesis: A public health risk assessment and management approach. WIT Transactions on Ecology and the Environment, 110(1–2), 71–79. https://doi.org/10.2495/ETOX080081.
Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K., & Sutton, D. J. (2012). Heavy metal toxicity and the environment. In EXS (Vol. 101, pp. 133–164). NIH Public Access. https://doi.org/10.1007/978-3-7643-8340-4_6.
Tomlinson, D. L., Wilson, J. G., Harris, C. R., & Jeffrey, D. W. (1980). Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresuntersuchungen, 33(1–4), 566–575. https://doi.org/10.1007/BF02414780.
US EPA. (2015). US EPA Integrated Risk Information System (IRIS). United States Environmental Protection Agency. Retrieved 16 April, 2020 from https://www.epa.gov/iris.
U.S. EPA. (2007). Estimation of relative bioavailability of lead in soil and soil-like materials using in vivo and in vitro methods. Washington, DC 20260. https://nepis.epa.gov/Exe/ZyNET.exe/93001C2U.TXT?ZyActionD=ZyDocument&Client=EPA&Index=2006+Thru+2010&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery.
U.S. EPA IRIS. (2011). Integrated Risk Information System. Integrated Risk Information System. Retrieved 16 April, 2020 from https://www.epa.gov/iris.
VROM. (2000). Circular on target values and intervention values for soil remediation. Netherlands Government Gazette, 2000, 1–12
Acknowledgements
This research was carried out at Kwame Nkrumah University of Science and Technology with funding from Danida through the SHEATHE Project (16-P03-GHA). The authors thank Canadian Foundation for Innovation (CFI) for funding the analytical instrumentation and would like to express their gratitude to Royal Roads University, Canada, for providing funds for M Dodd’s trips to Ghana.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amponsah, L.O., Dodd, M. & Darko, G. Gastric bioaccessibility and human health risks associated with soil metal exposure via ingestion at an E-waste recycling site in Kumasi, Ghana. Environ Geochem Health 44, 497–509 (2022). https://doi.org/10.1007/s10653-020-00760-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-020-00760-7