Abstract
The aim of this study was to assess the ecotoxicological effects of water coming from untreated organic and conventional rice field production areas in the Ebro Delta (Catalonia, Spain) treated with the herbicides oxadiazon, benzofenap, clomazone and bensulfuron-methyl and the fungicides carbendazim, tricyclazole and flusilazole. Irrigation and drainage channels of the study locations were also included to account for potential toxic effects of water coming in and out of the studied rice fields. Toxicity tests included four species (Pseudokirchneriella subcapitata, Desmodesmus subcapitatus, Chlorella vulgaris and Daphnia magna), three endpoints (microalgae growth, D. magna mortality and feeding rates), and two trophic levels: primary producers (microalgae) and grazers (D. magna). Pesticides in water were analyzed by solid phase extraction-liquid chromatography-electrospray-tandem mass spectrometry (LC-ESI-MS/MS). Negative effects on algae growth and D. magna feeding rates were detected mainly after application of herbicides and fungicides, respectively, in the conventional rice field. Results indicated that most of the observed negative effects in microalgae and D. magna were explained by the presence of herbicides and fungicides. The above mentioned analyses also denoted an inverse relationship between phytoplankton biomass measured as chlorophyll a and herbicides. In summary, this study indicates that in real field situations low to moderate levels of herbicides and fungicides have negative impacts to planktonic organisms and these effects seem to be short-lived.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conventional agricultural practices have increased crop yields but also have caused severe environmental problems such as contaminated ground water, adverse health effects and changes in the habitat of many species (Hansen et al. 2001). In industrialised countries, there is an urgent need to reduce pollution by using less fertilisers and synthetic pesticides in intensive agriculture. In this regard, organic farming systems are considered an alternative to conventional agriculture to minimize pesticide/fertilizer negative impacts (Hole et al. 2005). In some European countries, up to 8% of the agricultural areas are managed organically according to European Union Regulation (EEC) No. 2092/91 (Hole et al. 2005). In terms of land area, Italy has by far the largest organic farming sector in Europe, followed by the UK, Germany, Spain and France (Häring et al. 2004).
In agricultural areas, pesticides can contaminate surface waters through drainage, run-off or directly by spray drift. At peak application time, water bodies discharging from several agricultural areas have been shown to transport a cocktail of pesticides (Kuster et al. 2008; Mañosa et al. 2001). Since pesticides are designed to exert toxic effects, it is reasonable to assume that they may also be hazardous to aquatic non-target organisms (Van den Brink et al. 2002; Junghans et al. 2006; Barata et al. 2007).
The Ebro Delta (Catalonia, Spain) is a protected area, in which agriculture (65% of its area is under rice cultivation) and wildlife are in continuous interaction (Fores and Comín 1992; Ibáñez and Prat 2003; Tarazona and Sanchez 2006). In this area more than 30 different pesticides are regularly detected in surface waters and new active products are assayed each year (Claver et al. 2006; Kuster et al. 2008; Hildebrandt et al. 2009). Some of the measured Delta del Ebro pesticides studied in the Ebro Delta (i.e. fenitrothion, endosulfan and diazinon) are known to be very toxic to planktonic species (Fernández-Casalderrey and Andreu-Moliner 1994; Sancho and Andreu-Molier 1996). However, many substances used as fungicides and herbicides can also be hazardous to non-target organisms, such as crustaceans and may also affect the structure and function of biological communities (Gustafsson et al. 2010). Van den Brink et al. (2000), Cuppen et al. (2000) and Slijkerman et al. (2004) showed that the fungicide carbendazim altered the composition of freshwater model ecosystems, being cladocerans one of the most susceptible zooplankton groups.
Algae growth inhibition responses have been commonly used as an important population-based parameter to evaluate pesticide toxic effects (Sabater and Carrasco 2001; De Lorenzo et al. 2002; Prado et al. 2009). Post-exposure feeding responses in Daphnia magna have also been used in laboratory and field studies to assess sublethal effects of toxic chemicals within industrial, domestic and agricultural complex effluents (Mc William and Baird 2002; Barata et al. 2007; Damásio et al. 2008; Bosch et al. 2009). Feeding rates in D. magna are physiologically linked with population growth rates (Barata and Baird 2000) and ecologically with top-down control of phytoplankton by zooplankton grazers (Sommer et al. 2003). Thus, measuring potential negative effects of pesticides on phytoplankton species and D. magna feeding rates can reflect responses occurring in natural populations (Mc William and Baird 2002).
In a previous study conducted in the Ebro Delta during the rice growing season, Barata et al. (2007) were able to identify exposure and effects associated to the most used pesticides in the field using in situ post exposure D. magna feeding assays. These include the herbicides betazone, propanil, molinate and the insecticide fenitrothion. The aim of the present study was to compare ecotoxicological effects of water collected from untreated (organic) and pesticide treated (conventional) rice fields from the Ebro Delta using both primary producers (microalgae growth) and grazers (D. magna feeding). Conventional rice fields were treated with five pesticide formulations, that although are being used in Ebro Delta rice fields, they are rarely detected in its surface waters (Kuster et al. 2008; Mañosa et al. 2001). The pesticide formulations applied were the herbicides were VIPER® and LONDAX®, whose active compounds are benzofenap, clomazone and bensulfuron-methyl, and the fungicides BIM® and PUNCH®, whose active compounds are carbendazim, tricyclazole and flusilazole. Oxadiazon (RONSTAR®) was applied before inundation as pre emergence herbicide. Field studies conducted with the above mentioned pesticides have reported low to moderate half lifes in water (2–10 days), high environmental levels (10–206 μg/l) in rice paddies surface waters during peak application and moderate to low levels in surrounding waters afterwards (<0.1–2.3 μg/l in rivers and lakes) (Quayle et al. 2006; Zanella et al. 2002; Hogendoorn et al. 2000; Armbrust 2000; Okamoto et al. 1998; Nicosia et al. 1991). However, these compounds can be accumulated in soil where the half life can be of several months (FAO and United Nations 1989). The reported toxicity of the studied formulations or active compounds is highly variable across studies and species (Table 1). In general the studied herbicides were more toxic to algae than to D. magna or other zooplankton species. Among herbicides, oxadiazon and bensulfuron-methyl were reported to inhibit algae growth or photosynthesis at exposure levels lower than 10 μg/l, clomazone at 50 μg/l and benzofenap at 140 μg/l. Fungicides were toxic to both phyto and zooplankton with carbendazim being the most toxic compound impairing D. magna survival, feeding rates or zooplankton and phytoplankton abundance within the 33–70 μg/l range. Tricyclazole and flusilazole were toxic to phyto and zooplankton in the range 1–10 mg/l (Table 1).
Materials and methods
Study area
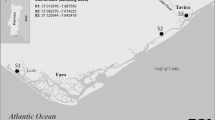
The Ebro Delta is located at the mouth of the largest river in Spain. It is the second most important wetland area in Spain, while simultaneously supports substantial rice production (Ibáñez and Prat 2003). The Ebro Delta receives a high load of pesticides as a consequence of the intensive agricultural activities developed upstream and in the delta itself (Mañosa et al. 2001; Claver et al. 2006; Terrado et al. 2006). Two main channels, one on each side of the river, bring the water from Xerta weir (30 km upstream) to the rice cultivation system. From these two channels, water is carried to and collected from the rice fields by a network of irrigation and drainage ditches, respectively. For this study, a conventional (C) and two organic (OA, OB) rice field plots were selected, as well as two additional sites located in irrigation (I) and drainage (D) channels (Fig. 1). The organic rice fields (without pesticide treatments) are part of a 54 ha farm, which applies the organic farming normative of the European Union (Regulation: CEE 2092/91).
Sampling
Water samples were collected to assess the potential toxicity of rice field water discharges following different pesticide applications (Table 2). Composite 5 l water samples of five 1 l sub-samples taken from the whole water column (10–40 cm deep) within a 10 m2 square were taken. The drainage channel was the deepest site sampled (120 cm), but the sample was taken from the first 10 cm of the water column. Water samples were collected simultaneously from 10 to 11 am at all sampling sites: prior to herbicide applications (beginning of June); 2, 7 days after herbicide application (in June); and 1 day after fungicide application in mid August. Once collected composite water samples were stored in 6 l glass containers and preserved on ice until arrival to the lab (30 min drive). Sub-samples of water of 2 l were stored in darkness at 4°C and toxicity tests were carried out within the next 4 days. Additional sub-samples of water of 1 and 2 l were also taken using pre-cleaned glass bottles and preserved at −20°C until chemical analyses.
Physico-chemical analyses
Physicochemical parameters such as dissolved oxygen (DO), water temperature, conductivity and pH were measured in situ with an YSI 556 multiprobe (YSI incorporated, Yellow Springs, USA). In the laboratory, total suspended solids, chlorophyll-a and pheopigments were determined by filtering 1 l of water using gravimetric and fluorometric methods, respectively (APHA-AWWA-WEF 1995; ASTM 1998; Jeffrey and Humphrey 1975).
Pesticide analysis
High purity (98–99.9%) standards of the three herbicides (oxadiazon, bensulfuron-methyl and clomazone) and three fungicides (carbendazim, flusilazole and tricyclazole) were purchased as powder from Sigma (St Louis, MO, USA). The herbicide benzofenap could not be included in the chemical analysis because it is no longer sold as individual active ingredient. The herbicide oxadiazon was not applied directly by the farmer in this crop season; however, it is widely used in the Ebro Delta rice fields as a pre-emergence herbicide and has been used before in the studied conventional rice fields. Stock standard solutions for each of the analytes were prepared in methanol at 10 mg/ml and stored in the dark at 4°C.
Sample extractions were conducted following previous procedures (Hildebrandt et al. 2007; Lacorte et al. 2000) with minor modifications. Water samples were filtered through 0.45 μm mesh glass fiber membrane filters from Whatman (Waters). A volume of ca 200 ml of water sample was extracted using OASIS HLB 200 mg solid-phase extraction cartridges. The cartridges were first conditioned with 10 ml of dichloromethane, 10 ml of methanol and 5 ml of HPLC water, then samples were percolated using a vacuum system at a flow of 6 ml/min. Afterwards, cartridges were rinsed with 1 ml of HPLC water, dried under a vacuum for 30 min and then immediately eluted with 20 ml of dichloromethane/methanol (1:1). Extracts were evaporated under a gentle stream of nitrogen to almost dryness and reconstituted to 250 μl with methanol. Standard solutions of the mixtures of all compounds at concentrations ranging between 0.1 and 10 μg/ml were prepared using appropriate dilution of the stock solutions in methanol. Standard mixtures were used as spiking solutions for preparing the calibration standards and external recovery studies. Three replicates of spiked water (0.1, 1 μg/l) for recovery studies and three blanks (HPLC water quality) were included in each extraction batch. Pesticide-grade solvents methanol and acetonitrile, and LC grade water were supplied by Merck (Darmstadt, Germany).
Ultra Performance Liquid Chromatography coupled with tandem Mass Spectrometry (LC-MS/MS) analyses were carried out in a Waters Alliance 2690 LC pump connected in series with the MS/MS detector. Instrument control, data acquisition and quantification were performed by Masslynx 4.0 software (Micromass, Manchester, UK). Chromatographic separation was performed using a capillary column of Merck RP-18e (12.5 mm × 2 mm) using the following gradient: 10 min starting from 20% acetonitrile in water, increasing to 80% acetonitrile in 5 min and continuing to 100% in 30 min. During the following 5 min the column was cleaned with 100% acetonitrile, readjusted to the initial conditions in 2 min, and equilibrated for a further 13 min. Acquisition was performed in Selected Reaction Monitoring (SRM) using 2 transitions per compound.
The recovery values of pesticides from spiked water samples were 91.56 ± 16.84% for bensulfuron-methyl, 94 ± 14% for clomazone, 111 ± 7% for oxadiazon, 105 ± 8% for carbendazim, 127 ± 33% for tricyclazole and 85 ± 17% for flusilazole. Detection limits (LOD) were calculated at a signal to noise ratio of 3, using spiked samples at 0.1 μg/l. Measured pesticide concentrations in water did not inform us about their potential toxicity. To do so pesticide levels (Ci) were transformed to toxic units (TU) following Liess and Von der Ohe (2005) procedures (Eq. 1).
where ECq i is the reported effect concentration (EC50, LOEC) for the measured compounds to phyto and zooplankton species (from Table 1).
Organisms and toxicity testing
Cultures of Chlorella vulgaris, Pseudokirchneriella subcapitata and Desmodesmus subcapitatus were performed in 250 ml Erlenmeyer flasks with 100 ml of sterilized medium (ISO 8692: 1989). Photoperiod was set to 12:12-h light:dark, temperature to 20 ± 1°C and light intensity to120 μ/Es m2. Algae growth tests were conducted following the OECD guidelines (2002), using undiluted water samples that were filtered with a glass filter of 0.45 μm (Whatman). Initial cell densities were adjusted to 1 × 104 cells/ml for P. subcapitata and D. subcapitatus, and to 3 × 104 cells/ml for C. vulgaris. Measurements were taken using a calibration curve of chlorophyll absorbance (λ = 665) nm versus cell number and appropriate dilution of pre-cultures, in a dual-beam spectrophotometer (Hitachi U-2001). Algae bioassays were performed using fourth replicates per sample plus an algal medium treatment as control. Tests lasted 72 h and were conducted at the same conditions of algal cultures, except for photoperiod (24 h light).
D. magna cultures were maintained in bulk cultures of 30 animals in 4 l with ASTM hard synthetic water (ASTM 1998) as described by Barata et al. (2008). Acute D. magna tests were conducted in accordance with OECD guidelines (OECD/OCDE 2004). D.magna post exposure feeding inhibition were carried out with <24 h old juveniles obtained from 15 to 30 days old gravid females following previous procedures (Bosch et al. 2009; Barata et al. 2008) with only minor modifications. Groups of 70–80 juveniles were exposed to unfiltered water collected from each sampling point during 24 h, in amber glass bottles of 600 ml with continuous orbital agitation (SCTR4 stuart) at 1 rpm for 24 h and 20 ± 1°C, in order to maintain suspension of particles. A control with ASTM medium with algae was also included in each run. After exposure, feeding rates were determined in groups of 10 individuals that were left to feed for 5 h in 20 ml of ASTM medium with C. vulgaris at 5 × 105 cells/ml in glass flasks of 30 ml, in darkness. Six replicates per sampling site and a blank without individuals were used to establish the initial algal concentration in the treatments (Barata et al. 2007, 2008). Algal concentration was determined using a multi Coulter Multisizer (Beckman Counter).
Statistical analysis
Our experimental design did not to asses and hence to compare the water samples collected in different periods simultaneously. To solve these problem two analyses were performed. Firstly, within each sampling period one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison tests were performed to compare algae and D. magna performance in water samples against controls. Secondly, proportional algae growth and D. magna feeding responses relative to controls were compared by a two way ANOVA to tests the effect of the sampling period and site. Significant differences were established at P < 0.05. Prior to any analysis, row and proportional data were log and arsine transformed to meet ANOVA assumptions of normality and variance homoscedasticity (Zar 1996).
The relationships between the measured contaminants and biological responses were explored by multivariate Principal Component Analysis (PCA), and possible causal-effect relationships using correlation analyses of the PCA scores. Initially, physicochemical parameters and ecotoxicological data were compared in two separated Principal Component Analysis (PCA). These analyses allowed the identification of the main sources of variability of the environmental and biological parameters within the studied samples. Possible causal-effect relationships were then determined comparing PCA scores using Pearson correlation analysis. Since variables were very different and they were not measured using the same scale units, data was first log transformed and then auto-scaled prior to analysis (each element was subtracted by the mean and divided by the standard deviation of all results). Pesticide values under the limit of detection were assigned half of the detection limit.
Results
Physicochemical characterization
Physicochemical parameters of the studied sites denoted differences in photosynthetic pigments, suspended solids and oxygen levels among sites and sampling periods (Table 3). In June, chlorophyll a and pheopigment levels were highest in the irrigation channels and lowest at the conventional rice fields. In August there was not a clear pattern of the physicochemical parameters and pigment concentration. Suspended solids and oxygen levels varied across the irrigation and drainage channels irrespective of the sampling period and were probably related to local water management strategies. Indeed, the lack of water flow in conventional rice fields following herbicide application may explain the high temperatures registered at these sites (32–34°C). The pH and conductivity values varied between sites and sampling times, pH varied from 7 to 9, and the highest conductivity values were found in the conventional rice fields and drainage channel (Table 3). No pesticides were detected in water samples, except in those collected 2 and 1 days after herbicide and fungicide applications, respectively (Table 3).
Bi-plots of loadings and sample scores of the first two PCA components denoted different patterns of contamination (Fig. 2). The first and second PCA components explained up to 65.4% of data variability and showed high positive loadings for herbicide and fungicide levels (Fig. 2). PCA on physicochemical parameters differentiated the pesticides found that showed similar loadings in the PC1 from those of suspended solids, chlorophyll pigments and conductivity (PC2) (Fig. 2).
PCA bi-plots of loadings and sample scores for physico-chemical parameters of the studied water samples. 0, H2, H7, F1 are samples collected before (O) and following 2, 7 days of herbicide and 1 day of fungicide treatments. Percentages of data variance explained for each of the two PCA components are depicted. Abbreviations are explained in Table 2
Predicted toxicity
Disparity of reported concentration effects in lab and field studies made difficult to estimate their expected toxicity from the exposure levels detected in water (Table 1). To overcome this problem we selected the mean, minimum and maximum concentration effect levels reported in Table 1 for phyto and zooplankton species to apply them into Eq. 1. The obtained results predicted a mean (minimum and maximum) toxicity of the measured chemical mixtures 2 days after herbicide application to algae and zooplankton of 0.3 (0.1–16.0) and 0.4 (0.2–12.0) TU, respectively. Predicted toxicity following fungicide application to algae and zooplankton was 0.5 (0.3–21.6) and 5 (2.2–30.1) TU, respectively. Due to the large variability reported in terms of toxicity of the studied compounds (Table 1), it was difficult to select those compounds with the largest contribution to the estimated TU of the mixtures. Only within the fungicide mixture, carbendazim was clearly identify as the compound more toxic to D. magna (4.9 (2.2–24.5) TU). Water samples with TU ≥ 1 should be highly toxic to the studied algae and D. magna.
Ecotoxicological responses
Within each sampling period algae growth rates of the three studied species were significantly affected in field collected water samples in 9 out of 12 comparisons (P < 0.05, one way ANOVA; Table 4). Significant (P < 0.05) lower algae growth rates than control treatments were observed in 12 water samples during the pre-treatment and 2 days after herbicide application periods. These included four and five water samples from conventional rice fields and drainage channels, respectively, two water samples from irrigation channels and one from organic fields A and B (Table 4). Significantly (P < 0.05) higher algae growth rates than control treatments were observed in C. vulgaris cultured in water samples collected seven after herbicide and 1 day after fungicide application and in D. subcapitatus exposed to water samples 1 day after fungicide application.
In D. magna no or negligible (<5%) mortality occurred in the acute tests performed with the studied water samples. Post-exposure D. magna feeding rates were significantly (P < 0.05) impaired relatively to control treatments in six water samples collected from irrigation and conventional rice fields during pre-treatment, 2 and 1 days after herbicide and fungicide application (Table 4).
Two way ANOVA and post hoc Tukey’s test results performed on relative responses indicated significant differences in microalgae growth and D. magna feeding responses within and across sites and periods (Table 5). The greatest inhibitory effects on algae growth rates were observed in water from conventional fields 2 days after herbicide application, whereas for D. magna feeding rates the most toxic waters were those obtained from conventional rice fields 1 day after fungicide application.
Bi-plots of loadings and scores of the first PCA components showed a strong relationship (high positive loadings to the first component; PC1 Tox) between the growth rates of the three studied algae species, whereas D. magna feeding loadings were only high in the second component (PC2 Tox 2) (Fig. 3). Most sample scores were grouped around the origin, indicating low to marginal levels of toxicity. The exception being those samples collected at the irrigation channel and at rice fields treated with herbicides and fungicides at days 2 and 1 after applications, respectively.
PCA bi-plots of loadings and sample scores for toxicity parameters of the studied water samples. 0, H2, H7, F1 are samples collected before (O) and following 2, 7 days of herbicide and 1 days of fungicide treatments. Percentages of data variance explained for each of the two PCA components are depicted. Abbreviations are explained in Table 2. For clarity not all water sample codes are depicted
Testing causal-effect relationships
Bivariate Pearson correlation values across toxicological and physicochemical PCA sample scores denoted only significant (P < 0.05) relationships between algae growth (PC1 Tox) and pesticides (PC1 Chem; −0.81, n = 21) and between D. magna post exposure feeding inhibition (PC2 Tox), conductivity and chlorophyll levels (PC2 Chem; 0.56, n = 21). The above mentioned relationships were mostly explained by water samples collected 2 and 1 days after herbicide and fungicide applications, respectively, in treated rice fields.
Discussion
Most of the reported physicochemical differences in the studied water samples were probably related to local rice field management strategies. By opening and closing floodgates, farmers can control incoming and outgoing water flows to rice fields. In this particular study, following the recommended managing procedure, the farmer closed the conventional rice plots during pesticide applications until day 7, when the floodgate to the drainage channel was opened. Therefore, observed changes in suspended solids, oxygen levels and temperature in June may be related to this type of management. Primary production is very intense in the first phases of rice growing leading to oxygen supersaturation during the day (Fores and Comín 1987; Fernández-Valiente and Quesada 2004). Water in the irrigation channels is initially collected in the Ebro River, 30 km upstream of the Delta, and when introduced into the rice fields, it becomes more salty due to the influence of the soil, causing an increase in water conductivity in rice fields and drainage channels. Changes in other parameters such as suspended solids and chlorophyll pigments should also be expected to occur in rice fields due to biological and water flow changes.
The presence of the studied herbicides and fungicides in water samples were only detected at high levels following the application of commercial formulations, indicating a low persistence of the compounds in the studied rice fields. Pesticide residue levels in water after pesticide application did not exceed regulatory limits for surface waters and agriculture effluents (i.e. 100–800 μg/l for tricyclazole, 4,000 μg/l for bensulfuron-methyl, Hamilton et al. (2003), and were in agreement with reported studies. The observed low environmental persistence of the studied herbicides (<7 days) was also in agreement with previous results. Zanella et al. (2002), studying the environmental fate of clomazone in rice field waters, observed a rapid, four fold decrease within 7 days. Clomazone concentrations in water reported by Quayle et al. (2006) reached maximum levels of 202 μg/l following herbicide application and decreased to 2.9 μ/l after 21 days. Nicosia et al. (1991) and Okamoto et al. (1998) found in rice fields peak concentrations of 29–106 μg/l of bensulfuron-methyl and half-lives of 1.5–2.9 days. Information for carbendazim residues in rice paddy waters is scarce, but a study conducted by Hogendoorn et al. (2000) reported peak carbendazim residues in ditch field water of 128.1 μg/l just after herbicide application and relative low levels after 3 days (<2.9 μg/l).
Predicted toxicity of the measured pesticide levels in water 2 and 1 days after herbicide and fungicide applications denoted high toxicity (TU ≥ 1) to algae and D. magna. Interestingly, predicted mean toxicity TU of herbicides was similar for phyto (0.3) and zooplankton (0.4) species; but for of fungicides it was one order of magnitude greater for zooplankton (5) than for phytoplankton (0.5). In an early study performed in the Ebro Delta rice fields using in situ tests Barata et al. (2006) found that pesticide sublethal effects on transplanted D. magna were likely to occur at TU > 0.1. Therefore the observed detrimental effects on microalgae and D. magna feeding rates of water samples collected following herbicide and fungicide application are mostly due to be related to their measured residue levels. Other factors that may enhance toxicity in real field situations are synergic effects of pesticide mixtures, the presence of more toxic metabolites, and/or additive effects of pesticides and other environmental factors (Barata et al. 2006, 2007, 2008). The presence of toxic adjuvants in commercial pesticide formulations may also increase toxicity. Marques et al. (2009) found that the behaviour of the earthworm Esenia andrei was affected under VIPER® exposures in soils, the formulated product being even more repulsive for E. andrei than the active ingredients benzofenap and clomazone. In our study, predicted TU, conductivity and suspended solids were at the upper tolerance range of the studied species (Barata et al. 2006, 2009) and other unmeasured pesticides could also be present (Kuster et al. 2008). Thus, many factors could have contributed to the observed toxicity of water samples. Our experimental design, however, allowed the estimation of the possible pesticide effects of by simultaneously testing the toxicity of pesticides that could enter to the rice fields from irrigation channels (organic rice fields) and the combined effect of pesticides entering from the irrigation channel, plus those applied in the studied fields. The inclusion of water samples from drainage channels allowed for estimating the extent to which some pesticides applied in rice fields could contaminate other aquatic systems of the Ebro Delta (Mañosa et al. 2001; Kuster et al. 2008). None of the water samples from the drainage channels had detectable levels of the studied pesticides, thus suggesting that the measured compounds did not significantly pollute surrounding areas. Nevertheless, in two out of the four sampling periods, water from drainage channels had negative effects to algae or/and D. magna. The studied drainage channel also collected water effluents from other rice fields and, according to previous work, it could contain more than 30 different pesticides (Kuster et al. 2008) that were toxic to D. magna (Barata et al. 2006).
PCA and correlation analyses of physico-chemical and toxicity data clearly evidenced two associations: microalgae growth inhibition was related to high levels of herbicides and conductivity values, and detrimental effects on post exposure feeding rates in D. magna were associated with high levels of fungicides and suspended solids. Previous studies have also shown that salinity and suspended solids are hazardous to freshwater microalgae and D. magna (Bosch et al. 2009; Barata et al. 2006, 2008). Interestingly, herbicide levels appeared to be inversely related to those of chlorophyll pigments, suggesting that herbicides, by inhibiting phytoplankton growth, may affect phytoplankton biomass in the studied conventional rice fields. This finding supports previous studies that also related temporary abundance alterations of phytoplankton communities in the Ebro Delta to herbicide usage (Mañosa et al. 2001). However, high temperatures could also contribute to phytoplankton inhibition (Lesser 1996; Osmond 1981). Rice fields are highly dynamic ecosystems, where the chemical characteristics of the water change drastically in relatively short time periods (Fores and Comín 1987; Fernández-Valiente and Quesada 2004). A recent review by Relyea and Hoverman (2006) determined that the majority of previous research has been focused on the effects of insecticides on freshwater ecosystems. We believe that more research is needed on wildlife and environmental impacts caused by other type of pesticides (in particular, herbicides and fungicides), especially during the growing seasons. In concurrence with Ibáñez et al. (in press), the introduction of alternative farming schemes, specifically agro-environmental measures and organic farming, is needed to increase the sustainability of agricultural practices in rice fields.
Conclusions
This study indicated that in real field situations low to moderate levels of herbicides and fungicides had negative effects to planktonic organisms and these effects seem to be short-lived but could be propagated to higher trophic levels. Microcosm studies using one of the studied pesticides (carbendazim) reported that transient detrimental effects on Cladocera grazing rates enhanced algae biomass (Van den Brink et al. 2002). Alternatively, the same authors reported that negative effects on algae growth decrease and change the abundance and community composition of grazers. Our results also suggest that organic management strategies are the least hazardous option for the environment, but the use of pesticides with low environmental impact to surrounding areas, used at recommended doses and combined with good water management practices, may also reduce the environmental hazards of pesticides.
References
APHA-AWWA-WEF (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Association/America Water Works Association/Water Environment Federation, Washington, DC
Armbrust KL (2000) Pesticide hydroxyl radical rate constants: measurements and estimates of their importance in aquatic environments. Environ Toxicol Chem 19(9):2175–2180 APHA
ASTM (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC
Barata C, Baird D (2000) Determining the ecotoxicological mode of action of chemicals from measurements made on individuals: results from instar-based tests with Daphnia magna Straus. Aquat Toxicol 48(2–3):195–209
Barata C, Baird DJ, Nogueira AJA, Soares AMVM, Riva MC (2006) Toxicity of binary mixtures of metals and pyrethroid insecticides to Daphnia magna Straus. Implications for multi-substance risks assessment. Aquat Toxicol 78:1–14
Barata C, Damásio JB, López MA, Kuster M, López de Alda M, Barceló D (2007) Combined use of biomarkers and in situ bioassays in Daphnia magna to monitor environmental hazards of pesticides in the field. Environ Toxicol Chem 26:370–379
Barata C, Alañón P, Gutierrez-Alonso S, Riva MC, Fernández C, Tarazona JV (2008) A Daphnia magna feeding bioassay as a cost effective and ecological relevant sublethal toxicity test for environmental risk assessment of toxic effluents. Sci Total Environ t405:78–86
Bosch C, Olivares A, Faria M, Navas JM, Olmo I, Grimalt JO, Piña B, Barata C (2009) Identification of water soluble and particle bound compounds causing sublethal toxic effects. A field study on sediments affected by a chlor-alkali industry. Aquat Toxicol 94:16–27
Claver A, Peña O, Rodríguez L, Ovelleiro JL (2006) Study of the presence of pesticides in surface waters in the Ebro river basin (Spain). Chemosphere 64(9):1437–1443
Cuppen JGM, Van den Brink PJ, Camps E, Uil KF, Brock TCM (2000) Impact of the fungicide carbendazim in freshwater microcosms. I. Water quality, breakdown of particulate organic matter and responses of macroinvertebrates. Aquat Toxicol 48:233–250
Damásio J, Tauler R, Teixido E, Rieradevall M, Prat N, Riva MC, Soares AMVM, Barata C (2008) Combined use of biomarkers and in situ bioassays in Daphnia magna to monitor environmental hazards of pesticides in the field. Aquat Toxicol 87:310–320
De Lorenzo ME, Taylor LA, Lund SA, Pennington PL, Strozier ED, Fulton MH (2002) Toxicity and bioconcentration potential of the agricultural pesticide endosulfan in phytoplankton and zooplankton. Arch Environ Contam Toxicol 42:173–181
FAO and United Nations (1989) Expert group on pesticide residues. Geneva, 18–27 Oct
Fernández-Casalderrey A, Andreu-Moliner E (1994) Effect of sublethal concentrations of pesticides on the feeding behaviour of Daphnia magna. Ecotoxicol Environ Saf 27:82–89
Fernández-Valiente E, Quesada A (2004) A shallow water ecosystem: rice-fields. The relevance of cyanobacteria in the ecosystem. Limnetica 23(1–2):95–108
Fores E, Comín FA (1987) Chemical characteristics of the water in the rice fields of the Ebro delta (N.E. Spain). Arch Hydrobiol 11(1):15–24
Fores E, Comín FA (1992) Ricefields, a limnological perspective. Limnetica 8:101–109
Gustafsson K, Blidberg E, Elfgren IK, Hellstrom A, Kylin H, Gorokhova E (2010) Direct and indirect effects of the fungicide azoxystrobin in outdoor brackish water microcosms. Ecotoxicology 19:431–444
Hamilton DJ, Ambrust A, Dieterle RM, Felsot AS, Harris CA, Holland PT, Katayama A, Kurihara N, Linders J, Unsworth J, Wong SS (2003) Regulatory limits for pesticide residues in water (IUPAC Technical report). Pure Appl Chem 75(8):1123–1155
Hansen B, Alroe HF, Kristensen ES (2001) Approaches to assess the environmental impact of organic farming with particular regard to Denmark. Agric Ecosyst Environ 83:11–26
Häring AM, Dabbert S, Aurbacher J, Bicher B, Eichert C, Gambelli D, Lampkin N, Offermann F, Olmos S, Tuson J, Zanoli R (2004) Organic farming and measures of European agricultural policy. Organic Farming in Europe: Economics and Policy 11
Hildebrandt A, Lacorte S, Barceló D (2007) Assessment of priority pesticides, degradation products, and pesticide adjuvants in groundwaters and top soils from agricultural areas of the Ebro river basin. Anal Bioanal Chem 387(4):1459–1468
Hildebrandt A, Lacorte S, Barceló D (2009) Occurrence and fate of organochlorinated pesticides and PAH in agricultural soils from the Ebro river basin. Environ Contam Toxicol 57:247–255
Hogendoorn EA, Westhuis K, Dijkman E, Heusinkveld HAG, Chamraskul P, Biadul P, Baumann RA, Cornelese AA, Van Der Linden MA (2000) Determination of carbendazim in water, soil and sediment samples by RPLC with and without column switching and various spectrometric detection modes. Int J Environ Anal Chem 78(1):67–85
Hole DG, Perkins AJ, Wilson JD, Alexander IH, Grice EAD (2005) Does organic farming benefit biodiversity? Biol Conserv 122:113–130
Ibáñez C, Prat N (2003) The environmental impact of the Spanish National Hydrological Plan on the lower Ebro river and delta. Water Res Dev 19(3):485–500
Ibáñez C, Curcó A, Riera X, Ripoll I, Sánchez C (in press) Influence on birds of rice field management practices during the growing season: a review and an experiment. Waterbirds
Jeffrey S, Humphrey G (1975) New spectrophotometric equations for determining chlorophylls a, b, c, and c1 in higher plants, algae and natural phytoplankton. Biochem Physiol 167:191–194
Junghans M, Backhaus T, Faust M, Scholze Mc, Grimme LH (2006) Application and validation of approaches for the predictive hazard assessment of realistic pesticide mixtures. Aquat Toxicol 76:93–110
Kuster M, de Alda MJL, Barata C, Raldúa D, Barceló D (2008) Analysis of 17 polar to semi-polar pesticides in the Ebro river delta during the main growing season of rice by automated on-line solid-phase extraction-liquid chromatography-tandem mass spectrometry. Talanta 75:390–401
Lacorte S, Guiffard I, Fraisse D, Barceló D (2000) Broad spectrum analysis of 109 priority compounds listed in the 76/464/CEE Council Directive using solid phase extraction and GC-EI/MS. Anal Chem 72(7):1430e1440
Lesser M (1996) Elevated temperatures and ultraviolet radiation cause oxidative stress and inhibit photosynthesis in symbiotic dinoflagellates. Limnol Oceanogr 41(2):271–283
Liess M, Von der Ohe C (2005) Analyzing effects of pesticides on invertebrate communities in streams. Environ Toxicol Chem 24:954–965
Ma J, Zheng R, Xu L, Wang S (2002) Differential sensitivity of two green algae, Scenedesmus obliqnus and Chlorella pyrenoidosa, to 12 pesticides. Ecotoxicol Environ Saf 52(1):57–61
Mañosa S, Mateo R, Guitart R (2001) A review of the effects of agricultural and industrial contamination on the Ebro delta biota and wildlife. Environ Monit Assess 71:187–205
Marques C, Pereira R, Gonçalves F (2009) Using earthworm avoidance behaviour to assess the toxicity of formulated herbicides and their active ingredients on natural soils. J Soil Sediment 9:137–147
Mc William RA, Baird DJ (2002) Post-exposure feeding depression: a new toxicity endpoint for use in laboratory studies with Daphnia magna. Environ Toxicol Chem 21:1198–1205
Nicosia S, Collison C, Lee P (1991) Bensulfuron methyl dissipation in California rice fields, and residue levels in agricultural drains and the Sacramento river. Environ Toxicol Chem 47:131–137
OECD (2002) Guideline for testing of chemicals. Proposal for updating guideline 201, freshwater alga and cyanobacteria, growth inhibition test
OECD/OCDE (2004) Guideline for testing of chemicals Daphnia sp acute immobilisation test 202
Okamoto Y, Fisher RL, Armbrust KL, Peter J (1998) Surface water monitoring survey for bensulfuron methyl applied in paddy fields. J Pestic Sci 23:235–240
Osmond CB (1981) Photorespiration and photoinhibition; some implications for the energetic of photosynthesis. Biochem Biophys Acta 639:77–98
Prado R, García R, Rioboo C, Herrero C, Abalde J, Cid A (2009) Comparison of the sensitivity of different toxicity test endpoints in a microalgae exposed to the herbicide paraquat. Environ Int 35(2):240–247
Quayle WC, Oliver DP, Zrna S (2006) Field dissipation and environmental hazard assessment of clomazone, molinate, and thiobencarb in Australian rice culture. J Agric Food Chem 54:7213–7220
Relyea R, Hoverman J (2006) Assessing the ecology in ecotoxicology: a review and synthesis in freshwater systems. Ecol Lett 9(10):1157–1171
Sabater C, Carrasco JM (2001) Effects of pyridaphenthion on growth of five fresh water species of phytoplankton. A laboratory study. Chemosphere 44:1775–1781
Sancho F, Andreu-Molier E (1996) Chronic toxicity of fenitrothion to an algae (Nannochloris oculata), a rotifer (Brachionus calyciflorus), and the cladoceran (Daphnia magna). Ecotoxicol Environ Saf 35:112–120
Slijkerman DME, Baird DJ, Conrad A, Jak RG, van Straalen NM (2004) Assessing structural and functional plankton responses to carbendazime toxicity. Environ Toxicol Chem 23:455–462
Sommer U, Sommer F, Santer B, Zollner E, Jurgens K, Jamieson C, Boersma M, Gocke K (2003) Daphnia versus copepod impact on summer phytoplankton: functional compensation at both trophic levels. Oecologia 135:639–647
Tarazona JV, Sanchez P (2006) Development of an innovative conceptual model and a tiered testing strategy for the ecological risk assessment of rice pesticides. Paddy Water Environ 4(1):1611–2490
Terrado M, Barceló D, Tauler R (2006) Identification and distribution of contamination sources in the Ebro river basin by chemometrics modelling coupled to geographical information systems. Talanta 70:691–704
Tomlin CDS (2009) The pesticide manual, a world compendium, 15th edn. Crop Protection Publications, Farnham, Surrey, UK
Van den Brink PJ, Hattink J, Bransen F, Van Donk E, Brock TCM (2000) Impact of the fungicide carbendazim in freshwater microcosms. II. Zooplankton, primary producers and final conclusions. Aquat Toxicol 48:251–264
Van den Brink PJ, Hattink J, Bransen F, Van Donk E, Brock TCM (2002) Effects of a mixture of two insecticides in freshwater microcosms: II. Responses of plankton and ecological risk assessment. Ecotoxicology 11:165–180
Van Wijngaarden RPA, Crum SJH, Decraene K, Hattink J, Van Kammen A (1998) Toxicity of Derosal® (active ingredient carbendazim) to aquatic invertebrates. Chemosphere 37:673–683
Zanella R, Primel EG, Machado SLO, Gonçalves FF, Marchezan E (2002) Monitoring of the herbicide clomazone in environmental water samples by solid-phase extraction and high performance liquid chromatography with ultraviolet detection. Chromatographia 55:573–577
Zar JH (1996) Biostatistical analysis. Prentice-Hall International, Inc, New Jersey
Acknowledgments
This study was carried out with the financial support of the Spanish Ministry of Environment and the Catalan Water Agency (ACA), Department of Environment, Government of Catalonia (MOBITROF project). We thank for field and laboratory assistance to Mireia San Lorenzo, David Mateu and Lluís Llornet. We are grateful to Rafael Verdiell and Riet Vell S.A. (owners of the rice fields) for their collaboration. We also thank Katherine Sharpe for helping to improve the English and providing useful comments on a previous version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suárez-Serrano, A., Ibáñez, C., Lacorte, S. et al. Ecotoxicological effects of rice field waters on selected planktonic species: comparison between conventional and organic farming. Ecotoxicology 19, 1523–1535 (2010). https://doi.org/10.1007/s10646-010-0537-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-010-0537-5