Abstract
Several research groups have studied new biopesticides which are less toxic to the environment and capable of controlling the vectors of parasitic diseases, especially in aquatic ecosystems. Pest control by photodynamic substances is an alternative to chemical or other measures, with chlorophyll and its derivatives as the most studied substances supported by their easy availability and low production costs. The impact of chlorophyll derivatives on four different species, a small crustacean (Daphnia similis), a unicellular alga (Euglena gracilis) and two species of fish (Astyanax bimaculatus and Cyprynus carpio) were tested under short-term conditions. In addition, the effects of long-term exposure were evaluated in D. similis and E. gracilis. In short-term tests, mortality of D. similis (EC50 = 7.75 mg/L) was most strongly affected by chlorophyllin, followed by E. gracilis (EC50 = 12.73 mg/L). The fish species showed a greater resistance documented by their EC50 values of 17.58 and 29.96 mg/L in C. carpio and A. bimaculatus, respectively. A risk quotient is calculated by dividing an estimate of exposure by an estimate of effect. It indicated that chlorophyll derivatives can be applied in nature to control the vectors of parasitic diseases under short-term conditions, but long-term exposure requires new formulations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vector-borne diseases are a global burden and are estimated to cause several million deaths and countless cases of diseases each year. More than 70 parasitic diseases caused by protozoa have been described in humans. The search for new insecticides capable of controlling different parasites combined with a low toxicity to the environment has been conducted by several research groups (Erzinger 2011).
Chlorophyll derivatives, such as chlorophyllin and pheophorbide can be used in photodynamic reactions that destroy parasites found in aquatic ecosystems. These substances can be produced from chlorophyll extracted from green plant material. By simple chemical modifications the hydrophobic chlorophyll can be made water-soluble in the form of chlorophyllin, which, in turn, can be modified to pheophorbide by acidification (Erzinger 2011).
Experiments with photodynamic substances yielded very promising results. The study by Abdel-Kader et al. (1999) demonstrated that Culex larvae are sensitive to a concentration of 0.07 mM chlorophyllin and Musca domestica larvae could be killed with this substance at a concentration of 10 µM. El-Tayeb (2003) demonstrated the effectiveness of hematoporphyrin against Culex eggs and the snail Lymnea natalensis (vector of the trematode Fasciola hepatica). The uptake of the photosensitizer into the organism has been shown by fluorescence microscopy as well as a pronounced effect on the ultrastructure of internal organs and muscles (El-Tayeb 2003, Abdel-Kader and El-Tayeb 2009).
Wohllebe et al. (2009) using chlorophyllin/pheophorbide under laboratory conditions as photodynamic substances for pest control showed that this treatment is not only effective and environmentally beneficial but also cost effective. The EC50 value (50 % mortality in test organisms) in Culex sp. larvae was about 6.88 mg/L and in Chaoborus sp. larvae approximately 24.18 mg/L. The EC50 values determined for pheophorbide were 8.44 mg/L in Culex and 1.05 mg/L in Chaoborus. The results presented by Wohllebe et al. (2009) show that chlorophyllin is more effective than hematoporphyrin or methylene blue by a factor of 100, which were previously tested for the same purpose.
Erzinger et al. (2011) showed that in Chaoborus sp. a dark incubation period of about 3 h is sufficient to induce mortality of about 90 % and ≥6 h resulted in nearly 100 % mortality when the organisms were exposed to light after the dark incubation. The temperature did not significantly influence the mortality of larvae in a treatment of 6 h in darkness and subsequent incubation for 3 h in light. At 10, 20C or 30 °C between 80 and 100 % of the treated larvae were killed when the intensity of light from a solar simulator was higher than 30 W/m2. Lower irradiances were less effective.
Several authors have reported that chlorophyll derivatives have the ability to control different parasites in aquatic systems. Wohllebe et al. (2012) found that the protozoan parasite Ichthyophthirius mulftifiliis (Fouquet), which causes the white spot disease in many freshwater fish species, was killed (EC50 = 0.67 mg/L). Mahmoud et al. (2013) reported the molluscicidal activity (EC50 = 30 mg/L) for the snails Lymnaea stagnalis, Biomphalaria spp. and Physa marmorata.
The possibility of developing new products to control aquatic pests resulted in intellectual property registration of two new products based on chlorophyll derivatives. Abdel-Kader et al. (2005a, b, 2006, 2008a, b, 2009) obtained several patents using chlorophyll derivatives to control different parasites. El-Tayeb and Abdel-Kader (2009) registered the intellectual property called “Field application for malaria vector control using sunlight active formulated extract which is based on using plant extracts containing chlorophyll derivatives. In 2010 Erzinger and Häder developed a new biopesticide, which is nationally and internationally patented (Erzinger and Häder 2009, Häder and Erzinger 2011). The product is capable to eliminate multiple vectors of parasites that have an aquatic life stage. Its mechanism of activity is based on the release of singlet oxygen (1O2) through the interaction with light, which in turn results in the activation of the caspase cascade (Wohllebe et al. 2011), producing signaling proteins, which finally induce apoptosis of cells in the intestine endothelium, resulting in the death of the parasites. This was elucidated in mosquito larvae which had been exposed to low concentrations of chlorophyllin. After several hours of uptake in darkness the chlorophyllin could be located in the intestine by fluorescence microscopy. The use of acridine orange staining demonstrated that some cells of the intestine endothelium had undergone necrosis induced by 1O2. Subsequent staining with propidium iodide and Hoechst 33342 showed that the cell nuclei of the intestine endothelium had entered apoptosis (Wohllebe et al. 2011).
In 2014, Kashiyama and Tamiaki (2014) published a review, which described various strategies by which organisms avoid phototoxicity of chlorophylls, emphasizing the costs of adaptive mechanisms against this risk of these biological processes. The objective of the present study was to evaluate the potential risk of environmental toxicity caused by the application of chlorophyll to control pest organisms in aquatic systems.
The objectives of the present research are to assess the ecotoxicological consequences of the application of chlorophyll derivatives to control vectors of human and animal parasites in aquatic ecosystems. Since these substances have been found to effectively kill mosquito larvae and fish parasites at low concentrations it is of importance to evaluate if they affect other organisms in aquatic ecosystems.
Materials and methods
Preparations of chlorophyllin
Chlorophyllin was prepared from deep frozen spinach (Spinacia oleracea L). Chlorophyll was extracted from the leaves in a water bath at 65 °C using 96 % ethanol, after adding 0.1 M CaCO3, which prevents the formation of pheophytin (Rahmani and Csallany 1991). The extract was filtered and petroleum ether (50–100 °C) was added to the liquid fraction at room temperature. After shaking and separation, the upper lipophilic phase was removed and saponified with methanolic 1 M KOH. This treatment converts chlorophyll into the water-soluble chlorophyllin (Schertz 1928). The chlorophyllin concentration was determined using a spectrophotometer (Shimadzu UV-2501) as described by Porra et al. (1989). The extract was stored in a dark flask at 4 °C under N2 atmosphere (Erzinger et al. 2011).
In the short-term experiments stock solutions of chlorophyllin were used at concentrations of 100 mg/L dissolved in methanolic KOH. The methanol was evaporated in darkness on a heating plate (80 °C) under airflow.
Organisms
Cell culture and growth conditions of Euglena gracilis
All experiments were performed using an axenic culture of the freshwater flagellate E. gracilis Klebs (strain Z) obtained from the culture collection in Göttingen (Germany). The cells were grown in a mixture of a complex and mineral medium described previously (Checcucci et al. 1976). New cultures were inoculated with 25 % from an exponential culture and grown at 22 °C under 20 W/m2 continuous white light from fluorescence tubes in stationary 100-mL Erlenmeyer flasks.
Daphnia similis
D. similis cultures were grown in 1 L-glass beakers containing 800 mL of culture medium and 20 Daphnia. The culture medium was renewed and the offspring produced was removed twice a week. Brood Daphnia were removed after 4 weeks in culture and replaced with neonatal organisms. Cultures were maintained at 21 ± 0.5 °C under 12 h light/12 h dark. Cultured Daphnia were fed with a suspension of the unicellular green alga Raphidocelis subcapitata (formerly called Selenastrum capricornutum) twice daily. A peristaltic pump controlled by a timer delivered the food. The feeding rate was about 2 × 105 cells/ml per days.
Fish species
For the tests two fish species, Astyanax bimaculatus and Cyprinus carpio, were selected. A. bimaculatus is a genus native to the Amazon basin; it has a geographical distribution in tropical and subtropical America and represents a significant food source for fish holding higher positions in the food chain. Ordinarily, many small Characiformes that inhabit rivers, reservoirs and lakes are called minnows in Brazil. A. bimaculatus, popularly known as red-tailed tetra, reaches an average length of 10 cm and is caught by small business companies. It is widely distributed in South and Central America, and it is abundant in several watersheds in Brazil. It has a broad food spectrum, so it can adapt to whatever food is available. In addition, it produces small and numerous eggs that develop rapidly (Dias et al. 2005), warranting their success in colonizing dammed environments (Silva et al. 2010). Among the native species, the yellow-tailed tetra (A. bimaculatus) has a potential for aquaculture. It is an omnivorous species that feeds mainly on insects (Reis et al. 2003, da Silva 2009). It is well accepted as a fish snack and used as bait for sport fishing (Hayashi et al. 2004).
C. carpio, although not native to Brazil, was selected because it is used for cultivation in aquaculture especially in the southern states of the country. Both fish species were obtained from aquaculture in Municipal Foundation for Rural Development July 25, Joinville, Brazil. All fish stored in individual glass tanks for a period of 3 days before the experiment with optimal cultivation conditions in order to adapt the fish to the new environment and reduce stress induced by catching and transferring them to deionized water with the appropriate concentration of chlorophyllin. The water was buffered to maintain of pH of 7.2–7.5, an alkalinity of 30–35 mg/L, and a hardness of 40–50 mg/L as CaCO3. The water was mixed thoroughly and aerated before transfer into the test chambers. Fish are acclimated to the test water by gradually changing the water in acclimation tanks from 100 % well water to 100 % reconstituted water over a 4- to 6-h period at 25 ± 1 °C as required by the test procedures (ABNT 2007, ABNT 2011). Toxicity tests were conducted under static conditions without aeration and the organisms were not fed during the test.
Judging from the behavior of the fish this acclimation period was sufficient. For both species juvenile specimens which showed good viability of about 6 cm length were selected.
Short- and long-term tests of toxicity in Daphnia similis
The methodology for the short-term tests with D. magna is described in the standard NBR 12713 (ABNT 2003). Neonates of D. similis, 2–26 h old, were exposed to diluted samples of chlorophyllin for a period of 48 h (Flohr et al. 2005).
Dilution water was reconstituted with a total hardness of 40–48 mg/L of CaCO3, pH 7.2 at 7.6 and conductivity of approximately 160 µS/cm. The quality control of each batch was carried out by a feasibility test, where a population of test organisms was exposed to the dilution water under the test conditions. The batch dilution water is considered acceptable for use if the immobility rate and/or mortality did not exceed 10 % over a period of 48 h.
Neonate D. similis were taken from the cultures and placed in new bottles with the same dilution water plus the appropriate concentration of chlorophyllin. Six neonates were used per sample with an age of 24 h which is considered a very sensitive stage in this species. The sensitivity of the test organisms was evaluated by determining the EC50 after 48 h. In parallel the response to potassium dichromate (K2Cr2O7) was tested, which is reference substance commonly used for this species. The acceptable range of K2Cr2O7 for D. similis was 0.04–0.17 mg/L, according to the methodology described in Araujo (2005).
In the long-term toxicity tests of (21 days) chlorophyllin concentrations were 0 (control), 1, 2, 2.5, 3, 3.5 and 4 mg/L. The fertility of three generations (the end of 21 days) was calculated from the number of offsprings. Each concentration sample contained one female Daphnia in 50 mL and there were 10 replicates for each concentration ISO 10706 (ISO 2000).
Short- and long-term tests of toxicity in Euglena gracilis
For the short-term tests the cells were exposed to chlorophyllin at the desired concentration for 24 h (one light/dark cycle). The first test was done 30 min after transferring the cells to the observation chamber where they were kept in darkness. The second test was performed after 24 h during which the cells again were kept in darkness. The endpoints of these tests were precision of gravitactic orientation, alignment in the water column, percentage of motile cells, mean velocity and form factor (compactness), all of which were determined using the automatic bioassay NGTOX (Erzinger et al. 2010), which is the second generation of the biomonitoring instrument ECOTOX (Tahedl and Häder 1999, 2001). The device consists of a miniaturized microscope, a Firewire camera (DMK 21F04, Imaging Source, Bremen, Germany), a stainless steel cuvette with glass windows, an infrared monitoring light source (infrared LED) and three stepper motor pumps to transfer water, samples and cultures to the observation cuvette. The internal functions are controlled by a built-in microprocessor linked to an external host computer to ensure a fully automatic performance.
In the test with E. gracilis, evaluating the motility and orientation parameters described above, the effect was considered significant if the inhibition values of all three replicates exceeded the threshold value of the NG-TOX for a given parameter (11.4 % for motility, 6.8 % for velocity, 3.4 % for compactness, 12.3 % for r-value and 3.1 % for upward swimming) (Azizullah et al. 2011). The highest concentration of a chemical with no significant effect is described as NOEC (no observed effect concentration). The highest concentration, at which no significant effect was observed for a given parameter, was considered as the G-value for that parameter (Azizullah et al. 2011).
The biotest NGTOX is programmed to work in several modi. The single toxin mode is used to produce a series of an automatic dilution (1:2, 1:4, 1:8, 1:16 and 1:32) of the toxin stock solution. Control measurements were done using cell cultures mixed with water instead of toxins. In addition, monitoring of a fixed toxin concentration over longer incubation times can be done using the control mode. In the current experiments measurements were performed immediately after exposure to the toxins and subsequently daily for up to 1 week. All measurements were performed in darkness to avoid interaction of gravitaxis and photoorientation.
For the long-term toxicity tests cultures of E. gracilis were grown in media with different concentrations of chlorophyllin (0, 1, 3, 7, 9, 13 and 16 mg/L) added, and the effect on the various movement parameters was observed after 7 days of growth. Cultures were grown in three independent replicates in 100-mL flasks with 50 ml of total volume in each flask. All cultures were inoculated with 10 mL at an initial cell density of about 80,000 cells per mL. The other growth conditions were as described above. There is no standardized method for quality control for the motility and orientation in Euglena. However, the minimum requirement was that >80 % of the cells were motile (Ahmed and Häder 2010).
Effects of chlorophyllin in fish in vivo
Three replicates with 10 young fish each were prepared for each concentration of chlorophyllin in 1000 ml of water at 20 °C and each experiment was repeated independently three times. KOH-treated samples (corresponding to the highest chlorophyllin concentration) served as controls and were compared with samples in water. All samples were incubated in darkness for 3 h. After incubation three samples were irradiated with simulated solar radiation (Sol 1200 W, mercury lamp 0383; Dr. Hönle, Martinsried, Germany) for another 3 h. The lamp output was PAR 149.66 W/m2, UV-A 32.67 W/m2, and UV-B 0.77 W/m2 (determined with a spectroradiometer OL 754; Optronics, USA). The emission spectrum of the lamp has been published elsewhere (Klisch et al. 2001). Three parallel samples were kept in darkness as dark controls. This scheme was performed for all chlorophyllin concentrations. After irradiation the vitality of the fish was determined. Abiotic parameters such as temperature, pH and dissolved oxygen were measured before and after of each analysis using a Hanna Multiprobe.
The endpoint for the fish tests was mortality. Individuals showing no vital signs (movements, reflexes after tipping with a needle) were counted as dead, while unaffected organisms and organisms with reduced viability (compared to the untreated controls) were counted as survivors (ABNT 2011).
Data analysis
Statistical analyses were performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Statistical analyses were also performed using one-way ANOVA. When the ANOVA showed significant significance, a Tukey test was to determine which concentrations were significantly different from the control group and the other treatments. This procedure allowed the determination of the standard NOEC (No Observed Effect Concentration) and LOEC (Lowest Observed Effect Concentration) values. To detect any significant differences in individual growth, the nonparametric Mann–Whitney test (MW) was used. A value of p ≤ 0.05 was considered to indicated significance in all tests.
The median effective concentration (EC50) were estimated using Eq. (1) fitted to the dataset through the least squares method (Tahedl and Häder 1999):
where y is the response variable (percentage of dead organism), c the chlorophyll concentration, y0 is the response when the concentration tends to infinity and b is a scale factor.
Confidence intervals were calculated from the covariance matrix at a two-side error level of 5 %. All data are expressed as mean ± standard deviation (SD).
Results
Initial experiments evaluated the toxicity in short-term experiments for the micro crustacean D. similis, the alga E. gracilis and two fish species. In all experiments the control samples (treated with KOH or water) showed no physiological modification or mortality in the studied species. The investigated risk factor was the hydrophilic chlorophyll derivative chlorophyllin. In long term studies no breakdown product of this substance has been found to have adverse effects (Wohllebe 2010). The other potential toxic substances, such as ethanol, methanol and petroleum ether used for extraction and chemical modification, were removed from the chlorophyllin by evaporation before application of the photosensitizer. The other substance to be considered is KOH; this was also tested and found not to induce any toxicity at the highest concentration used in the dilution series or any measureable change in the water pH. These evidences rule out the assessment of other risk factors, and the concentrations indicated below refer to chlorophyllin.
Evaluation of short-term toxicity
The effects of chlorophyllin in E. gracilis on motility, cell velocity, gravitaxis and cell compactness (=cell shape) were studied immediately after incubation, and EC50 values were calculated for all parameters and summarized Fig. 1.
In the short-term exposure experiments with chlorophyllin, all parameters gave a dose-dependent response but varied in their sensitivity giving different EC50 values. Alignment was the least sensitive parameter with an EC50 value of 16.33 mg/L (Fig. 1c) followed by the precision of gravitational orientation (r-value) with an EC50 of 14.70 mg/L (Fig. 1b) and compactness with an EC50 value of 14.00 mg/L (Fig. 1d). The velocity showed a low EC50 value (9.81 mg/L) (Fig. 1e). Motility was the most sensitive parameter with an EC50 value of 8.1 mg/L (Fig. 1a). The mean EC50 value obtained for the algae and derived from the five physiological parameters showed a sensitivity of 12.73 mg/L. The value obtained for D. similis was 7.77 mg/L (Fig. 2).
When exited with blue light, chlorophyllin emits a red fluorescence. Because the carapace of Daphnia is transparent, incorporated chlorophyllin showed an intensive fluorescence of the intestine which could be visualized using epifluorescence microscopy (NOVA 606, Nova Optical Systems) with no camera attached.
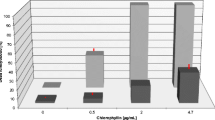
The effects of different concentrations of chlorophyllin in short-term toxicity tests in fish are summarized in Fig. 3. A. bimaculatus showed greater resistance to chlorophyllin (EC50 = 29.96 mg/L) which was higher than that of C. carpio (EC50 = 17.58 mg/L).
Percent mortality of C. carpio a (EC50 = 17.58 mg/L) and A. bimaculatus b (EC50 = 29.96 mg/L) exposed to chlorophyllin, incubated in the dark for 3 h and subsequently for 3 h in simulated sunlight. Each point corresponds to ten fishes. The values shown are mean ± SD of the three replicates. One-way ANOVA was calculated for a 95 % confidence level. For all tests p < 0.001 was calculated
Evaluation of long-term toxicity
In order to assess the toxicity in long-term tests, we used the invertebrate D. similis and the alga E. gracilis. The precision of gravitactic orientation of E. gracilis, as quantified by the r-value (Häder et al. 1995), was less sensitive than the other parameters with a NOEC of 8.7 mg/L, followed by velocity with a NOEC of 7.0 mg/L (Table 1). The mean NOEC value obtained for the long-term tests of algae of the five physiological parameters showed a sensitivity of 4.7 mg/L.
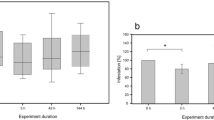
The test acceptability criteria according to the standard NBR 12713 (2003) were met for the chronic Daphnia toxicity test. A chronic test of D. similis was also established as a benchmark to compare the lethal concentration (EC50) observed in the acute tests. Figure 4 shows the total number of neonates per adult in the long-term bioassay of D. similis exposed to different concentrations of chlorophyllin.
Total number of neonates per adult in the long-term bioassay of D. similis exposed to different concentrations of chlorophyllin. Values given are mean ± standard deviation of three independent replicates. *indicates values significantly different (p < 0.05) from the controls as calculated by one-way ANOVA and Tukey test
After 21 days exposure, no immobility was observed in the control group, validating the test. Data distribution was normal and homogeneous (ANOVA and Tukey test). The fecundity of D. similis exposed to chlorophyllin concentrations >3.0 mg/L was significantly different from that in the control significant (p = 0.0361). At concentrations above 4 mg/L there was no yield of offspring due to the toxicity of the medium. In the long-term tests NOEC for fecundity was 2.05 mg/L and LOEC was 3.81 mg/L. The Maximum Acceptable Toxicant Concentration (MATC) (Eq. 2) was calculated and reported from the results of a number of standard procedures designed by the United States Environmental Protection Agency (US EPA) and other organizations to maintain high accuracy and precision among all toxicity tests for regulatory purposes. The MATC is the geometric mean between these two values, such that:
In this study, the MATC obtained for the chlorophyllin was 2.79 mg/L.
Discussion
According to Wohllebe et al. (2009) upon irradiation solubilized chlorophyll transfers its excitation energy to oxygen resulting in the formation of singlet oxygen (1O2) and other reactive oxygen species (ROS) that have the potential to damage and kill specific developmental stages of pest organisms or their vectors. The application of very low concentrations of chlorophyllin to water inhabited by parasites reliably kills these larvae via the photodynamic activity of the substance in the presence of light. This same phenomenon was observed in acute and chronic tests for both D. similis and E. gracilis. Since the organisms were exposed to light during or after the incubation to chlorophyllin, the observed effects can be due to a photodynamic effect, i.g. formation of ROS. Future research will clarify if this is true for the test organisms in this study or whether another mechanism is involved.
Fundel et al. (1998) conducted studies on the effect of chlorophyll in Daphnia. At concentrations between 1.2 and 16.3 mg/L, 50 % of the chlorophyllin was observed to change into pheophytin in the absence of light; in the presence of light the conversion was significantly lower. This mechanism occurs during ingestion of chlorophyllin by phytoplankton cells (Strom 1993).
According to U.S. EPA (2004, 2007) the environmental toxicity can be evaluated by defining a risk quotient (RQ) which is calculated by dividing a tabulated value (provided by the agency for various test organisms) by the measured value of the effect.
where EEC is the expected environmental concentration (mg/L). EC50 is the estimated mean lethal concentration (mg/L). The quotient RQ identifies situations of high or low risk. According to Goktepe et al. (2004), RQ values below <0.05 suggest that the chlorophyllin used in the study is of no concern for Euglena. RQ values between 0.05 and 0.5 indicate that the compounds could present some hazard that may be mitigated by restricted use. Daphnia gave a value of 0.08 in the short-term tests. Finally, RQ values above >0.5 require that further testing be conducted before taking regulatory action for the use of pesticides or compounds. According to the National Council for the Environment Resolution 357 (CONAMA 2005) the maximum chlorophyll concentration established for waters intended to supply drinking water for humans or life stock or used for recreation after conventional or advanced treatment is 60 µg/L.
In the short-term tests with Daphnia RQ values >0.05 were found (D. similis RQ = 0.077) while the tests with E. gracilis (RQ = 0.047), C. carpio (RQ = 0.002) and A. bimaculatus (RQ = 0.003) were lower. The finding that A. bimaculatus showed a greater resistance to chlorophyllin than C. carpio could be due to the fact that the former is a species capable to survive in adverse conditions (Paiva et al. 2006).
In order to determine the coefficient of environmental risk in long-term tests we also used the methodology described by U.S. EPA (2004, 2007), which requires to divide the 21 days average concentration in the water by the long-term NOEC of the aquatic invertebrates. The RQ for D. similis was 0.975. For algae, the RQ level of screening was routinely based on the lowest EC50 relative to NOEC. The RQ value for E. gracilis was 0.369.
In contrast to the micro-crustacean D. similis and the alga E. gracilis, where photodynamic activity was the main cause of mortality, the tested fish are not transparent so that light does not penetrate into their bodies. The possible cause of mortality could be a toxic effect of chlorophyllin on the gills of the fish. Gomes et al. (2012) conducted studies on A. bimaculatus in which they monitored anomalies of fish gills to assess fresh water quality. One of the stressors studied was the presence of chlorophyll derivatives. According to Shimada Borges et al. (2013), in studies with Nile tilapia (Oreochromis niloticus), the gills of teleost fish are potentially useful to assess eutrophication, because these organs are in direct contact with the water and act as a selective interface between the organisms and the environment. Besides their respiratory function the gills of fish are also responsible for the acid–base balance, osmoregulation, excretion and reception of stimuli (Sayer and Davenport 1987, Laurent and Perry 1990, Randal and Wright 1990, Bailly et al. 1992, Partearroyo et al. 1992). However, it was found that the gill covers in young fish can transmit a considerable fraction of the impinging light (Wohllebe 2010) which is sufficient to activate the photosensitizer chlorophyllin. Therefore the chlorophyllin effect on young fish in this study could well be due to a photodynamic reaction on the gills.
Stringfellow et al. (2009) described that chlorophyll in the water can reduce dissolved oxygen, however, the effective concentrations being used for the control of pest organisms are so minute that they could not measurably reduce the oxygen concentration of the water body.
Concentrations for chlorophyllin have been determined to eliminate mosquito larvae as vectors for human illnesses such as malaria, dengue, yellow fewer and others (Wohllebe et al. 2009, Erzinger et al. 2011, Wohllebe et al. 2011). Likewise, chlorophyllin concentrations have been measured to kill economically important fish parasites (Wohllebe et al. 2012). Comparing those concentrations with those found in this study to affect ecologically important vertebrate and invertebrate test organisms indicates that the use of chlorophyllin in short-term applications does not pose an ecological risk for aquatic ecosystems. In contrast, long-term exposure of these test organisms to chlorophyllin requires some caution. One way of solving this problem could be the application in a specific formulation, which is taken up preferentially by the target organisms, while others are not affected. This is facilitated by the fact that many organisms in the food web feed by particle size (Fenchel 1980).
Conclusions
The potential of chlorophyll and its derivatives to control parasites and pest organisms in aquatic ecosystems is an interesting alternative to chemical or other forms of remedification. But using these substances requires some caution and requires an ecological risk assessment with a proper quality control. Judging from the RQ values and adopting the U.S. EPA recommendations, chlorophyll derivatives (chlorophyllin) can apparently be used to aquatic ecosystems in short-term applications without risk for the environment as indicated by invertebrate and vertebrate test organisms. However, for long-term applications some caution is warranted. Further research of specific formulations is necessary to reduce the potential risk of the environment and increase the stability of the biocide and to prevent its rapid degradation in the presence of light.
References
Abdel-Kader MH, El-Tayeb TA (2009) Field application for malaria vector control using sunlight active formulated extract. Treaty PC. Austria. WO 2009(149720):A1
Abdel-Kader MH, Al-Sherbini ASAM, El Tayeb TAA (1999). Sunlight and photosensitizers for insect control. The 9th International Conference on ‘‘Environmental Protection is a must”, Alexandria University
Abdel-Kader MH, El-Sherbini SA, Balal MH, El-Tayeb TA, Ayoub S, E-F SA (2005a) Using sunlight and the derivatives of porphyrine and phthalocyanine to control the stages of whitefly (Bemisia tabaci). Office EP, Egypt 23385
Abdel-Kader MH, El-Sherbini SA, El-Tayeb TA, Mandour OS (2005b) Photosensitizers for control of Schistosoma hematobium and Schistosoma mansoni cercaria and eggs. Office EP, Egypt 23397
Abdel-Kader MH, El-Sherbini SA, El-Tayeb TA, Jori G, Ben-Amor T (2006) Using environmentally friendly and solar activated compounds for control of Musca domestica. Academy of Scientific Research and Technology, Ministry of High Education & Research. Office EP. Egypt 23571
Abdel-Kader MH, El Sherbini SA, El-Tayeb TA, Hassan E, El-Emam M, El-Taraky A (2008a) Using photo-oxidation reactions by photosensitizer for control of Schistosoma’s snail vector. Office EP, Egypt 23969
Abdel-Kader MH, Jori G, El-Sherbini SA, El-Tayeb TA (2008b) Using environmentally friendly and solar activated compounds for control of Culex pipiens larvae (mosquito). Office EP, Egypt 34113
ABNT (2003) Ecotoxicologia Aquática - Toxicidade crônica - Método de ensaio com Ceriodaphnia spp. (Crustacea, Cladocera). 13.373. Técnicas ABDN. Rio de Janeiro
ABNT (2011) Aquatic Ecotoxicology - Acute test method with fish. 15088. Técnicas ABDN. Rio de Janeiro
Ahmed H, Häder D-P (2010) Rapid ecotoxicological bioassay of nickel and cadmium using motility and photosynthetic parameters of Euglena gracilis. Env Exp Bot 69:68–75
Araujo RPdA (2005) Testes de toxicidade como instrumento na avaliação dos sedimentos de água doce do estado de São Paulo, Universidade de São Paulo
Azizullah A, Richter P, Häder D (2011) Ecotoxicological evaluation of wastewater samples from Gadoon Amazai Industrial Estate (GAIE), Swabi, Pakistan. Int J Environ Sci Technol 1:1–17
Bailly Y, Dunel-Erb S, Laurent P (1992) The neuroepithelial cells of the fish gill filament: indolamine-immunocytochemistry and innervation. Anat Rec 233:143–161
Checcucci A, Colombetti G, Ferrara R, Lenci F (1976) Action spectra for photoaccumulation of green and colorless Euglena: evidence for identification of receptor pigments. Photochem Photobiol 23:51–54
CONAMA (2005) Dispõe sobre a classificação dos corpos de água e diretrizes ambientais para o seu enquadramento, Conselho Nacional do Meio Ambiente. 357
da Silva RRP (2009) Avaliação da toxicidade aguda e genotoxicidade de extrato de floração de Microcystis spp. para peixes de água doce. Instituto de Biologia. Brasília, Universidade de Brasília,. Ph.D
Dias RM, Bailly D, Antônio RR, Suzuki HI, Agostinho AA (2005) Colonization of the Corumbá Reservoir (Corumbá River, Paraná River Basin. Goiás State, Brazil) by the “lambari” Astyanax altiparanae (Tetragonopterineae; Characidae). Braz Arch Biol Techonol 48:467–476
El-Tayeb TAA (2003) Laser scanning microscopy for determination of the efficiency of hematoporphyrin in control of Culex pipiens larvae and the snail vector of Fasciola gigantica. National Institute of Laser Enhanced Sciences. Cairo, Cairo University 1-216
El-Tayeb TA, Abdel-Kader MH (2009) Field application for malaria vector control using sunlight Active Formulated Extract Office EP. Egypt. WO2009149720
EPA US (2004) Overview of the Ecological Risk Assessment Process in the Office of Pesticide Programs
EPA US (2007) Interim policy: acute endangered species level of concern (LOC) for terrestrial invertebrates
Erzinger GS (2011) New perspectives for the control of parasitic diseases through the use of photodynamic products. Pharma Anal Acta 9:1–3
Erzinger GS, Häder D-P (2009) Bioinsecticide nontoxic biodegradable. Property NIoI, Brasil 020090120220
Erzinger GS, Ciampo LF, Häder D-P (2010) Equipamento e processo para análise de toxicidade em ambientes aquáticos. Property NIoI. Brasil. PI1102317-1
Erzinger GS, Wohllebe S, Vollrath F, Souza SC, Richter P, Lebert M, Häder D-P (2011) Optimizing conditions for the use of chlorophyll derivatives for photodynamic control of parasites in aquatic ecosystems. Parasitol Res 109:781–786
Fenchel T (1980) Suspension feeding in ciliated protozoa: functional response and particle size selection. Microbial Ecol 6:1–11
Flohr F, Brentano DM, Carvalho-Pinto CRS, Machado VM, Matias WG (2005) Classificação de resíduos sólidos industriais com base em testes ecotoxicológicos utilizando Daphnia magna: uma alternativa. Biotemas 18:7–18
Fundel B, Stich HB, Schmid H, Maier G (1998) Can phaeopigments be used as markers for Daphnia grazing in Lake Constance? J Plankton Res 20:1449–1462
Goktepe Ipek, Portier Ralph, Ahmedna Mohamed (2004) Ecological risk assessment of neem-based pesticides. J Env Sci Health B 39(2):311–320
Gomes ID, Nascimento AA, Sales A, Araújo FG (2012) Can fish gill anomalies be used to assess water quality in freshwater neotropical systems? Env Monit Assess 184:5523–5531
Häder D-P, Erzinger GS (2011) Non-toxic anbd biodegradable insecticide formulation. Organisation WIP, USA
Häder D-P, Rosum A, Schäfer J, Hemmersbach R (1995) Gravitaxis in the flagellate Euglena gracilis is controlled by an active gravireceptor. J Plant Physiol 146:474–480
Hayashi C, Meurer F, Boscolo WR, Lacerda CHF, Kavata LCB (2004) Feeding frequency for yellow tail lambari (Astyanax bimaculatus) fingerlings. Rev Brasil Zootecnia 33:21–26
ISO (2000) Water quality - Determination of long term toxicity of substances to Daphnia magna Straus (Cladocera, Crustacea). Zurich, Switzerland
Kashiyama Y, Tamiaki H (2014) Risk management by organisms of the phototoxicity of chlorophylls. Chem Lett 43:148–156
Klisch M, Sinha RP, Richter PR, Häder D-P (2001) Mycosporine-like amino acids (MAAs) protect against UV-B-induced damage in Gyrodinium dorsum Kofoid. J Plant Physio 158:1449–1454
Laurent P, Perry SF (1990) Environmental effects of fish gill morphology. Physiol Zool 358:4–25
Mahmoud MS, Richter P, Shalaby HAM, Kandil OM, Häder D-P (2013) Molluscicidal activity of chlorophyll extraction against the freshwater snails. J Coast Life Med 1:85–88
Paiva SR, Dergam JA, Machado F (2006) Determining management units in southeastern Brazil: the case of Astyanax bimaculatus (Linnaeus, 1758) (Teleostei: Ostariophysi: Characidae). Hydrobiol 560:393–404
Partearroyo MA, Pilling SJ, Jones MN (1992) The effects of surfactants on the permeability of isolated perfused fish gills to urea. Comp Biochem Physiol 101A:653–659
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochem Biophys Acta 975:384–394
Rahmani M, Csallany AS (1991) Chlorophyll and β-carotene pigments in Moroccan virgin olive oils measured by high-performance liquid chromatography. J Am Oil Chem Soc 68:672–674
Randal D, Wright PA (1990) Gill water flow and the chemistry of the boundary layer. Physiol Zool. 64:26–38
Reis RE, Kullander SO, Ferraris CJJ (2003) Checklist of freshwater fishes of South and Central America. Porto Alegre, Brasil, pp 106–109
Sayer MDJ, Davenport J (1987) The relative importance of the gills to ammonia and urea excretion in five seawater and one freshwater teleost species. J Fish Biol 31:561–570
Schertz FM (1928) The extraction and separation of chlorophyll (α + β) carotin and xanthophyll in fresh green leaves, preliminary to their quantitative determination. Plant Physiol 3:211
Shimada Borges JC, Salimbeni Vivai ABB, Branco PC, Silva Oliveira M, Cunha Machado, da Silva JR (2013) Effects of trophic levels (chlorophyll and phosphorous content) in three different water bodies (urban lake, reservoir and aquaculture facility) on gill morphology of Nile tilapia (Oreochromis niloticus). J Appl Ichthyol 29:573–578
Silva JPA, Muelbert AE, de Oliveira EC, Fávaro LF (2010) Reproductive tactics used by the lambari Astyanax aff. fasciatus in three water supply reservoirs in the same geographic region of the upper Iguaçu River. Neotropical Ichthyol 8:885–892
Stringfellow W, Herr J, Litton G, Brunell M, Borglin S, Hanlon J, Chen C, Graham J, Burks R, Dahlgren R, Kendall C, Brown R, Quinn N (2009) Investigation of river eutrophication as part of a low dissolved oxygen total maximum daily load implementation. Water Sci Technol 59:9–14
Strom SL (1993) Production of pheopigments by marine protozoa: results of laboratory experiments analysed by HPLC. Deep Sea Research Part I: Oceanographic Res Pap 40:57–80
Tahedl H, Häder D-P (1999) Fast examination of water quality using the automatic biotest ECOTOX based on the movement behavior of a freshwater flagellate. Water Res 33:426–432
Tahedl H, Häder D-P (2001) Automated biomonitoring using real time movement analysis of Euglena gracilis. Ecotoxicol Environ Safety 48:161–169
Wohllebe S (2010) Bekämpfung von Parasiten in aquatischen Ökosystemen mittels natürlicher Photosensitizer. Biology. Erlangen, Germany, Friedrich-Alexander Universität. PhD, Dept
Wohllebe S, Richter R, Richter P, Häder D-P (2009) Photodynamic control of human pathogenic parasites in aquatic ecosystems using chlorophyllin and pheophorbid as photodynamic substances. Parasitol Res 104:593–600
Wohllebe S, Ulbrich C, Grimm D, Pietsch J, Erzinger G, Richter P, Häder D-P (2011) Photodynamic treatment of Chaoborus crystallinus larvae with chlorophyllin induces necrosis and apoptosis. Photochem Photobiol 87:1113–1122
Wohllebe S, Richter P, Häder D-P (2012) Chlorophyllin for the control of Ichthyophthirius multifiliis (Fouquet). Parasitol Res 111:729–733
Acknowledgments
The authors thank the National Counsel of Technological and Scientific Development (CNPq) and Foundation for Research and Innovation State of Santa Catarina (FAPESC) for partially supporting this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Erzinger, G.S., Souza, S.C., Pinto, L.H. et al. Assessment of the impact of chlorophyll derivatives to control parasites in aquatic ecosystems. Ecotoxicology 24, 949–958 (2015). https://doi.org/10.1007/s10646-015-1437-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-015-1437-5