Abstract
Water-soluble chlorophyll (chlorophyllin) was used in a phototoxic reaction against a number of fish ectoparasites such as Ichtyobodo, Dactylogyrus, Trichodina, and Argulus. Chlorophyllin is applied to the water at concentrations of several micrograms per milliliter for a predefined incubation time, and afterwards, the parasites are exposed to simulated solar radiation. Application in the dark caused only little damage to the parasites; likewise, light exposure without the addition of the photosensitizer was ineffective. In Ichthyobodo, 2 μg/mL proved sufficient with subsequent simulated solar radiation to almost quantitatively kill the parasites, while in Dactylogyrus, a concentration of about 6 μg/mL was necessary. The LD50 value for this parasite was 1.02 μg/mL. Trichodina could be almost completely eliminated at 2 μg/mL. Only in the parasitic crustacean Argulus, no killing could be achieved by a photodynamic reaction using chlorophyllin. Chlorophyllin is non-toxic, biodegradable, and can be produced at low cost. Therefore, we propose that chlorophyllin (or other photodynamic substances) are a possible effective countermeasure against several ectoparasites in ponds and aquaculture since chemical remedies are either forbidden and/or ineffective.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Extensive aquaculture with dense fish populations is prone to be affected by ectoparasites (Blaylock and Bullard 2014). Thousands of pandemic fish parasites have been identified ranging from bacteria, flagellates, and ciliates to crustaceans (Cross and Matthews 1992), which prey on fish feeding on the epidermis, eyes, and gills, damaging or even killing their hosts (Reichenbach-Klinke and Elkan 2013). Some of the economically important species affecting ornamental fish and fish reared for human consumption include Ichthyophthirius multifiliis, Trichodina sp., Dactylogyrus spp., Gyrodactylus sp., Argulus sp., and Ichtyobodo necator (Fu et al. 2015; Kayis et al. 2013; Syihab et al. 2015).
I. necator (formerly known as Costia necatrix Leclerq) is a heterotrophic flagellate which affects numerous fish species in a wide array of climatic zones (Ridanovic et al. 2015). This small ectoparasite (10–20 μm) can be detected since it renders the fish epidermis opaque. It often occurs in low numbers on fish which it weakens but usually does not kill because it is controlled by the immune defense system unless there are adverse environmental conditions; however, in fish eggs and young larvae, it can result in massive losses (Wohllebe 2010). The almond-shaped parasite anchors itself on an epidermal cell and feeds on it. The cellular lesions in the form of epithelium hyperplasia compromise the efficiency as a barrier to external impact and other parasites (Phromkunthong et al. 2013). The use of only a limited number of chemicals is permitted such as copper sulfate and potassium permanganate to fight this parasite in marine and freshwater fish intended for human consumption, which, however, are not very effective (Farmer et al. 2014).
Four species of the 2-mm-long trematode Dactylogyrus spp., commonly known as gill flukes, are known in Germany. They affect several fish species and cause hypertrophic epithelium thickening and gill tip necrosis and often affect young carps (6 weeks old with a body weight of 1 g) and other Cyprinidae where these worms attach to the gills (Koyun 2013). They use two pairs of anchors to attach to the gills of usually freshwater hosts. The hermaphroditic adults shed their eggs into the water where they develop into ciliated larvae which swim and attach to the gills of other fish (Koskivaara et al. 1991). The gills are inflamed and show excessive mucous secretion and accelerated respiration. The fish show signs of lethargy swimming near the surface and lose their appetite (Hanzelova and Zitnan 1985). At 22 °C, the life cycle is completed within a few days, while at colder temperatures, this may require 5 to 6 months (Reed et al. 2009). In addition to the direct damage, they are vectors for bacteria such as Aeromonas (Cusack and Cone 1986). The densest populations of these parasites are found in late autumn and early winter. In goldfish (Dactylogyrus intermedius) infections are treated with petroleum ether, chloroform, acetone, and methanolic extracts of medicinal plants such as Dryopteris, Kochia, or Polygala (Lu et al. 2012).
Trichodina sp. Ehrenberg is a ciliate with a characteristic UFO-like cell shape (Lom and Dyková 1992). This 60-μm-long protist is typically found in small numbers on the epidermis, gills, and fins of many fresh- and saltwater fishes which it weakens even though many species of this genus feed exclusively on bacteria and not on the fish skin cells. In addition, this peritrichous flagellated facultative ectoparasite can attack several amphibian species (Lom and Dyková 1992). In contrast to the sessile peritrichs such as Vorticella, the over 150 members of this genus move on the epidermis in a rotary fashion shaving off cells by its cytoskeletal denticles on the adoral surface. However, some of the members of this genus also enter the urogenital system. In contrast to Ichthyophthirius, its life cycle is very simple without an alternation of generations or asexual replication. The cells divide resulting in two daughter cells with only half of the denticles and subsequently generate the missing ones. Infection can occur from one fish to another. Under eutrophication or with poor water quality, they can grow to large numbers.
About 130 species are known in the crustacean Argulus (family Argulidae), commonly known as fish lice, which pose a major threat to aquaculture in both marine and freshwater fish since they are some of the most widespread ectoparasites (Kumar et al. 2012). One of the three Argulus species known in central Europe is Argulus foliaceus which affects not only carps but also other freshwater fish and amphibia larvae (Wohllebe 2010). The parasites attach to their host with suction cups, penetrate the skin with their sharp stylets, and suck the blood. During this process, they can also transmit bacteria and viruses resulting in further infections. The crustaceans use vision, smell, and mechanical sensation to locate their hosts (Guha et al. 2013). Almost every fish species including carp and minnows as well as salmons, eels, and trout within the habitat of Argulus has been found to be host for this parasite, and even frogs and toads can be attacked (Alsarakibi et al. 2014).
Many of the remedies against these fish ectoparasites such as the application of sodium chloride, peroxide, and formaldehyde are not very effective (Sudova et al. 2007). Formerly, malachite green had been successfully employed against Ichthyophthirius. But its use has been suspended in the European community for fish intended for human consumption since it is suspected to be carcinogenic (Rintamäki-Kinnunen et al. 2005). According to the fish health service (Fischgesundheitsdienst, personal communication), chemicals are not allowed, e.g., in Germany to treat ectoparasites on fish for human consumption. Calcium oxide, calcium hydroxide, formaldehyde, and sodium chloride can be used if they are specifically certified for this purpose (ad us. vet.); but these substances are prohibitively expensive and not very effective. Thus, there is an urgent need for a non-toxic, biodegradable, inexpensive remedy which should be highly effective against target parasites.
One possible approach is the use of photodynamic substances, which are not toxic per se in the dark but when activated by light, they enter the reactive triplet state (1T). This in turn transfers its activation energy to triplet oxygen resulting in the production of the very reactive singlet state (1O2) which, even though it has a very short lifetime, is highly cytotoxic (DeRosa and Crutchley 2002). The application requires a period of time during which the photosensitizer is ingested into the cells which are subsequently irradiated to induce the photodynamic response.
A number of colored chemicals have been used successfully as sensitizers driving phototoxic reactions. Several protoporphyrins have been used in the photodynamic therapy of cancer (Hasan et al. 2003). Chemicals with phototoxic properties have been employed against mosquito larvae (reviewed by Ben Amor et al. 2000). However, many of these substances are excessively expensive which prevents their use in aquaculture. In contrast, chlorophyll and its derivatives have been found to display effective photodynamic properties (Park et al. 1989; Scherz et al. 1994).
We successfully applied chlorophyllin, a water-soluble form of chlorophyll to control the parasitic ciliate I. multifiliis, which causes the white spot disease in many ornamental and food fish (Häder and Erzinger 2011; Häder et al. 2015). In several recent projects, Wohllebe et al. characterized the effects of chlorophyllin on mosquito larvae (Wohllebe et al. 2009; Wohllebe et al. 2011) as well as on fish parasites (Erzinger et al. 2011; Wohllebe 2010; Wohllebe et al. 2012). The cellular mechanism includes both necrosis and apoptosis in the affected tissue as shown in the larvae of the mosquito Chaoborus (Wohllebe et al. 2011).
In isolated trophonts of Ichthyophthirius, an LD50 of 0.67 mg/L chlorophyllin was determined after exposure to simulated solar radiation (Wohllebe et al. 2012). In tomites, the sensitivity against photoactivated chlorophyllin was found at <2 mg/L. Motivated by these very promising results, we tested the effects of photodynamic treatment using chlorophyllin on a number of common fish ectoparasites.
Material and methods
Isolation of chlorophyllin and preparation for the experiments
Frozen spinach was extracted in a water bath at 55 °C with 100 % ethanol to solubilize the chlorophyll. One gram CaCO3 was added to 1 kg plant material which prevents transformation of chlorophyll into pheophytin. After filtration of the extract, the same volume of petroleum benzene was added to the liquid and well shaken. The upper lipophilic phase was removed, and 20 mL methanolic NaOH per 1 L of the benzene phase was added to convert the contained chlorophyll into the water-soluble chlorophyllin, the concentration of which was determined with a spectrophotometer according to equations of Lichtenthaler and Wellburn (1983). The chlorophyllin was stored at 4 °C in the dark for later use. The methanol was removed from the chlorophyllin solution by evaporation on a hot plate (45 °C) under laminar air-flow. The resulting chlorophyllin was transferred to water and used in the experiments at desired concentration. NaOH was added to the control samples at a concentration which corresponded to that at the highest chlorophyll concentration.
Fish and parasites
All animal experiments were approved by the Government of Central Franconia (request-numbers 4–2532.1-18/09 and 54–2532.1-1/11) according to § 8 Protection of Animals Act. All experiments were carried out with the common carp (Cyprinus carpio) at the Bavarian State Research Center for Agriculture, Institute for Fisheries, Höchstadt, Germany.
In order to obtain I. necator (formerly C. necatrix Leclerq), mucus was carefully removed from the skin of freshly dead carps with a cover glass and transferred to a glass dish with 5 mL tap water (Wohllebe 2010). To determine their density, the parasites were killed by adding 0.2 μL 2 % formaldehyde and counted under the microscope at ×400 magnification. For the experiments with chlorophyllin, 45 live flagellates each were transferred into glass dishes and incubated for 60 min in darkness with the photosensitizer added at concentrations between 0 and 4.7 μg/mL. Controls were kept in darkness in triplicates, and another set of dishes was exposed under a solar simulator lamp (Sol 1200 W, mercury lamp 0383, Dr. Hönle, Martinsried, Germany), which simulates solar radiation (Klisch et al. 2001). Light intensities were photosynthetic active radiation (PAR, 400–700 nm) 149.66 W/m2, UV-A (315–400 nm) 32.67 W/m2, and UV-B (280–315 nm) 0.77 W/m2. In addition, six NaOH controls were run in parallel, three of which were kept in darkness. All experiments were repeated three times. Living and dead parasites were counted under the microscope after the end of light or dark exposure.
The trematode Dactylogyrus sp. was harvested from live carp obtained from the fish health service. The fish were killed after narcosis by cutting their neck, the gill arcs removed, and three of them transferred into Petri dishes with 30 mL tap water each. The number of worms attached to the gills was determined under a stereo microscope. Chlorophyllin was added at concentrations between 0 and 7 μg/mL, and NaOH controls (0.1 and 8 μg/mL) were carried out in parallel. All samples were incubated with chlorophyllin for 150 min in darkness, and half of the samples were exposed to the simulated solar radiation for 2.5 h. Dark incubation and light exposure had been predetermined in preliminary experiments. After the end of the experiments, the number of live and dead parasites was determined.
Trichodina sp. was obtained by removing slime from the skin of freshly dead carp with a scalpel, and the number of parasites was determined under the microscope. Live parasites were transferred into glass dishes, 0, 2, or 4.7 μg/mL chlorophyllin was added and kept in darkness for 30 min to allow the ciliates to ingest the photosensitizer. Subsequently, they were exposed for 30 min to simulated solar irradiation. Dark and NaOH controls were exposed in parallel, and all experiments were done in triplicates. Afterwards, the percentage of live and dead Trichodina was determined by microscopy.
The crustacean Argulus sp. was collected from 3-year-old carps which were already heavily infected. Ten parasites each were transferred into disks with 30 mL tap water, and between 0 and 10 μg/mL chlorophyllin was added and helt in darkness overnight. Subsequently, they were either kept in darkness or exposed to simulated solar radiation for 3 h and then the percentage of live and dead parasites determined. All experiments and the controls were carried out in triplicates. The uptake of chlorophyllin into the intestine was determined under a fluorescence microscope.
In another experiment, the swim bladder was removed from a 3-year-old carp, filled with pork blood containing 10 μg/mL chlorophyllin with a syringe and transferred into a beaker with 150 mL tap water to which 20 Argulus had been added. The number of surviving parasites was determined at 1-h intervals, and the uptake of chlorophyllin was evaluated under a fluorescence microscope.
Statistics
All data were subjected to the Kolmogorov-Smirnov test. If a normal distribution was found, a paired t test was carried out to determine statistically significant differences. The level for significance was chosen to be p < 0.05 and for high significance as p < 0.01.
Results
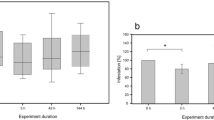
Without application of chlorophyllin and simulated solar irradiation, 98 % of I. necator survived the experimental period (Fig. 1). Sixty-minute incubation in 0.5 μg/mL chlorophyllin killed 5 % during the following dark phase, while 2 μg/mL and a subsequent dark phase induced 20 % mortality and the same procedure with 4.7 μg/mL resulted in 35 % mortality. In contrast, application of 0.5 mg/mL of the photosensitizer with subsequent 30 min simulated solar irradiation left 40 % of the parasites dead while 2 μg/mL or 4.7 μg/mL killed 100 % of the flagellates.
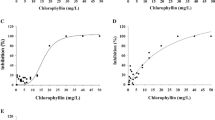
In the worms Dactylogyrus spp., a chlorophyllin concentration of 0.1 μg/mL had no effect on their survival when subsequently exposed to darkness; 90 % survived applications of 2.5 or 5 μg/mL in the dark (Fig. 2a). Even at 7 μg/mL, only 15 % of the parasites were killed. In contrast, application of 0.1 μg/mL with subsequent simulated solar irradiation killed 34 % of Dactylogyrus and 75 % were killed at a concentration of 2.5 μg/mL. At 7 μg/mL, no parasite survived. Using all data for chlorophyllin and light treatment, an LD50 value of 1.02 μg/mL was calculated (Fig. 2b).
a Percentage of Dactylogyrus killed by application of chlorophyllin at different concentrations followed by dark incubation (front row) or exposure to simulated solar radiation (rear row). Red lines indicate standard deviation. b Effect-concentration curve for the effect of chlorophyllin (150-min incubation in the dark) followed by 150-min exposure to simulated solar irradiation on the killing of Dactylogyrus with an LD50 value of 1.02 μg/mL. The black line shows the sigmoidal approximation to the measured values (black dots), and the red lines and dots show the upper and lower boundaries for the calculated 95 % confidence range
All Trichodina survived the treatment without chlorophyllin and irradiation, but 15-min exposure to irradiation killed 5 % in the absence of chlorophyllin (Fig. 3). Likewise, 5 % of the parasites were killed by the application of 2 μg/mL in the dark while 20 % died at a chlorophyllin concentration of 4.7 μg/mL in darkness. In contrast, at a concentration of 2 μg/mL, 70 % died after simulated solar irradiation and 100 % were killed at 4.7 μg/mL.
In contrast to the other three parasites, Argulus sp. was not affected by the application of chlorophyllin, irradiation, or the combination of both. In all experiments, only two animals died and there was no correlation between the treatment and the death of the individuals. Photos taken under a fluorescence microscope did not reveal any visible damage, only the carapace was stained by chlorophyllin (Fig. 4). In the intestine, there was no red fluorescence indicating that there was no ingestion of the photosensitizer.
The experiment with Argulus in water in a beaker with the blood-filled swim bladder with 10 μg/mL chlorophyllin added showed a slightly different result. After a short period of time, most Argulus were attached to the swim bladder. In fact, fluorescence microscopic analysis revealed a small concentration of chlorophyllin inside the intestine which had been ingested together with the blood. However, simulated solar irradiation of these animals did not kill them; all parasites survived the exposure.
Discussion
The treatment of fish parasites, especially the ciliate I. multifiliis, using the phototoxic chemical phloxin B is covered by a US patent (US 6,506,791 B2). Earlier experiments had shown that water-soluble chlorophyllin can be efficiently used to destroy tomites and isolated trophonts of the ciliate I. multifiliis, which causes the white spot disease in numerous ornamental and food fish (Häder et al. 2015). More than 50 % of the trophonts could be removed from infested fish by incubation at concentrations of 2 or 4 μg/mL with a subsequent exposure to simulated solar irradiation. These results demonstrated that this widespread fish disease can be treated effectively using photodynamic reactions with chlorophyll derivates after malachite green was banned in the treatment of fish for human consumption (Maceda-Veiga and Cable 2014; Rintamäki-Kinnunen et al. 2005).
Chlorophyll is non-toxic and is certified as a food additive (E140, E141), e.g., to stain pasta green. Chlorophyll derivates are easily biodegradable and leave no toxic intermediates (Heaton and Marangoni 1996). This natural substance has been reported to reduce toxic effects of aflatoxin in trouts (Breinholt et al. 1999), probably by aflatoxin complexion or due to reduced absorption of the toxin in the intestine. Previous experiments with a number of photosensitizers such as hematoporphyrin or rose bengal, to fight water-borne parasites, demonstrated the suitability of the treatment (Abdel-Kader and El-Tayeb 2009; Abdel-Kader et al. 2007; Abdel-Kader et al. 2008), but the high prices for these substances prevented their usage in large-scale applications. In contrast, chlorophyll can be extracted from all sorts of plants, weeds, and green residues at low costs. The conversion into water-soluble chlorophyllin is chemically not challenging. The low concentrations needed to effectively kill the fish parasites allow the application to ponds or aquaculture installations.
The phototoxic reaction which eventually kills the parasites requires the irradiation with solar radiation in the field. Fish ponds can be murky with high turbidity. However, experiments with mosquito larvae (Chaoborus crystallinus) have shown that an irradiance of about 36 W/m2 of visible daylight is sufficient to effectively destroy the parasites (Erzinger et al. 2011), which is about 10 % of solar radiation on a sunny day in central Europe (Häder et al. 1999). Wohllebe (2010) determined the transparency in four different carp ponds to find the depth at which light is attenuated to 10 % of its surface value. In two of the tested ponds, 10 % of the incident irradiation reached a depth of 2 m while in the other two, it was attenuated to this value at 20–30 cm depth.
These promising results motivated the employment of this procedure against other fish parasites. The current results indicate that chlorophyllin can be employed to effectively destroy a number of common fish parasites including flagellates, ciliates, and worms at low concentrations comparable to those found to be effective against I. multifiliis. Dactylogyrus lives on the gills under the operculum where it is protected from incident light. However, measurements have shown that the operculum in 1-year-old carp transmits about 12 % of the incident light, which is sufficient to kill the worms in the phototoxic reaction using chlorophyllin (Wohllebe 2010). The operculum of 2-year old-carps transmitted 1.3 % and that of 3-year-old ones 0.4 % of the incident light.
Only in the case of Argulus, the treatment proved ineffective which can be easily explained by the fact that this crustacean does not ingest water but feeds on the blood of their host (Mirzaei and Khovand 2015).
One important question remains whether the application of chlorophyllin affects the treated fishes or their brood and larval fish. Wohllebe (2010) had demonstrated that the highest chlorophyllin concentrations sufficient to eliminate fish parasites did not affect eggs, larval stages, and adults of common carps (C. carpio), rainbow trouts (Oncorhynchus mykiss), and graylings (Thymallus thymallus). However, chlorophyllin induced mortality in fish brood, killing about 50 % of the eggs at 10 mg/L. At that concentration, it induced strong slime production in larval gills leading to suffocation. Other studies revealed that other aquatic organisms such as the flagellate Euglena and the microcrustacean Daphnia as well as the fish Astyanax bimaculatus and C. carpio were not affected at the concentrations used to kill fish parasites (Erzinger et al. 2015). Thus, it is safe to suggest the usage of solubilized chlorophyll against a number of common fish parasites in ponds and aquaculture.
Conclusions and perspectives
In conclusion, the data demonstrate that several fish ectoparasites can be effectively killed with chlorophyllin in combination with irradiation in a photodynamic reaction at low concentrations of the photosensitizer. Only fish eggs and larval stages of some fish species can be sensitive to higher concentrations of chlorophyllin.
Chlorophyll is non-toxic and certified as a food additive (E140). It is photodegradable without leaving toxic residues.
Chlorophyll can be produced at low costs and can be easily converted into water-soluble chlorophyllin.
The phototoxic reaction can be induced at low light intensities so that it can be applied even in turbid ponds and aquaculture installations.
Thus, we conclude that chlorophyllin can be applied against a number of fish ectoparasites in fish farming and intensive aquaculture with a high density of fish.
References
Abdel-Kader MH, El-Tayeb TA (2009) Field application for malaria vector control using sunlight active formulated extract. Austria Patent
Abdel-Kader MH, El Sherbini SA, El-Tayeb TA, Hassan E, El-Emam M, El-Taraky A (2008) Using photo-oxidation reactions by photosensitizer for control of Schistosoma’s snail vector. Egypt Patent
Alsarakibi M, Wadeh H, Li G (2014) Parasitism of Argulus japonicus in cultured and wild fish of Guangdong, China with new record of three hosts. Parasitol Res 113(2):769–775
Ben Amor T, Bortolotto L, Jori G (2000) Porphyrins and related compounds as photoactivatable insecticides. 3. Laboratory and field studies. Photochem Photobiol 71:124–128
Blaylock RB, Bullard SA (2014) Counter-insurgents of the blue revolution? Parasites and diseases affecting aquaculture and science. J Parasitol 100(6):743–755
Breinholt V et al (1999) Chlorophyllin chemoprevention in trout initiated by aflatoxin B 1 bath treatment: an evaluation of reduced bioavailability vs. target organ protective mechanisms. Toxicol Appl Pharmacol 158(2):141–151
Cross ML, Matthews RA (1992) Ichthyophthiriasis in carp, Cyprinus carpio L.: fate of parasites in immunized fish. J Fish Dis 15:497–505
Cusack R, Cone DK (1986) A review of parasites as vectors of viral and bacterial diseases of fish. J Fish Dis 9(2):169–171
DeRosa MC, Crutchley RJ (2002) Photosensitized singlet oxygen and its applications. Coord Chem Rev 233:351–371
Erzinger GS et al (2011) Optimizing conditions for the use of chlorophyll derivatives for photodynamic control of parasites in aquatic ecosystems. Parasitol Res 109(3):781–786
Erzinger GS et al (2015) Assessment of the impact of chlorophyll derivatives to control parasites in aquatic ecosystems. Ecotoxicology 24(4):949–958
Farmer BD, Straus DL, Mitchell AJ, Beck BH, Fuller SA, Barnett LM (2014) Comparative effects of copper sulfate or potassium permanganate on channel catfish concurrently infected with Flavobacterium columnare and Ichthyobodo necator. J Appl Aquac 26(1):71–83
Fu Y-W, Zhang Q-Z, Xu D-H, Wang B, Liang J-H, Lin D-J (2015) Cynatratoside-C efficacy against theronts of Ichthyophthirius multifiliis, and toxicity tests on grass carp and mammal blood cells. Dis Aquat Org 117(1):13–20
Guha A, Aditya G, Saha SK (2013) Survivorship and fecundity of Argulus bengalensis (Crustacea; Branchiura) under laboratory conditions. Invertebr Reprod Dev 57(4):301–308
Häder D-P, Erzinger GS (2011) Non-toxic and biodegradable insecticide formulation. USA Patent
Häder D-P, Lebert M, Marangoni R, Colombetti G (1999) ELDONET—European Light Dosimeter Network hardware and software. J Photochem Photobiol B Biol 52:51–58
Häder D-P, Schmidl J, Hilbig R, Oberle M, Wedekind H, Richter P (2015) Treatment of ichthyophthiriasis with photodynamically active chlorophyllin. Parasitology Research:1–9
Hanzelova V, Zitnan R (1985) Epizootiologic importance of the concurrent monogenean invasions in the carp. Helminthologia 22(4):277–283
Hasan T, Ortel B, Solban N, Pogue B (2003) Photodynamic therapy of cancer. Cancer Med 7:537–48
Heaton JW, Marangoni AG (1996) Chlorophyll degradation in processed foods and senescent plant tissues. Trends Food Sci Technol 7(1):8–15
Kayis S, Balta F, Serezli R, Er A (2013) Parasites on different ornamental fish species in Turkey. J Fish Sci 7(2):114
Klisch M, Sinha RP, Richter PR, Häder D-P (2001) Mycosporine-like amino acids (MAAs) protect against UV-B-induced damage in Gyrodinium dorsum Kofoid. J Plant Physiol 158:1449–1454
Koskivaara M, Valtonen E, Prost M (1991) Dactylogyrids on the gills of roach in Central Finland: features of infection and species composition. Int J Parasitol 21(5):565–572
Koyun M (2013) Seasonal distribution and ecology of some Dactylogyrus species infecting Alburnus alburnus and Carassius carassius (Osteichthyes: Cyprinidae) from Porsuk River, Turkey. Afr J Biotechnol 10(7):1154–1159
Kumar A et al (2012) Antiparasitic efficacy of piperine against Argulus spp. on Carassius auratus (Linn. 1758): in vitro and in vivo study. Parasitol Res 111(5):2071–2076
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Lom J, Dyková I (1992) Protozoan parasites of fishes, vol 26. Elsevier Science Publishers, Amsterdam
Lu C, Zhang H-Y, Ji J, Wang G-X (2012) In vivo anthelmintic activity of Dryopteris crassirhizoma, Kochia scoparia, and Polygala tenuifolia against Dactylogyrus intermedius (Monogenea) in goldfish (Carassius auratus). Parasitol Res 110(3):1085–1090
Maceda-Veiga A, Cable J (2014) Efficacy of sea salt, metronidazole and formalin-malachite green baths in treating Ichthyophthirius multifiliis infections of mollies (Poecilia sphenops). Bull Eur Assoc Fish Pathol 34(5):182–186
Mirzaei M, Khovand H (2015) Prevalence of Argulus foliaceus in ornamental fishes [goldfish (Carassius auratus) and Koi (Cyprinus carpio)] in Kerman, southeast of Iran. J Parasit Dis 39(4):780–782
Park YJ, Lee WY, Hahn BS, Han MJ, Yang WI, Kim BS (1989) Chlorophyll derivatives—a new photosensitizer for photodynamic therapy of cancer in mice. Yonsei Med J 30(3):212–218
Phromkunthong W, Nuntapong N, Boonyaratpalin M, Kiron V (2013) Toxicity of melamine, an adulterant in fish feeds: experimental assessment of its effects on tilapia. J Fish Dis 36(6):555–568
Reed PA, Francis-Floyd R, Klinger RC (2009) FA28/FA033: Monogenean parasites of fish. In: Electronic Data Information Source UF/IFAS Extension. http://edis.ifas.ufl.edu/FA033
Reichenbach-Klinke H, Elkan E (2013) The principal diseases of lower vertebrates. Elsevier, Amsterdam
Ridanovic S, Nedic Z, Ridanovic L (2015) First observation of fish condition from Sava river in Bosnia and Herzegovina. J Surv Fish Sci 1(2):27–32
Rintamäki-Kinnunen P, Rahkonen M, Mannermaa-Keränen A-L, Suomalainen L-R, Mykrä H, Valtonen ET (2005) Treatment of ichthyophthiriasis after malachite green. I. Concrete tanks at salmonid farms. Dis Aquat Org 64(1):69–76
Scherz A, Salomon Y, Fiedor L (1994) Chlorophyll and bacteriochlorophyll derivatives, preparation and pharmaceutical compositions comprising them as photosensitizers for photodynamic therapy. Chem Abstr 120:386
Sudova E, Machova J, Svobodova Z, Vesely T (2007) Negative effects of malachite green and possibilities of its replacement in the treatment of fish eggs and fish: a review. Vet Med 52(12):527
Syihab MIMT, Suryanto D, Harahap ZA, Dhuha OR (2015) Bacterial isolate in gourami (Osphronemus gouramy) as the result of the infestation of ectoparasites Argulus sp. Aquacostamarine 8(3):14
Wohllebe S (2010) Bekämpfung von Parasiten in aquatischen Ökosystemen mittels natürlicher Photosensitizer. PhD thesis, Friedrich-Alexander Universität
Wohllebe S, Richter R, Richter P, Häder D-P (2009) Photodynamic control of human pathogenic parasites in aquatic ecosystems using chlorophyllin and pheophorbid as photodynamic substances. Parasitol Res 104(3):593–600
Wohllebe S et al (2011) Photodynamic treatment of Chaoborus crystallinus larvae with chlorophyllin induces necrosis and apoptosis. Photochem Photobiol 87(5):1113–1122
Wohllebe S, Richter P, Häder D-P (2012) Chlorophyllin for the control of Ichthyophthirius multifiliis (Fouquet). Parasitol Res 111(2):729–733
Acknowledgments
This work was supported by the Bundesministerium für Ernährung und Landwirtschaft, Bundesprogramm Ökologischer Landbau (FKZ 08OE040). The authors thank S. Wohllebe for her excellent work during the experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal experiments were approved by the Government of Central Franconia (request-numbers 4–2532.1-18/09 and 54–2532.1-1/11) according to § 8 Protection of Animals Act. All experiments were carried out with the common carp (C. carpio) at the Bavarian State Research Center for Agriculture, Institute for Fisheries, Höchstadt, Germany.
Rights and permissions
About this article
Cite this article
Häder, DP., Schmidl, J., Hilbig, R. et al. Fighting fish parasites with photodynamically active chlorophyllin. Parasitol Res 115, 2277–2283 (2016). https://doi.org/10.1007/s00436-016-4972-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-4972-y