Abstract
The city of Candiota contains a great amount of coal resources. Coal activities, including coal combustion, are major releasers of polycyclic aromatic hydrocarbons (PAHs). The PAHs are considered priority air pollutants regarding their large carcinogenic potential. So, the carcinogenic risk assessment of populations living near areas with PAH sources is mandatory. This study aimed to evaluate the carcinogenic health risk of the PAH inhalation exposure of individuals living in Candiota City. A total of 158 individuals were enrolled in the study. Monitoring of PAH and meteorological parameters were carried out, and the health risk assessment was determined through the benzo(a)pyrene equivalent toxic equivalent quotient (BaP-TEQ) and the incremental lifetime cancer risk (ILCR) estimation. The coal activity area of Candiota demonstrated an annual PAH concentration of 27.7 ng/m3, PM10 concentration of 26.3 µg/m3, SO2 concentration of 9.5 µg/m3, a BaP-TEQ value of 0.3 ng/m3, and a daily inhalation of 62.4 ng/day. The comparison among seasons showed no difference in PAH concentration and BaP-TEQ. It was observed ILCR values of 2.8 × 10–6 and 2.6 × 10–6 for estimation based on reference and real values, respectively, and these levels were above the reference limit of 10–6, indicating cancer risk. Therefore, an epidemiological survey of cancer cases in the region and its relationship with environmental exposure and air pollutants levels must be required.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The relationship between environmental issues and the abrupt development of the modern world is one of the leading issues of the present time, as its progression has been followed by an accelerated increase in energy demand (Kaygusuz, 2012). In present days, the production of energy is mostly by burning fossil fuels such as coal, oil, and natural gas (Veziroglu & Sahin, 2008). Coal is a relevant resource that is abundant world widely, providing approximately 40% of the world’s electricity, and the cheapest source of energy (Franco & Diaz, 2009; World Environment Council, 2016). Coal activities, including its pyrolysis, are a great source of polycyclic aromatic hydrocarbons (PAHs) releasing and are linked with several environmental problems (WHO, 2000; Abdulazeez & Fantke, 2017).
Polycyclic aromatic hydrocarbons (PAHs) consist of two or more fused aromatic rings made entirely from carbon and hydrogen. These substances represent carcinogenic risks to humans, once PAHs are a major group of carcinogens and mutagens in the environment (Straif et al., 2005, Kelly et al., 2021). PAH includes an ample variety of compounds that are emitted from several anthropogenic activities, such as the coal activities, including mining process, combustion, and dust resuspension; also, PAH can be found in the gas phase or well-bonded to particulate matter (PM) (Akhbarizadeh et al., 2020; Kong et al., 2010; Zhu et al., 2014). PAHs are persistent and broadly dispersed in the atmosphere, being able to be transported over long distances; also, they are toxic and difficult to degrade. These substances were one of the first atmospheric pollutants being identified as a suspected carcinogen when inhaled (Abdulazeez & Fantke, 2017). The US Environmental Protection Agency (USEPA) had listed 16 PAHs as priority pollutants because of the greater chance of exposure by humans and their high concentrations in the environment, in which benzo[a]pyrene (B[a]P) is the best known (USEPA, 2014). B[a]P is a suitable marker due to its stability and relatively constant contribution to the carcinogenic activity of particle-bound PAH, being considered highly carcinogenic and mutagenic (Abdulazeez & Fantke, 2017; European Communities, 2001). B[a]P is often used as an indicator of human exposure to PAHs, and the toxicity of other PAHs is converted into toxicity equivalency factors (TEFs) to B[a]P to evaluate their relative toxicities (Lee, 2010).
Coal activity is a hazardous activity, not restricted to underground miners but extends to surface mining workers and the surrounding communities that live near the mine (Laney & Weissman, 2014; Perret et al., 2017). In the course of the coal mining process, coal dust can spread to the air through mining operations and transport activities. Moreover, generous quantities of mining wastes are produced and stocked around the coal mine (Stout & Emsbo-Mattingly, 2008). Thereby, emissions of coal dust particles from coal mines are absolutely serious, as well as the pyrolysis of coal for energy production. In Brazil, there is the second-largest recoverable coal reserve in the Western Hemisphere, totaling 11.9 billion metric tons (BRASIL, 2008). It comprises the Rio Bonito Formation of the Paraná Basin, located in the southernmost region of Brazil, including the States of Paraná, Santa Catarina, and the Rio Grande do Sul. Still, the greater amount of coal resources occur in the Rio Grande do Sul (89%), more specifically in the city of Candiota (Chaves et al., 2018). Several studies in Candiota’s region have already demonstrated an increased risk of mortality and/or morbidity, alterations in hematological parameters, liver function, and genotoxicity and mutagenicity in human cells among residents in the vicinity of the coal usage (Bigliardi et al., 2021; Cortes-Ramirez et al., 2018; da Silva Júnior et al., 2018). Coal mining and combustion contribute to local PAH emission, with documented genotoxic effects and increased risks for cancer, cardiovascular disease, and respiratory disease, among mineworkers and community populations (Espitia-Pérez et al., 2018; Ghose & Majee, 2007; Kurth et al., 2015).
Therefore, the knowledge about human health risks related to environmental exposure to hazardous chemical agents is a current and valuable concern (Ghasemi et al., 2020; Idani et al., 2020). Assessing the environmental impact of coal activity areas is a fundamental tool to periodically monitor human health, even more, when considering the potential carcinogenic effects of PAH exposure. Therefore, the present study aimed to evaluate the carcinogenic health risk of the PAH inhalation exposure of individuals living in Candiota City, the biggest coal activity area in Brazil.
Methods
Study area
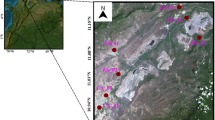
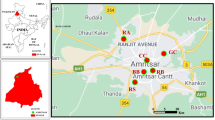
Candiota city (31° 33′ 28″ S 53° 40′ 22″ W) is located in Campaign Region in the southeast of the state of Rio Grande do Sul, covering an area of 933,834 km2 (Fig. 1). This city has approximately 9.406 inhabitants (9.39 83 people per km2) and has a human development index (HDI) of 0.698. Candiota is situated 197 heights above mean sea level and is characterized by a humid subtropical climate (Köppen climate classification) (Prefeitura de Candiota, 2013). Candiota coalfield is the largest mineral coal reserve in Brazil, situated in a location with the most favorable geological conditions in the country to superficial coal extraction (up to 50 m deep) (Secretaria do Meio Ambiente e Infraestrutura RS). The extracted coal is used locally to generate electricity at a thermal power complex, called Presidente Medici, which is the largest coal-fired power station. The coal from Candiota´s mine is considered low-quality regarding calorific power, although it has a content of more than 50% fly ash, which is a fine powder formed from the mineral matter in coal consisting of the noncombustible matter, and a sulfur concentration higher than 1.5% (BRASIL, 2008; Pires et al., 2001). Therefore, it is assumed that the emission of atmospheric pollutants from this coal is higher than that from similar plants worldwide. A conceptual chart illustrating the methodology approaches is presented in Supplementary material (Fig. S1).
Data collection of the individuals
Data from individuals living in the study area were used to achieve more realistic and regional parameters, as recommended by USEPA (2005) and Dos Santos et al (2021). Data from 158 male workers in the coal sector of the municipality of Candiota. Data of age (y), body mass (kg), and height (m) were collected from 158 individuals working in the coal sector and living in the municipality of Candiota (age: 44.6 ± 9.5 years; body mass: 85.4 ± 15.2 kg; height: 1.7 ± 8.1 m; BMI: 28.4 ± 4.1 kg/m2). More detailed information on these subjects is available in previous publications (da Silva Júnior et al., 2018; dos Santos et al., 2019). This study comprised all male coal mining workers in the municipality. The body mass was assessed using a digital scale (Model: 210Q, Geratherm®, range 0.1–180 kg), and height was measured using a stadiometer with the individuals instructed to stay in a standing position without shoes. The body mass index (BMI) was calculated by dividing the body mass (kg) by the square of height (m2) (de Onis & Habicht, 1996). This study followed the principles of the Declaration of Helsinki, and it was approved by Research Ethics Committee in Health 036/2013 (CEPAS/FURG). All subjects agreed to be included in our study by signing the informed consent.
Air pollutants monitoring and meteorological parameters
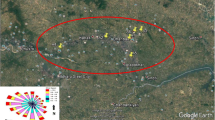
PAH analysis was assessed in particulate matter with an aerodynamic diameter equal to or less than 10 µm (PM10). The sampling location was situated in the district of Dario Lassance, which is a continuous monitoring point of the air quality of the Thermal Power Generation Company (Companhia de Geração Térmica de Energia Elétrica—CGTEE). This site was chosen because it is located close to the industrial settlements of CGTEE and within an urban area, which has high vehicle-fleet traffic liable to receive the effects of atmospheric contaminants. Monitoring campaigns occurred from July 2012 to March 2013. Fiberglass filters (Energética®—203 mm × 254 mm) were used for PM10 sampling, which was balanced in a chamber filter for 24 h to stabilize temperature and humidity. After reaching equilibrium, the filters were weighed on a 5-digit analytical balance. These filters were used in a high-volume air sampler (Hi-Vol) (AGV MP10, Energética®) placed 1 m from the ground, for 24 h. Figures S2A and S2B illustrate the details of the equipment used in the study (Fig. S1. Supplementary material). After collection, the filters were removed and packed with aluminum foil, and taken to the laboratory where they remained in the same temperature and humidity conditions. The filter was weighed, and the concentration was expressed in μg/m3. After, they were stored at 4 °C for further analysis. The analyses of PAHs in PM10 were based on the methodology described by the United States Environmental Protection Agency (USEPA, 1999). Before the extraction process, 100 μl of an internal standard was added to the filters to measure the recovery of the extraction of hydrocarbons PIR HC (1—Eicosene, 1—Hexadecene, Androstanol, 1—Phenylnanodecene, Paraterphenyl—D14).
The extraction was carried out with the Soxhlet system adding 250 ml of dichloromethane for 12 h, at a temperature ranging 40–50 °C. About 2 ml of the samples were concentrated in a rotary evaporator and then placed in tubes. The extraction flask was washed with n-hexane (0.5–1 ml) three times, and the solvent was transferred to the same test tube as the samples. The cleanup was carried out using column adsorption chromatography in a glass column. The column was impregnated with sodium sulfate (Na2SO4), silica (SiO2), and alumina (Al2O3), using n-Hexane as solvent. The silica and alumina were previously heated at a temperature of 160 °C for 3 h. Then, they were deactivated at 5% of the previous weight with MiliQ water, pre-washed with n-hexane. The extract was added to the column and a sequence of solvents was added. First, 25 ml of n-Hexane (F1) was added to remove the fraction of aliphatic hydrocarbons. Then, a mixture of dichloromethane/n-hexane was used to separate the aromatic fraction of hydrocarbons. This extract was then concentrated in a rotary evaporator at 40–50 °C and then measured 1 ml with N2. Then, 50 μl of chromatographic standard was added, containing the following compounds: Acenaphetene—d10, Crysene—d12, Naphthalene—d8, Perylene—d12, Phenanthrene—d10 and 1,4 Dichlorobenzene—d4. For the determination of PAHs, a gas chromatograph (Perkin Elmer Clarus 600®) was used. The limits of detection and quantification and the recovery percent of measurement of the 16 PAHs are described in Table S2—Supplementary material.
The device was coupled with a mass spectrometer, with Elite-5MS column (5% Diphenyl—95% dimethylsiloxane) with a length of 30 m, 0.25 mm D.I., 0.25 μm DF, and an automatic sample injector. The used heating ramp was at 40 °C, increasing at a rate of 10 °C min−1 to 60 °C, continuing heating at a rate of 5 °C min−1 to 290 °C, being maintained for 5 min; changing the heating rate from 10 °C min−1 to 300 °C, maintained for 10 min. For the detector, it was used a source 29 in a temperature of 200 °C and a transfer line temperature of 280 °C, using an electronic impact of 70 eV. The samples were injected in Splitless mode, with a flow of 50 ml of helium, after 1 min. The volume of the sample injected was 1 μl, and the injector temperature was set at 280 °C with Helium gas as a carrier (1.5 ml min−1). The detection limit of the equipment was 1 μg kg−1. For the identification of PAHs, the SIR method (selected ion recording) was used.
Sulfur Dioxide (SO2) was measured by the Ultraviolet Fluorescence Method using a HORIBA brand ambient analyzer (model APSA-360). This method meets USEPA recommendations—Reference Method EQSA-0506-159.
The meteorological parameters of precipitation (mm), temperature (°C), humidity (%), and wind speed (m/s) were extracted from the National Institute of Meteorology (INMET). The seasons during the monitoring period were defined as Spring (September, October, and November), Summer (December, January, and February), Autumn (March, April, and May), and Winter (June, July, and August).
Health risk assessment
The health risk assessment was determined considering the US EPA values of body mass and inhalation rate to male eutrophic adults (reference values) and using the real value for the body mass of individuals living in the coal activity area (USEPA, 2011). We calculated the benzo(a)pyrene equivalent toxic equivalent quotient (BaP-TEQ) and the incremental lifetime cancer risk (ILCR) as measures to assess the health risk. The benzo(a)pyrene is the most toxic of the 16 HPAs with a well-established carcinogenic effect, once it is listed as a Group 1 carcinogen by the International Agency for Research on Cancer (IARC), and therefore, it is used to evaluate the risk of exposure to PAHs (IARC, 2013) The BaP-TEQ consists of the carcinogenic effect of the PAHs, and it was calculated considering each PAH concentration and the toxic equivalency factor (TEF) of PAHs, according to Eq. 1.
in which CHPA = concentration of PAHs (pg/m3); TEF = toxic equivalency factor (Table S1. Supplementary Material).
The BaP-TEQ of the annual mean of PAHs and each season of the year was used to calculate the daily inhalation of the BaP-TEQ, as seen in Eq. 2.
where BaP-TEQ: benzo(a)pyrene equivalent toxic equivalent quotient (ng/m3); IR: inhalation rate (m3/day) of the USEPA value for normal-weight male (21–61 years) (USEPA, 2011); ET: exposure times (hours/day), we considered 24 h.
The incremental lifetime cancer risk provides an estimation of the risk that the population living in the coal area is exposed to. The ILCR was estimated as annual and seasonal values, considering potential air pollutants and meteorological fluctuations. The equation to estimate the ILCR follows (Mo et al., 2019):
in which \({\text{BaP - TEQ}}:{\text{benzo(a)}}\,{\text{pyrene}}\,{\text{equivalent}}\,{\text{toxic}}\,{\text{equivalent}}\,{\text{quotient}}\left( {{\text{pg}}/{\text{m}}^{3} } \right);\) IR: inhalation rate (m3/day) of the USEPA value for normal-body mass male (21–61 years)—16.68 m3/day for reference values; and inhalation rate (m3/day) for normal-body mass (16.68 m3/day) and overweight (19.175 m3/day) individuals included in our study (USEPA, 2011).
CF = Conversion factor 10–9 (mg/pg); ED = Exposure duration (years)—70 years; ET = Exposure times (hours/day)—24 h; EF = Exposure frequency (days/year)—365 days; CSF = Cancer slope factor (mg/kg-day)−1—we adopted the value 3.14 (Chen & Liao, 2006); BM = Body mass (kg)—we used the body mass of male normal-body mass individuals of USEPA (70 kg) to calculate ILCR with the references values and using the real values of BM collected from the individuals living in the coal activity area; AT = Averaging time of the 365 days of the 70 years of exposure (hours)—70 years x 365 days x 24 h.
According to USEPA, the ILCR values above 10−6 suggest the point that implicates in potential cancer risk (USEPA, 2005).
Statistical analysis
All variables underwent to a Kolmogorov–Smirnov normality test. The comparison among seasons of the PAHs concentration (ng/m3), BaP-TEQ, SO2 concentration, and precipitation levels was analyzed using a nonparametric Kruskal–Wallis test followed by Dunn’s post hoc test. The incremental lifetime cancer risk among the seasons was compared using a one-way analysis of variance (ANOVA) followed by Bonferroni's post hoc test. To compare the meteorological parameters of temperature, humidity, and wind speed among the seasons, a one-way analysis of variance (ANOVA) was followed by Bonferroni's post hoc test. A Pearson correlation was performed between the total and individual concentrations of PAHs. A principal component analysis (PCA) was performed considering the meteorological data and the levels of total PAHs and PM10 with the analysis based on the correlation index. The data were plotted considering the two most significant axes (factors). Furthermore, bivariate analyzes and multiple linear regression were performed between each PAHs (dependent variable) and other environmental variables (PM10, SO2, precipitation, temperature, humidity, and wind speed) (independent variables). Only data from those HPAs that had a significant correlation (bivariate analysis) with some environmental parameters were presented. The statistical analysis was carried out using Statistica 8.0 (Statistica Software, Tulsa, Oklahoma, USA) and GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, USA). A value of p ≥ 0.05 was considered statistically significant.
Results
Descriptive analyses of the epidemiological data of the sample are summarized in Table 1. The PAHs concentration (ng/m3), BaP-TEQ, SO2 concentration, and precipitation levels did not demonstrate a parametric distribution. The incremental lifetime cancer risk, the meteorological parameters of temperature, humidity, and wind speed had a parametric distribution. The annual concentrations of PAHs, PM10, and SO2 were, respectively, 27.7 ng/m3, 26.3 µg/m3, and 9.5 µg/m3, and the concentrations along the monitoring campaigns are illustrated in Fig. 2. The concentration of the 16 PAHs, the toxic equivalent factor of the components, and the respective BaP-TEQ values are described in Table S1 (Supplementary material). During the one-year air pollution monitoring in the coal activity area of Candiota, the BaP-TEQ value was 0.2 ng/m3, the daily inhalation was 62.4 ng/day, the ILCR calculated with reference values of USEPA was 2.8 × 10–6, while the ILCR estimated using the real values of Candiota`s inhabitants was 2.6 × 10–6 (Fig. 3). The PM10 concentration among the seasons did not differ (p = 0.23) (Table 2). The SO2 concentration was higher in Summer and Autumn compared to Spring and Winter, and in Winter compared to Spring (p < 0.001) (Table 2). The ILCR estimated based on reference values of USEPA was 3.8 × 10–7 in Spring, 1.6 10–6 in Summer, 8.7 × 10–6 in Autumn, and 1.7 × 10–6 in Winter (Table 2).
The seasonal comparison of a PAH concentration; b BaP-TEQ; c Daily inhalation; d ILCR. Data of PAH concentration and BaP-TEQ are presented as mean ± SEM. Difference between groups verified by the Kruskal–Wallis test followed by Dunn’s post hoc (p < 0.05). Descriptive data of daily inhalation are presented as mean. Data of ILCR are presented as mean ± SD. The difference among groups verified by the ANOVA test followed by (p < 0.05). *Denotes statistical difference compared to Spring. †Denotes statistical difference compared to Winter. ‡Denotes statistical difference compared to Summer. PAH: polycyclic aromatic hydrocarbons; BaP-TEQ: benzo(a)pyrene equivalent toxic equivalent quotient; ILCR: incremental lifetime cancer risk
The comparison among the seasons showed no difference in PAH concentration (p = 0.49) and BaP-TEQ values (p = 0.50) (Fig. 3a, b). The daily inhalation of BaP-TEQ was 8.4 ng/day in Spring, 36.5 ng/day in Summer, 193.2 ng/day in Autumn, and 37.3 ng/day in Winter (Fig. 3c). The ILCR using real values showed higher ILCR in the Autumn season compared to Spring, Summer, and Winter, and Summer and Winter had higher ILCR than Spring (p < 0.001) (Fig. 3d). The ILCR distribution among male adults from the coal activity area demonstrated a higher number of individuals with ILCR ranging from 2.2 × 10–6 to 2.8 × 10–6, consisting in 51.3% of our sample, while 86.1% was below the value of ILCR that considered the USEPA reference values for calculation (Fig. 4).
The meteorological parameters during the PAH monitoring period showed no difference in precipitation levels (p = 0.90) among seasons, temperature was lower in Autumn and Winter compared to Spring and Summer (p < 0.001). Humidity was higher in Winter compared to Spring (p = 0.008). The wind speed was lower in Autumn compared to Spring, Summer, and Winter, and Winter had a higher wind speed compared to Summer (p < 0.001) (Table 3).
There was a significant positive correlation between the total concentration of PAHs and Chrysene, Fluoranthene, Pyrene, Indeno(1,2,3)pyrene, Benzo(g, h, i)perylene, Dibenzo(a, h)anthracene, Benzo(a)pyrene, and Benzo(a)anthracene (Fig. 4a). The PCA showed a strong association between the concentration of total PAHs and PM10, and an association between the concentrations of total PAHs, PM10, and the concentration of SO2. Contrarily, the wind speed was negatively associated with the levels of PAHs and PM10. The significance of the axes was 30.6% (Factor 1) and 23.8% (Factor 2) (Fig. 4b).
Additionally, the acenaftilene had a negative correlation with temperature (r = − 0.7, p = 0.003) and a positive correlation with humidity (r = 0.6, p = 0.01), and benzo(a)pyrene had negative correlation with temperature (r = − 0.5, p = 0.04) and wind speed (r = − 0.7, p = 0.009). Then, the multiple linear regression demonstrated a relation of acenaftilene with the temperature (β = − 0.6, p = 0.05), and humidity (β = 0.2, p = 0.4), and a relation of benzo(a)pyrene with temperature (β = − 0.4, p = 0.39), and wind speed (β = − 0.6, p = 0.01) (Table 4) (Fig. 5).
Analysis of total PAH with each of the 16 individuals HPA and with environmental variables. a Correlation plot between total PAHs and each individual HPA. The respective correlation (r) and p-values were: PAHtotal:Chrysene: r = 0.7808; p = 0.0006; PAHtotal:Fluoranthene: r = 0.6655; p = 0.0068; PAHtotal:Pyrene: r = 0.7668; p = 0.0009; PAHtotal:B(b)f: r = 0.3573; p = 0.1911; PAHtotal:Indeno(1.2.3)pyrene: r = 0.9853; p = 0.0000; PAHtotal:Benzo(g, h, i)perylene: r = 0.9598; p = 0.00000002; PAHtotal:Dibenzo(a, h)anthracene: r = 0.9082; p = 0.000003; PAHtotal:Benzo(k)fluoranthene: r = 0.1216; p = 0.6660; PAHtotal:Acenaftilene: r = 0.0808; p = 0.7746; PAHtotal:Anthracene: r = − 0.1302; p = 0.6436; PAHtotal:Fluorene: r = − 0.0230; p = 0.9352; PAHtotal:Benzo(a)pyrene: r = 0.7050; p = 0.0033; PAHtotal:Benzo(a)anthracene: r = 0.7982; p = 0.0004; PAHtotal:Naphthalene: r = − 0.1989; p = 0.4772; PAHtotal:Phenanthrene: r = 0.2570; p = 0.3552; PAHtotal:Acenaphene: r = − 0.4039; p = 0.1354. b PCA of total PAHs and PM10 levels and meteorological data with the analysis based on the correlation index. The data were plotted considering the two most significant axes (factors), the significance of the axes was 30.6% (Factor 1) and 23.8% (Factor 2)
Discussion
In the present study, the air pollution monitoring in the coal activity area of Candiota demonstrated an annual PAH concentration of 27.7 ng/m3, PM10 concentration of 26.3 µg/m3, SO2 concentration of 9.5 µg/m3, a BaP-TEQ value of 0.2 ng/m3, a daily inhalation of 62.4 ng/day, and ILCR values of 2.8 × 10–6 and 2.6 × 10–6 for estimation based on reference and real values, respectively. The comparison among seasons showed no difference in PAH concentration and BaP-TEQ. However, it was observed a higher daily inhalation of BaP-TEQ and incremental lifetime cancer risk in the autumn season, with the majority of the ILCR of individuals included in this study ranging from 2.2 × 10–6 to 2.8 × 10–6. In the Autumn season, the temperature and wind speed were lower than the levels detected in the seasons' Spring and Summer. Also, the total PAHs were associated with PM10 and SO2 concentrations, the wind speed was negatively associated with the levels of PAHs and PM10, while acenaftilene had a negative correlation with temperature and a positive correlation with humidity, and benzo(a)pyrene had a negative correlation with temperature and wind speed.
Studies investigating the PM-bounded PAHs showed large heterogeneity of results related to the concentration of these air pollutants. This variability seems to be connected with several factors, as the geographic region, meteorological conditions and aerodynamic size of the pollutant at the moment of collection. A study conducted in Bangkok (Thailand) demonstrated that the annual mean of PM10 concentration of 3 polluted areas was 49.1 ug/m3. Despite the higher levels of PM10, the concentration of bounded PAH and BaP-TEQ values (3.7 ng/m3 and 0.4 ng/m3, respectively) was lower than that found in our study (Tadsanaprasittipol et al., 2021). Previously, another study also realized in Bangkok observed a PAH annual concentration of 12.6 ng/m3, approximately half of the concentration found in our study, but a BaP-TEQ value of 2.1 ng/m3 (Norramit et al., 2005). Still, in the same region, an investigation showed a PM10 annual concentration of 66.0 ug/m3 followed by a PAH concentration of 60.8 ng/m3 (Thongsanit et al., 2003). Regarding PAH bounded in PM2.5, a study from China showed a mean annual concentration of PM2.5 of 204 μg/m3 and PAH concentration of 66.2 ng/m3. As expected, the BaP-TEQ value also demonstrated a high value (16.6 ng/m3) at the expense of the great PM2.5 concentration (Chao et al., 2019).
Regarding the variability factors influencing the concentration of PM-bounded PAH, it is common to find a higher concentration of PAH in urban areas; however, other areas containing great sources of PAHs could equally demonstrate augment levels. This trend was observed by Tadsanaprasittipol et al. (2021), showing a higher BaP account in roadside sites than in urban and industrial ones, as observed in the geolocation of area Candiota City. Additionally, PAH emissions are closely related to the social-economic population level. In Candiota city, the per capita income was 2221$ in the period of study development (2013), which is substantially lower compared to the capital of Rio Grande do Sul State, Porto Alegre (6922$) (IBGE, 2021), so it indicates that Candiota City is a vulnerable area considering its social-economic status. Meteorological conditions also influence air pollutants levels, as significantly lower concentrations were observed in hot and humid periods, while higher average total carcinogenicity was observed during the winter (Tadsanaprasittipol et al., 2021). Several studies also found higher PAH concentrations in the coldest season, contributing to higher daily inhalation of BaP-TEQ and incremental lifetime cancer risk (Hsu, 2019; Jyethi et al., 2014; Stracquadanio et al., 2007; Tadsanaprasittipol et al., 2021). In our study, PAH concentration, daily inhalation of BaP-TEQ, and incremental lifetime cancer risk were higher in cold period, once the thermal inversion phenomenon increase the concentration of PM10 and PAH bounded to it.
In our study, a significant positive correlation was observed between the total PAHs and Chrysene, Fluoranthene, Pyrene, Indeno(1,2,3)pyrene, Benzo(g, h, i)perylene, Dibenzo(a, h)anthracene, Benzo(a)pyrene, and Benzo(a)anthracene. Those compounds had a high molecular weight and present 4 to 6 rings in their composition compared to other PAHs. Moreover, they are classified with increased carcinogenic potency (Norramit et al., 2005; USEPA, 2005). Likewise, Chrysene, Fluoranthene, Benzo(a)pyrene, and Benzo(a)anthracene are considered markers of coal combustion and were associated with the total PAH in our study, ensuring that the activities related to coal in Candiota area were the sources of the PAHs (Larsen & Baker, 2003, Abdulazeez & Fantke, 2017). Rekefu et al. (2018) demonstrated that similar annual concentrations of different PM sizes (PM2.5 20.9 ng/m3 and PM2.5–10 20.9 ng/m3) resulted in very distinct BaP-TEQ values (10.5 ng/m3 and 1.2 ng/m3, respectively). Once each PAH has a different carcinogenic potential, the carcinogenic risk of PAH is not necessarily associated with total pollutant concentration showing the relevance of dosing isolated compounds (Norramit et al., 2005).
The individuals living in the coal activity area of Candiota are exposed to potential cancer risk, once the ILCR values exceed the limit of 10–6 (USEPA, 2005). Individuals living in areas with coal extraction and combustion are more susceptible to developing cancer, once PAHs concentration, which is carcinogenic compounds, is higher because of the coal-related activities. Epidemiological studies, systematic reviews, and geospatial analysis of cancer risk showed that the PAH emissions from coal activity contributes to the etiology of cancer once it was observed a positive association between all cancer mortality, lung cancer incidence, and mortality with proximity to a coal mine. The carcinogenic effect caused by PAH emitted by coal activity could affect population due to occupational and environmental exposure, including the geographical proximity as a risk factor for the residents in these areas (Bigliardi et al., 2021; Cortes-Ramirez et al., 2018; da Silva Júnior et al., 2018; Fernández-Navarro et al., 2012; Jenkins et al., 2013; Mueller et al., 2015). The ILCR values estimated considering the reference values of USEPA for inhalation rate and body mass were higher than the ILCR using the real values (USEPA, 2011).
Our results showed that 86.1% of our sample was below the value of ILCR that considered the USEPA reference values for calculation. This difference indicates the importance to consider information from the local population. We used the body mass index of each individual included in our study to estimate their inhalation rate to calculate the ILCR. Overweight individuals have a higher inhalation rate than lean individuals, as defined by USEPA, 2011. However, overweight individuals did not present a higher exposure because the equation is relativized by the body mass.
As a limitation of our study, we cited the monitoring campaigns frequency, which did not have a homogeneous scenario during all the seasons of the one-year monitoring period. Moreover, there was a lack of data concerning coal mining and power plant activities. Although it was an advance to include local epidemiological data, it was limited to the only data available, a male adult population. Moreover, we did not investigated the dietary intake of the population enrolled in the study; therefore, it is not included the PAHs intake in the exposure. The technique to meal preparation with grilling and/or frying would produce high levels of PAHs, due to the pyrolysis of fat at higher temperature and adsorption of PAHs emitted from combustion process. Additionally, the PAHs concentration in raw foods may come from the deposition of ambient particles, contaminated soils and water (Alomirah et al., 2011; Duan et al., 2016).
Conclusion
Our study demonstrated that Candiota city area had substantial emissions of air pollutants, including toxic components with carcinogenic potentials, like PM10 and PAHs. This emission could be related to the coal activity and burning activities performed in this area, once these pollutants are emitted by coal combustion. Consequently, the studied population had ILCR levels above the reference limit of 10–6, indicating cancer risk. The observed risk in the population sets an alert for the harmful consequences of the use of this energy source. Therefore, an epidemiological survey of cancer cases in the region and its relationship with environmental exposure and air pollutants levels must be mandatory. Especially because there is a lack of public policies in Brazil focusing on air pollutants emissions monitoring, highlighting the need for free disclosure and interpretation of data collected by air quality monitoring stations administered by public or private companies.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdulazeez, T. L., & Fantke, P. (2017). Polycyclic aromatic hydrocarbons. A Review. Cogent Environmental Science, 3(1), 1339841.
Alomiraha, H., Al-Zenkia, S., Al-Hootia, S., Zaghloula, S., Sawayaa, W., Ahmedb, N., & Kannan, K. (2011). Concentrations and dietary exposure to polycyclic aromatic hydrocarbons (PAHs) from grilled and smoked foods. Food Control, 22(12), 2028–2035.
Akhbarizadeh, R., Dobaradaran, S., Torkmahalleh, M. A., Reza Saeedi, R., Aibaghi, R., & Ghasemi, F. F (2020). “Suspended fine particulate matter (PM2.5), microplastics (MPs), and polycyclic aromatic hydrocarbons (PAHs) in air: Their possible relationships and health implications. Environmental Research, 110339. https://doi.org/10.1016/j.envres.2020.110339.
Bigliardi, A. P., Fernandes, C. L. F., Pinto, E. A., Dos Santos, M., Garcia, E. M., Baisch, P. R. M., Soares, M. C. F., Muccillo-Baisch, A. L., & da Silva Júnior, F. M. R. (2021). Blood markers among residents from a coal mining area. Environmental Science and Pollution Research International, 28(2), 1409–1416.
BRASIL. (2008). Atlas de Energia Elétrica Elétrica Agência Nacional de Energia.
Chao, S., Liu, J., Chen, Y., Cao, H., & Zhang, A. (2019). Implications of seasonal control of PM2.5-bound PAHs: An integrated approach for source apportionment, source region identification and health risk assessment. Environmental Pollution, 247, 685–695.
Chaves, H. A. F., & RenéAde, M. V. B. (2018). Geochemical characterization of coal deposits of Candiota coalfield Rio Bonito formation. Journal of Sedimentary Environments, 3(1), 19–35.
Chen, S. C., & Liao, C. M. (2006). Health risk assessment on human exposed to environmental polycyclic aromatic hydrocarbons pollution sources. Science of the Total Environment, 366(1), 112–123.
Cortes-Ramirez, J., Naish, S., Sly, P. D., & Jagals, P. (2018). Mortality and morbidity in populations in the vicinity of coal mining: A systematic review. BMC Public Health, 18(1), 721.
Duan, X., Shen, G., Yang, H., Tian, J., Wei, F., Gong, J., & Zhang, J. J. (2016). Dietary intake polycyclic aromatic hydrocarbons (PAHs) and associated cancer risk in a cohort of Chinese urban adults: Inter- and intra-individual variability. Chemosphere, 144, 2469–75. https://doi.org/10.1016/j.chemosphere.2015.11.019. Epub 2015 Dec 6.
da Silva Júnior, F., Tavella, R. A., Fernandes, C., Soares, M., de Almeida, K. A., Garcia, E. M., da Silva Pinto, E. A., & Baisch, A. (2018). Genotoxicity in Brazilian coal miners and its associated factors. Human and Experimental Toxicology, 37(9), 891–900.
de Onis, M., & Habicht, J. P. (1996). Anthropometric reference data for international use: Recommendations from a World Health Organization Expert Committee. American Journal of Clinical Nutrition, 64(4), 650–658.
dos Santos, M., Penteado, J. O., Soares, M. C. F., et al. (2019). Association between DNA damage, dietary patterns, nutritional status, and non-communicable diseases in coal miners. Environmental Science and Pollution Research, 26, 15600–15607. https://doi.org/10.1007/s11356-019-04922-8
dos Santos, M., Ramires, P. F., Gironés, M. C. R., et al. (2021). Multiple exposure pathways and health risk assessment of selenium for children in a coal mining area. Environmental Science and Pollution Research, 28, 13562–13569. https://doi.org/10.1007/s11356-020-11514-4
European Communities. (2001). Ambient air pollution by Polycyclic Aromatic Hydrocarbons (PAH). Position Paper, European Communities.
Espitia-Pérez, L., Arteaga-Pertuz, M., Soto, J. S., Espitia-Pérez, P., Salcedo-Arteaga, S., Pastor-Sierra, K., Galeano-Páez, C., Brango, H., da Silva, J., & Henriques, J. A. P. (2018). Geospatial analysis of residential proximity to open-pit coal mining areas in relation to micronuclei frequency, particulate matter concentration, and elemental enrichment factors. Chemosphere, 206, 203–216.
Fernández-Navarro, P., García-Pérez, J., Ramis, R., Boldo, E., & López-Abente, G. (2012). Proximity to mining industry and cancer mortality. Science of the Total Environment, 435–436, 66–73.
Franco, A., & Diaz, A. R. (2009). The future challenges for “clean coal technologies”: Joining efficiency increase and pollutant emission control. Energy, 34, 348–354.
Ghasemi, F., Dobaradaran, S., Saeedi, R., et al. (2020). Levels and ecological and health risk assessment of PM2.5-bound heavy metals in the northern part of the Persian Gulf. Environmental Science and Pollution Research, 27, 5305–5313. https://doi.org/10.1007/s11356-019-07272-7
Ghose, M. K., & Majee, S. R. (2007). Characteristics of hazardous airborne dust around an Indian surface coal mining area. Environmental Monitoring and Assessment, 130(1–3), 17–25.
Hsu, C. (2019). Characterization of PM2.5-bound polycyclic aromatic hydrocarbons in Taiwan. Environmental Epidemiology, 3, 164–165.
IARC. (2013). Air pollution and cancer. W. H. O. WHO. France. IARC Scientific Publications World Health Organization (Vol. 161). IARC Scientific Publications.
IBGE. (2021). Cidades e Estados. Retreived December, 2021, from https://www.ibge.gov.br/cidades-e-estados/.
Idani, E., Geravandi, S., Akhzari, M., Goudarzi, G., Alavi, N., Yari, A. R., Mehrpour, M., Khavasi, M., Bahmaei, J., Bostan, H., Dobaradaran, S., Salmanzadeh, S., & Mohammadi, M. J. (2020). Characteristics, sources, and health risks of atmospheric PM10-bound heavy metals in a populated middle eastern city. Toxin Reviews, 39(3), 266–274. https://doi.org/10.1080/15569543.2018.1513034
Jenkins, W. D., Christian, W. J., Mueller, G., & Robbins, K. T. (2013). Population cancer risks associated with coal mining: a systematic review. PLoS ONE, 8(8), e71312.
Jyethi, D. S., Khillare, P. S., & Sarkar, S. (2014). Risk assessment of inhalation exposure to polycyclic aromatic hydrocarbons in school children. Environmental Science and Pollution Research International, 21(1), 366–378.
Kaygusuz, K. (2012). Energy for sustainable development: A case of developing countries. Renewable & Sustainable Energy Reviews 1116–1126.
Kelly, J., Ivatt, P., Evans, M. J., Kroll, J. H., Hrdina, A., Kohale, I. N., White, F. M., Engelward, B. P., & Selin, N. E. (2021). Global cancer risk from unregulated polycyclic aromatic hydrocarbons. GeoHealth, 5(9). https://doi.org/10.1029/2021GH000401.
Kong, S., Ding, X., Bai, Z., Han, B., Chen, L., Shi, J., & Li, Z. (2010). A seasonal study of polycyclic aromatic hydrocarbons in PM(2.5) and PM(2.5-10) in five typical cities of Liaoning Province, China. Journal of Hazardous Materials, 183(1–3), 70–80.
Kurth, L., Kolker, A., Engle, M., Geboy, N., Hendryx, M., Orem, W., McCawley, M., Crosby, L., Tatu, C., Varonka, M., & DeVera, C. (2015). Atmospheric particulate matter in proximity to mountaintop coal mines: Sources and potential environmental and human health impacts. Environmental Geochemistry and Health, 37(3), 529–544.
Laney, A. S., & Weissman, D. N. (2014). Respiratory diseases caused by coal mine dust. Journal of Occupational and Environmental Medicine, 56(Suppl 10), S18-22.
Larsen, R. K., & Baker, J. E. (2003). Source apportionment of polycyclic aromatic hydrocarbons in the urban atmosphere: A comparison of three methods. Environmental Science and Technology, 37(9), 1873–1881.
Lee, B.-K. (2010). Sources, distribution and toxicity of polyaromatic hydrocarbons (PAHs) in particulate matter. Air Pollution. London, IntechOpen 99–122.
Mo, Z., Wang, Z., Mao, G., Pan, X., Wu, L., Xu, P., Chen, S., Wang, A., Zhang, Y., Luo, J., Ye, X., Wang, X., Chen, Z., & Lou, X. (2019). Characterization and health risk assessment of PM. Science and Reports, 9(1), 7296.
Mueller, G. S., Clayton, A. L., Zahnd, W. E., Hollenbeck, K. M., Barrow, M. E., Jenkins, W. D., & Ruez, D. R. (2015). Manuscript title: Geospatial analysis of Cancer risk and residential proximity to coal mines in Illinois. Ecotoxicology and Environmental Safety, 120, 155–162.
Norramit, P., Cheevaporn, V., Itoh, N., & Tanaka, K. (2005). Characterization and carcinogenic risk assessment of polycyclic aromatic hydrocarbons in the respirable fraction of airborne particles in the Bangkok Metropolitan Area. Journal of Health Science, 51(4), 437–446.
Perret, J. L., Plush, B., Lachapelle, P., Hinks, T. S., Walter, C., Clarke, P., Irving, L., Brady, P., Dharmage, S. C., & Stewart, A. (2017). Coal mine dust lung disease in the modern era. Respirology, 22(4), 662–670.
Pires, M., Querol, X., & Teixeira, E. C. (2001). Caracterização do carvão de Candiota e de suas cinzas. Geochimica Brasiliensis, 15, 113–130.
Prefeitura de Candiota, P. D. (2013). Dados Gerais. Retrieved 2 June, 2021, from https://www.candiota.rs.gov.br/dados-gerais/.
Secretaria do Meio Ambiente e Infraestrutura RS. (2022). Minas. https://www.crm.rs.gov.br/minas.
Stout, S. A., & Emsbo-Mattingly, S. D. (2008). Concentration and character of PAHs and other hydrocarbons in coals of varying rank—Implications for environmental studies of soils and sediments containing particulate coal. Organic Geochemistry, 39, 801–819.
Stracquadanio, M., Apollo, G., & Trombini, C. (2007). A study of PM2.5 and PM2.5-associated polycyclic aromatic hydrocarbons at an urban site in the Po Valley (Bologna, Italy). Water, Air, and Soil Pollution, 179, 227–237.
Straif, K., Baan, R., Grosse, Y., Secretan, B., El Ghissassi, F., & Cogliano, V. (2005). Carcinogenicity of polycyclic aromatic hydrocarbons. The Lancet Oncology, 6(12), 931–932. https://doi.org/10.1016/s1470-2045(05)70458-7
Tadsanaprasittipol, A., Tonrub, P., Beverland, I. J., & Kalin, R. M. (2021). Characterization of PM10-bound polycyclic aromatic hydrocarbons and associated carcinogenic risk in Bangkok, Thailand. Applied Science, 11.
Thongsanit, P., Jinsart, W., Hooper, B., Hooper, M., & Limpaseni, W. (2003). Atmospheric particulate matter and polycyclic aromatic hydrocarbons for PM10 and size-segregated samples in Bangkok. Journal of the Air and Waste Management Association, 53(12), 1490–1498.
USEPA. (1999). Determination of polycyclic aromatic hydrocarbons (PAH) in ambient air using gas chromatography/mass spectrometry (GC/MS). United States Environmental Protection Agency
USEPA. (2005). Guidelines for Carcinogen Risk Assessment (Vol. 70 FR, pp. 17765–17817). United States Environmental Protection Agency.
USEPA. (2011). Exposure Factors Handbook. United States Environmental Protection Agency.
USEPA. (2014). Office of the Federal Registration (OFR) Appendix A: Priority pollutants. US Environmental Protection Agency.
Veziroglu, T. N., & Sahin, S. (2008). 21st Century’s energy: Hydrogen energy system Energy Conversion and Management. Energy Conversion and Management, 49, 1820–1831.
World Environment Council. (2016). World Energy Resources.
WHO, Air Quality Guidelines for Europe. (2000). In Air quality guidelines for Europe. World Health Organization Regional Office for Europe.
Zhu, Y., Yang, L., Yuan, Q., Yan, C., Dong, C., Meng, C., Sui, X., Yao, L., Yang, F., Lu, Y., & Wang, W. (2014). Airborne particulate polycyclic aromatic hydrocarbon (PAH) pollution in a background site in the North China Plain: Concentration, size distribution, toxicity and sources. Science of the Total Environment, 466–467, 357–368.
Acknowledgements
Bruna Marmett and Roseana Böek Carvalho are supported by doctoral fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Flávio Manoel Rodrigues da Silva Júnior is grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the PQ productivity scholarship (310856/2020-5).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or non-for-profit sectors.
Author information
Authors and Affiliations
Contributions
BM involved in conceptualization, methodology, formal analysis, writing—original draft, and writing—review and editing. RBC involved in conceptualization, methodology, formal analysis, writing—original draft, and writing—review and editing. ALM-B involved in methodology. PRMB involved in methodology. MS involved in methodology. EMG involved in methodology. CRR involved in conceptualization, writing—original draft, writing—review and editing. FMRSJ involved in conceptualization, methodology, formal analysis, writing—original draft, and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This study followed the principles of the Declaration of Helsinki and it was approved by the Research Ethics Committee in Health 036/2013 (CEPAS/FURG).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Marmett, B., Carvalho, R.B., Muccillo-Baisch, A.L. et al. Emissions monitoring and carcinogenic risk assessment of PM 10 -bounded PAHs in the air from Candiota’s coal activity area, Brazil . Environ Geochem Health 45, 899–911 (2023). https://doi.org/10.1007/s10653-022-01253-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-022-01253-5