Abstract
When used at low concentrations and added to the water body, water-soluble chlorophyllin (resulting from chlorophyll after removal of the phytol) and pheophorbid (produced from chlorophyllin by acidification) are able to kill mosquito larvae and other small animals within a few hours under exposure of solar radiation. Under laboratory conditions, the use of chlorophyllin/pheophorbid as photodynamic substances for pest control in water bodies promises to be not only effective and ecologically beneficial but also cheap. The LD50 (50% of mortality in the tested organisms) value in Culex sp. larvae was about 6.88 mg/l, in Chaoborus sp. larvae about 24.18 mg/l, and in Daphnia 0.55 mg/l. The LD50 values determined for pheophorbid were 8.44 mg/l in Culex, 1.05 mg/l in Chaoborus, and 0.45 mg/l in Daphnia, respectively. In some cases, chlorophyllin and pheophorbid were also found to be (less) active in darkness. The results presented in this paper show that chlorophyllin is about a factor of 100 more effective than methylene blue or hematoporphyrine, which were tested earlier for the same purpose. It is also much cheaper and, as a substance found in every green plant, it is 100% biodegradable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasitic diseases such as malaria and schistosomiasis are a plague for millions of people mainly in underdeveloped and developing countries and often prohibit the economic development of the affected areas. Great efforts have been made in the past to extinct or at least control these diseases by means of medical treatment, pesticides, exhausting wetland, etc. but with limited success. An important problem of underdeveloped areas suffering from parasitic diseases is poverty, which cuts off the supply of material necessary for countermeasures.
More than 300 parasitic worm diseases and 70 protozoan-elicited diseases are described for humans (Cox 2002). Host finding in parasites is based on a well-adapted sensory system (Haeberlein and Haas 2008; Haas et al. 2002; Haberl et al. 1995; Brachs and Haas 2008). A number of human parasites live in aquatic ecosystems at least for some time of their developmental cycle. The global distribution of these organisms is not limited to subtropical and tropical countries, but some are found and are medically important in mid-latitude waters such as in Europe. One of the effects of global warming will probably be the invasion of malaria in Germany, which is only inhibited by the temperate climate (Goklany 2004).
One of the most important tropical infectious diseases in the world is malaria (Trampuz et al. 2003; Sachs and Malaney 2002; Greenwood et al. 2005). At least 300 million cases of malaria occur each year, which causes the death of more than 1 million people (WHO 2007). Most cases of malaria occur in Africa (about two thirds of the cases and about 90% of the mortality); also the tropical regions of Asia and Latin America are partially severely affected (Anonymous 2007).
Malaria is a protozoan parasitosis, which is caused by different Plasmodium species (phylum Apicomplexa). Most important are Plasmodium malariae, Plasmodium ovale, Plasmodium vivax, and above all Plasmodium falciparum, the most severe representative. P. falciparum causes the greatest incidence of illness and death (about 80% of the malaria infections and 90% of deaths caused by malaria). Plasmodium shows alternation of generations, the human pathogenic species: Anopheles (final host) and man as intermediate host.
Also helminthosis caused by parasitic worms, such as nematodes, cestodes, and trematodes, cost many thousand lives every year. Bilharziosis (schistosomiasis) is a major problem in Egypt and other countries worldwide. It is a worm disease caused by the trematod Schistosoma (phylum Plathelmintes). Schistosoma mansoni (Africa and South America) and Schistosoma japonicum (Southeast Asia) cause gastrointestinal symptoms, while Schistosoma haematobium (Africa) causes mostly urogenital disorders. About 200 million people worldwide are infected, and between 200,000 to 1 million people die each year from the complications (Engels et al. 2002; WHO 2006). The adult worms live in the venous system of the liver and guts of man. A male and a female worm form a couple, where the female lives in a dorsal fold of the male and only separates from the male to lay eggs in the capillary system of the gut or the bladder (S. haematobium). The eggs elicit inflammation reactions and break through the vessels into the gut or bladder, respectively, and are set free with urine or feces. The alternate hosts of the sporocyst are snails (Biomphalaria for S. mansoni, Bilinus for S. haematobium, or Tricula aperta for S. japonicum).
The intention of this project was to develop methods for pest control, which are very inexpensive and non-toxic and, by this, also applicable for poor regions in the world. The methods are based on the photodynamic activity of certain colored organic substances, which, in the presence of light, produce singlet oxygen (1O2) and other reactive oxidative species (ROS) that have the potential to damage and kill specific developmental stages of pest organisms or their vectors, respectively (He and Häder 2002; Tominaga et al. 2004; Abdel-Kader et al., submitted for publication). Several organic substances induce photodynamic activity and produce reactive oxygen species such as the high reactive singlet oxygen and radicals, which have the potential to kill certain life stages of the parasites (cercaria) or the corresponding vectors (e.g., mosquito larvae). The most promising application of such substances is the suspension in parasite-contaminated water. The method strictly focuses on the expense factor. In contrast to previous attempts, inexpensive natural substances chlorophyll and its derivatives (chlorophyllin and phaeophorbid), which can easily be extracted from plants, were used. To make the hydrophobic substance water soluble, the phytol group responsible for hydrophobicity is removed by alkaline treatment (transfer into chlorophyllin). Slight acidification of the chlorophyll extracts results in a color change from green to olive-yellow (Richter 1996). This is an indication that pheophorbid (pheophytin without phytol) has been generated from chlorophyllin by hydrolysis. It is characterized by the lack of the central atom, magnesium, present in chlorophyll. The organic solvents ethanol and benzine can be recycled.
Materials and methods
Organisms
Culex sp. larvae were collected in a pond near the institute of biology in Erlangen and directly used for experiments. Chaoborus sp. larvae and Daphnia sp. were purchased from Zoo Baum (Nuremberg, Germany). Common carps (Cyprinus carpio) were provided from the Landesanstalt für Fischerei und Teichwirtschaft, Außenstelle Karpfenteichwirtschaft, Höchstadt Aisch, Germany.
Isolation of chlorophyll, modifications, storage, and material recycling
Different sources for isolation of chlorophyll were used, e.g., grass, stinging-nettle, dandelion, green cabbage, water hyacinth, spinach, etc. From all tested plants, deep-frozen spinach delivered the highest concentration of chlorophyll.
Chlorophyll was extracted in a waterbath at 55°C from the leaves, either with 96% ethanol or with 100% ethanol, after adding CaCO3, which prevents formation of pheophytin. The extract was filtered and petroleum benzene was added. The upper lipophilic phase was taken and saponified with methanolic KOH. This treatment converts chlorophyll into water-soluble chloropyllin. KOH precipitation yields high chlorophyllin concentrations. To calculate the concentration, this fallout was measured with a spectrophotometer. The extract was stored in a dark flask at 4°C. In some experiments, the effect of saponified pheophorbid (pheophytin without phytol) was determined. Pheophorbid was produced by acidification (HCl) of chlorophyllin. Before use, the stock solution was adjusted to pH 7.0. Petroleum benzine, which was used for isolation, could be reused several times. Nearly all ethanol could be recycled by distillation. The water content of the recycled ethanol was about 4%. The recycled EtOH was fully suitable to extract chlorophyll from plant material.
Sample treatment and determination of LD50 values
Defined amounts of chlorophyllin dissolved in methanolic KOH were evaporated in darkness on a heating plate under air flow. Each concentration was applied in three repetitions and three independent approaches. Water and KOH-treated (corresponding to highest chlorophyllin concentration) samples served as controls. Each chlorophyllin sample was dissolved in 30 ml of water, and subsequently, ten organisms were added. One-year-old carps were incubated in 10-l water basins with a chlorophyllin concentration of 15 mg/l. Carp larvae were incubated in chlorophyllin-containing water with 15 mg/l. All samples were kept in darkness overnight for incubation. After incubation, three samples were irradiated with simulated solar radiation (Sol 1200 W, mercury lamp 0383, Dr. Hönle, Martinsried, Germany) for 3–4 h. The lamp output was PAR: 149.66 W/m2, UV-A 32.67 W/m2, and UV-V 0.77 W/m2 (determined with spectroradiometer OL 754, Optronics, USA). The emission spectrum of the lamp is shown elsewhere (Klisch et al. 2001). Three parallel samples were kept in darkness as dark controls. This scheme was performed for all concentrations. After irradiation, the vitality of the organisms was determined. Individuals showing no vital signs (movements, reflexes after tipping with a needle) were counted as dead, while not affected organisms and organisms with reduced viability (compared to untreated control) were counted as survivors. Lethal-concentration curves of mortality (LD curves) were calculated. They have a sigmoidal form described by the following equation (Eq. 1; Tahedl and Häder 1999), described as in previous work (Tahedl and Häder 1999; Willemann 2002; Millán de Kuhn et al. 2006) to interpret the experimental data:
where y is the response variable (percentage of dead organisms), c the chlorophyllin concentration, y 0 is the response when the concentration tends to infinity, and b is a scale factor.
The data were processed using the software SigmaPlot for Windows 2000 by SPSS. This model corresponds with the equation proposed by Emmens (Tahedl and Häder 1999) to interpret effect–concentration relationships. The software fits the values with a nonlinear regression. The SigmaPlot curve fitter uses the Marquardt–Levenberg algorithm to determine the parameters of the independent variables that give the best fit between the equation and the data. This algorithm determines the values of the parameters iteratively so that the sum of the squared differences between the values of the observed and predicted values of the dependent variable is minimized.
where \(\bar y_i \) is the observed and y i the predicted value. The parameters EC50, b, and y o (see Eq. 1) are optimized in order to minimize SS. Confidence intervals of the optimized parameter set are calculated from the covariance matrix at a two-side error level of 5%.
Results
Initial experiments in which organisms were immediately irradiated after addition of chlorophyllin did not yield high mortality. This is most likely due to the fact that chlorophyllin was bleached before it was incorporated by the organisms. Several hours of dark incubation led to a significant accumulation of chlorophyllin inside mosquito larvae, which could be documented by means of fluorescence microscopy (data not shown). Chlorophyllin accumulation was also observed in cercaria of different trematodes and in yeast cells (data not shown).
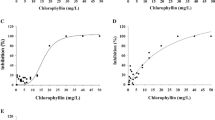
After incubation, chlorophyllin was found to kill different organisms at remarkably low concentrations. The LD50 value in Culex sp. larvae was about 6.88 mg/l, in Chaoborus sp. larvae about 24.18 mg/l, and in Daphnia 0.55 mg/l (Figs. 1, 2, 3, 5 and 8). For pheophorbid, the LD50 values were 8.44 mg/l in Culex, 1.05 mg/l in Chaoborus, and 0.45 mg/l in Daphnia (Figs. 2 and 4, data for Daphnia not shown). In some cases, chlorophyllin and pheophorbid were also found to be active in darkness. Pheophorbid resulted in LD50 values of 8.8 mg/l Culex, which is close to the determined value of the light-exposed samples. In Chaoborus LD50 values were five times higher (5.31 mg/l) compared to those in light-exposed samples, and in Daphnia, 50% mortality was found to be in a range of 8.8 mg/l (Figs. 2, 4, and 8). It was found that during the puparium, mosquito larvae are relatively insensitive against chlorophyllin treatment. During metamorphosis, the chrysalis is encapsulated and totally stops food uptake. Most likely due to this reason, the accumulation of chlorophyllin inside the organism is limited and the photodynamic effect is reduced. For comparison, the efficiency of two other photodynamic substances, hematoporphyrin and methylene blue, was tested in Chaoborus larvae. The LD50 for hematoporphyrin was 157.78 mg/l (darkness, 365.1 mg/l), while methylene blue was not effective at all concentrations tested (Figs. 6 and 7). All LD50 values are summarized in Table 1.
Percentage of dead mosquito larvae (Culex) in dependence of the chlorophyllin concentration after 3 h of light exposure (left) or 3 h in darkness (right). Each sample (ten larvae) is represented by a single dot. The black line is the sigma-shaped fit curve obtained by Sigma Plot, and the gray ones are the corresponding upper and lower borders of a 95% confidence level
Percentage of dead mosquito larvae (Culex) in dependence of the pheophorbid concentration after 3 h of light exposure (left) or 3 h in darkness (right). Each sample (ten larvae) is represented by a single dot. The black line is the sigma-shaped fit curve obtained by Sigma Plot, and the gray ones are the corresponding upper and lower borders of a 95% confidence level
Percentage of dead mosquito larvae (Chaoborus) in dependence of the chlorophyllin concentration after 3 h of light exposure (left) or 3 h in darkness (right). Each sample (ten larvae) is represented by a single dot. The black line is the sigma-shaped fit curve obtained by Sigma Plot, and the gray ones are the corresponding upper and lower borders of a 95% confidence level
Percentage of dead mosquito larvae (Chaoborus) in dependence of pheophorbid concentration after 3 h light exposure (left) or 3 h in darkness (right). Each sample (ten larvae) is represented by a single dot. The black line is the sigma-shaped fit curve obtained by Sigma Plot, and the gray ones are the corresponding upper and lower borders of a 95% confidence level
LD50 curve of Chaoborus larvae in presence of KOH after 4 h light exposure (left) or 3 h in darkness (right). Each sample (ten larvae) is represented by a single dot. The black line is the sigma-shaped fit curve obtained by Sigma Plot, and the gray ones are the corresponding upper and lower borders of a 95% confidence level
Percentage of dead mosquito larvae (Culex) in dependence of hematoporphyrin concentration after 4 h of light exposure (left) or 4 h in darkness (right). Each sample (ten larvae) is represented by a single dot. The black line is the sigma-shaped fit curve obtained by Sigma Plot, and the gray ones are the corresponding upper and lower borders of a 95% confidence level
Percentage of dead mosquito larvae (Culex) in dependence of methylene blue concentration after 3 h of light exposure (left) or 3 h in darkness (right). Each sample (ten larvae) is represented by a single dot. The black line is the sigma-shaped fit curve obtained by Sigma Plot, and the gray ones are the corresponding upper and lower borders of a 95% confidence level
Percentage of dead Daphnia in dependence of the chlorophyllin concentration after 3 h of light exposure (left) or 3 h in darkness (right). Each sample (ten larvae) is represented by a single dot. The black line is the sigma-shaped fit curve obtained by Sigma Plot, and the gray ones are the corresponding upper and lower borders of a 95% confidence level
One-year-old common carps (C. carpio) were not affected, while carp larvae did not survive the dark incubation with chlorophyllin.
Discussion
Abdul Kader and his research group of the National Institute of Laser Enhanced Science (NILES) performed very promising experiments using hematoporphyrin for pest control. In a recent work performed by Abdel-Kader et al. (2008a, b), it was demonstrated that larvae of Culex are sensitive against hematoporphyrin (0.07 μmol/ml). Also larvae of Musa domestica could be killed with this substance (10 μmol/ml). In the course of his PhD thesis, Allah El Tayeb (El Tayeb 2003) could demonstrate the effectiveness of hematoporphyrin against Culex and eggs of the snail Lymnea natalensis (vector of the trematode Fasciola hepatica). In this work, the penetration of the photosensitizer inside the organism was shown by means of fluorescence microscopy and the pronounced effects on the ultrastructure of inner organs and muscles by means of TEM.
The intention of the chlorophyllin experiments was to find a cheap and practical method to control parasites or their vectors, respectively, for large scale application. For bulk experiments, we consider several natural photosensitizers derived from plants including chlorophyll derivatives. Chlorophyll can easily be extracted by organic solvents from local weeds, such as Eichornia crassipes, which grows fast in the water canals in Egypt. The extraction of chlorophyll from Eichhornia was tested and was easily possible (but with relatively low chlorophyll concentration per fresh weight) by means of the method described above (unpublished results). Alternative sources for chlorophylls are byproducts from food processing plants, which extract and discard chlorophyll. Other potential photosensitizers might include cytochromes, hemoglobin, and other cyclic and linear tetrapyrroles, such as phycobilins, which can easily be extracted from cyanobacteria. But the use of the later substances was not the scope of the research.
The data clearly show the potential of chlorophyllin and pheophorbid as a possible counter measure against pest organisms. Prerequisite for successful application is the incorporation of the substance inside the organism, where chlorophyllin can exert its photodynamic property. The results show that dark incubation for several hours is necessary for accumulation inside the pest organism. This can be achieved by the application of the chlorophyllin during the night. After sunrise, the photodynamic action will start. Photodynamic effects of chlorophyll and its derivates (pheophytin and conjugates) are also of interest, e.g., in photodynamic therapy research (Lee et al. 2004; Kessel and Smith 1989). By means of fluorescence techniques and biochemical methods, it was found that chlorophyllin induces apoptosis, necrosis, and expression of heat shock proteins in Chaoborus larvae (Wohllebe and Grimm, manuscript in preparation).
The experiments show that chlorophyll derivates are much more effective to kill mosquito larvae than other photoactive substances like methylene blue or hematoporphyrin. From the curves and the LD50 values, we obtained evidence that chlorophyllin is much more effective than the two other chemicals in the tested concentration range. Unfortunately, other small animals like Daphnia and fish larvae are also affected by chlorophyllin, which makes some precautions necessary before application. An advantage is the easy and cheap way of producing the chlorophyll derivates and the ability to recycle all extraction substances needed. Preliminary experiments indicate that chlorophyll and its derivates may also be an antiparasite measure in fish farming. Intensive fish cultivation with high density of animals is often the cause of massive appearance of fish parasites such as Ichthyophtirius or Myxobolus (Haas et al. 1998; Kallert et al. 2005, 2007). Unlike fish larvae, elder fish were unharmed and survived chlorophyllin treatment at concentrations, where, e.g., Culex larvae are severely affected. In the course of a future project, the applicability of chlorophyll derivates in aqua culture is going to be investigated.
References
Abdel-Kader MH, El Sherbini AAM, El Tayeb TAA, Jori G, Rueck A (2008a) Sunlight and environmentally friendly sensitizer for mosquito control. in press
Abdel-Kader MH, El Sherbini EAM, El Tayeb TAA, Jori G, Ben Amor T (2008b) Environmentally friendly photopesticides. in press.
Brachs S, Haas W (2008) Swimming behavior of Schistosoma mansoni cercariae: responses to irradiance changes and skin attractants. Parasitol Res 102:685–690
Cox FEG (2002) History of human parasitology. Clin Microbiol Rev 15:595–612
El Tayeb TAA (2003) Laser scanning microscopy for determination of the efficiency of hematoporphyrin in control of Culex pipiens larvae and the snail vector of Fasciola gigantica. PhD thesis. National Institute of Laser Enhanced Sciences (NILES), Cairo University, pp 1–216
Engels D, Chitsulo L, Montresor A, Savioli L (2002) The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Tropica 82:139–146
Goklany IM (2004) Climate change and malaria. Science 306:55–56
Greenwood BM, Bojang K, Whitty CJM, Targett GAT (2005) Malaria. Lancet 365:1487–1498
Haas W, Haberl B, Hofmann M, Kerschensteiner S, Ketzer U (1998) Theronts of Ichthyophtirius multifiliis find their fish hosts with complex behavior patterns and in response to different chemical signals. Tokai J Exp Clin Med 23:329–331
Haas W, Grabe K, Geis C, Päch T, Stoll K, Fuchs M, Haberl B, Loy C (2002) Recognition and invasion of human skin by Schistosoma mansoni cercariae: the key-role of L-arginine. Parasitology 124:153–167
Haberl B, Kalbe M, Fuchs H, Ströbel M, Schmalfuss G, Haas W (1995) Schistosoma mansoni and S. haematobium: miracidial host-finding behavior is stimulated by macromolecules. Int J Parasitol 25:551–560
Haeberlein S, Haas W (2008) Chemical attractants of human skin for swimming Schistosoma mansoni cercariae. Parasitol Res 102:657–662
He Y-Y, Häder D-P (2002) Involvement of reactive oxygen species in the UV-B damage to the cyanobacterium Anabaena sp. J Photochem Photobiol B Biol 66:73–80
Kallert D, Eszterbauer E, El-Matbouli M, Erséus C, Haas W (2005) Life cycle studies of Myxobolus parviformis sp. n. (Myxozoa, Myxobolidae) from bream. Dis Aquat Org 66:233–243
Kallert D, Ponader S, Eszterbauer E, El-Matbouli M, Haas W (2007) Myxozoan transmission via actionospores: new insights in mechanisms and adaptations for host invasion. Parasitology 134:1741–1750
Kessel D, Smith K (1989) Photosensitization with derivatives of chlorophyll. Photochem Photobiol 49:157–160
Klisch M, Sinha RP, Richter PR, Häder D-P (2001) Mycosporine-like amino acids (MAAs) protect against UV-B-induced damage in Gyrodinium dorsum Kofoid. J Plant Physiol 158:1449–1454
Lee W-Y, Lim D-S, Ko S-H, Park Y-J, Ryu K-S, Ahn M-Y, Kim Y-R, Lee DW, Cho C-W (2004) Photoactivation of pheophorbid a induces a mitochondrial-mediated apoptosis in Jurkat leukaemia cells. J Photochem Photobiol B Biol 75:119–126
Millán de Kuhn R, Streb C, Breiter R, Richter P, Neeße T, Häder D-P (2006) Screening for unicellular algae as possible bioassay organisms for monitoring marine water samples. Water Res 40:2695–2703
Richter G (1996) Biochemie der Pflanzen. Thieme, Stuttgart, New York
Sachs J, Malaney P (2002) The economic and social burden of malaria. Nature 415:680–685
Tahedl H, Häder D-P (1999) Fast examination of water quality using the automatic biotest ECOTOX based on the movement behavior of a freshwater flagellate. Water Res 33:426–432
Tominaga H, Kodama S, Matsuda N, Suzuki K, Watanabe M (2004) Involvement of reactive oxygen species (ROS) in the induction of genetic instability by radiation. J Radiat Res 45:181–188
Trampuz A, Jereb M, Muzlovic I, Prabhu RM (2003) Clinical review: Severe malaria. Crit Care 7:315–323
WHO (2006) Schistosomiasis and soiltransmitted helminth infections—preliminary estimates of the number of children treated with albendazole or mebendazole. Weekly Epidemiol Rec 81(16):145–164
WHO (2007) Malaria. Fact sheet no. 94. Geneva, Switzerland
Willemann RL (2002) Development of an application of the ECOTOX system in the estuarine zone of the Baía da Babitonga, SC, Brazil, Diplom, Friedrich-Alexander Universität, Erlangen-Nürnberg, pp1–72
Acknowledgments
We gratefully acknowledge the friendly and competent support of Dr. Martin Oberle and his team, LfL Fischerei Höchstadt, who provided the carps and supported this work with valuable ideas and fruitful discussions. All performed experiments comply with the current laws in Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wohllebe, S., Richter, R., Richter, P. et al. Photodynamic control of human pathogenic parasites in aquatic ecosystems using chlorophyllin and pheophorbid as photodynamic substances. Parasitol Res 104, 593–600 (2009). https://doi.org/10.1007/s00436-008-1235-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-008-1235-6