Abstract
Background, aim, and scope
Soil quality has been threatened by intensive agricultural practises, namely those relying on the application of pesticides, such as herbicides. Among the non-target terrestrial organisms exposed to such scenarios, earthworms are key ecological receptors widely used in ecotoxicological studies. As such, this work aims to assess the effects of two herbicide active ingredients (a.i.)—sulcotrione and penoxsulam—and their respective commercial formulations—MIKADO® and VIPER® (referred as Mikado and Viper)—on the avoidance behaviour of Eisenia andrei. In an attempt to enhance the ecological relevance of the generated toxicity data, the avoidance tests were run with standard (LUFA 2.2; L) and natural soils (from corn and rice fields), as long as their habitat function did not constrain the earthworm behaviour.
Methodology
Earthworms were bred in the lab before test conductance. The natural soils used as substrates were collected before the cropping season on corn (C) and rice (R) fields, which are integrated in a wide area exploited for agriculture. Their physico-chemical characterization evolved the determination of pH (H2O, KCl), conductivity, organic matter (OM) and clay/silt contents, and water-holding capacity (WHC). The avoidance tests intended to ascertain (1) the random distribution of earthworms in the natural soils C and R (dual-control tests), (2) the habitat function of natural soils against each other and against L soil, (3) the effect of active ingredients and formulated herbicides on E. andrei behaviour. Avoidance tests with the a.i.s were only performed in L soil. Data evaluation followed ISO (2005) guidelines.
Results
C and R soils presented higher OM (5.1% and 4.5%, respectively) and clay/silt (53.3 and 43.1, respectively) contents and WHC (107.2 and 109.9%, respectively) than L soil (4.1, 21.4 and 48.0%, correspondingly). Earthworms distributed randomly in dual-control tests, but preferred R soil significantly, relative to L or C soils. The LOEC and EC50 values calculated for sulcotrione (>1,000.0 and 1,263.3 mg a.i. kg-1, respectively) and Mikado (1,012.8 and 1,301.3 mg a.i. kg-1, respectively) were much higher than those calculated for penoxsulam (100 and 80.6 mg a.i. kg-1, respectively) or Viper (52.7 and 51.5 mg a.i. kg-1, respectively), when L soil was used as substrate. Moreover, the habitat function of L soil contaminated with the formulated herbicide Viper was more constrained relative to that of the a.i. penoxsulam. Viper induced higher % avoidance on E. andrei exposed to the contaminated L soil compared to that under the R soil.
Discussion
The response of earthworms to R (attraction) and C (avoidance) soils could be related, not only to the quantity of OM content, but also to the quality of organic and inorganic fractions of soil, beyond other intrinsic properties of soils. Both Mikado and sulcotrione impacted the behaviour of E. andrei only slightly. This endpoint was more affected under penoxsulam or Viper exposures on L soil, being the latter-formulated product even more repellent for E. andrei than the a.i. The effect of adjuvants added to the commercial formulation of Viper, may have increased the toxicity of the a.i. Thereby, our results reinforce the need for a careful assessment of the impacts of formulated products. Furthermore, since there was a reduction in earthworm % avoidance under Viper exposures on the natural soil R, it was possible that pesticide bioavailability had been reduced by its sorption to OM and clay mineral sorption sites.
Conclusions
Though the standard L soil should be used for reproducibility and comparison means, other natural soils should be added to the assessment of chemicals, for sake of ecological relevance. Both herbicides induced avoidance behaviour on E. andrei, albeit stronger effects were denoted by penoxsulam and its respective formulated product, Viper. Overall, avoidance tests provided a sensitive, valuable and feasible response either to compare the habitat function of different standard and agricultural natural soils or to test the effect of herbicides.
Recommendations and perspectives
An effort should be made to enlarge the terrestrial ecotoxicological database as a way to fulfil the huge lack of information available for this ecosystem. In this context, additional research congregating a potential linkage between physiological activities sustaining the regular metabolism of earthworms and their avoidance behaviour or even their reproductive effects would be welcomed, especially in what regards formulated pesticides. Such approach would provide a robust and comprehensive understanding of chemical effects. Furthermore, it is encouraged that natural soils should be used to improve the reliability of chemical testing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
2 Backgroud, aim, and scope
Recent awareness regarding the urgent need for soil protection (CEC 2006) encouraged the development of frameworks for the prospective risk assessment (EC 2003) of new and existing chemicals, as well as pesticides (EEC 1991; EC 2002). Such an assessment approach suggests the performance of tests with earthworms to study the toxicity of pesticides upon the use of standard acute and chronic tests (EEC 1991; EC 2002, 2003).
Indeed, a wealth of literature points out for earthworms, as a key ecological receptor widely used in ecotoxicological studies and that they are also one of the terrestrial organisms potentially exposed to the presence of pesticides in soil (Muthukaruppan et al. 2005; Reinecke et al. 2002; Reinecke and Reinecke 2007; Römbke 2006). This can be attributed, on the one hand, to their ecological role in the maintenance of soil structure and functioning, mainly sustained by their burrowing activities, and to their breakdown of organic matter (Lavelle et al. 2006; Römbke et al. 2005). On the other hand, earthworms are sensitive to the presence of chemicals in the soil due to the chemoreceptors distributed on their body surface (Reinecke et al. 2002). This characteristic associated with their locomotory abilities, renders them the chance to avoid contaminated areas where soil habitat function has been affected (Reinecke et al. 2002; Yeardley et al. 1996).
As a matter of fact, the avoidance behaviour of earthworms has been defended as an ecologically relevant endpoint (e.g. Hund-Rinke and Wiechering 2001; Amorim et al. 2005) to be used as an indicator of soil quality in a sublethal test—the earthworm avoidance test (ISO 2005). The advantages of avoidance tests rely on their short duration and reduced effort comparative to the acute or chronic tests, being generally more sensitive than the acute tests, while, according to some authors, they respond similarly to the reproduction tests (Achazi 2002; Garcia et al. 2008; Hund-Rinke and Wiechering 2001; Hund-Rinke et al. 2005; Yeardley et al. 1996).
Earthworms have been demonstrated to avoid soils contaminated with pesticides, mainly with fungicides (e.g. Garcia et al. 2008; Natal-da-Luz et al. 2008; Zhou et al. 2007) and insecticides (e.g. Reinecke and Reinecke 2007), but there is not much published information about detrimental impacts triggered by herbicide applications on behavioural endpoints. In spite of this, large quantities of herbicides are used worldwide and, in 2002, they represented ca. 35% of the pesticides used in Europe (ECPA 2003). Although herbicides are not to be designated for the control of animal pests, the bioavailability of their residues in the soil matrix may threaten the maintenance of earthworm and other soil invertebrates.
On these grounds, the aim of the present study was to assess the effects of two herbicide active ingredients (a.i.)—sulcotrione and penoxsulam—and their respective commercial formulations—MIKADO® and VIPER® (hereinafter referred to as Mikado and Viper)—on the avoidance behaviour of Eisenia andrei. In an attempt to enhance the ecological relevance of the generated toxicity data, the avoidance tests were run with standard (LUFA 2.2; L) and agricultural [from corn (C) and rice (R) fields] natural soils.
This work makes part of a more comprehensive study, concerning an agricultural area intensively exploited for corn and especially rice production, in which Mikado and Viper are applied, respectively. They are relatively new herbicides on the European market (Meazza et al. 2002; Bird et al. 2006) and the related, available ecotoxicological studies are scarce, as far as authors are aware. Although the registration process complies with the evaluation of the active ingredient and the ‘lead formulation’ (EEC 1991), the available ecotoxicological information of the commercialised pesticides relies mainly on the acute effects induced by the a.i.s. According to Tominack (2000) and Cox and Surgan (2006), the toxicity of adjuvants added to pesticide formulations is often more toxic to non-target living organisms than the a.i.; what strengthens these formulations should be carefully assessed and the data communicated. Therefore, it is quite noteworthy to compare and produce toxicity data based on rapid sublethal endpoints that could additionally provide a more ecologically sound outcome of potential damages on non-target organisms. On the other hand, the present work will contribute to enlarge the terrestrial ecotoxicological database, which is considerably poor and needs urgent updates for the derivation of soil quality thresholds that are useful for the protection of terrestrial ecological receptors (O’Halloran 2006).
3 Materials and methods
3.1 Test organisms
The epigeic earthworm E. andrei (Lumbricidae) was bred in large plastic boxes containing a mixture of horse manure, dried leaves and potting soil as substrate, which was regularly moistened and monitored for pH levels. The culture was maintained at temperature 20 ± 2°C and photoperiod 16L:8D. One day prior to the beginning of the test, adult worms presenting developed clitella with an average weight of 300–600 mg, were selected and kept in the pre-moistened standard soil L for acclimatisation.
3.2 Soils
In the present study, two natural soils and one standard natural soil (hereinafter referred to as standard soil) were used. The natural soils were collected in the 0–20-cm soil surface layer from corn (C) and rice (R) fields, before the cropping season to guarantee that there was no recent input of pesticides. These fields are integrated in a wide area extensively exploited for agriculture in the lower Mondego river valley, which is located in the centre of Portugal (40°2′N, 8°43′W). In the laboratory, both soils were air-dried, homogenised and sieved (2-mm mesh) before their characterization and the performance of avoidance tests.
The standard soil used was LUFA 2.2 (commercially available at Agricultural Research Centre, Speyer, Germany). In temperate regions, this European soil is widely accepted as a suitable and reference soil for conducting ecotoxicological assays with invertebrates (Løkke and van Gestel 1998), namely avoidance tests (Garcia et al. 2008). The physico-chemical characterization of L soil shown in Table 1 was provided by Agricultural Research Centre, Speyer, Germany.
Relative to the characterization of natural soils, ten replicates were used to measure the pH (H2O) (FAOUN 1984), pH (KCl) (ISO 2005), conductivity (FAOUN 1984) and organic matter content (OM) (SPAC 2000). The pH (H2O or KCl) and the conductivity were determined in a soil suspension of 1:5 (w/v) soil:water (or KCl 1 M). After 30 min of shaking thoroughly, the suspension was left to rest for 1 h before measuring the pH of the overlying solution with a WTW 330/SET pH metre. On the day after, the conductivity was recorded with the WTW LF/330 metre. The OM content of each replicate was obtained by ignition loss at 450°C during 8 h. The maximum water-holding capacity (WHC; ISO 2005) was determined in three replicates for each soil type. Soil samples were introduced in plastic vessels and immersed in tap water for 3 h. Afterwards, they were drained for 2 h, weighed, dried at 105°C until the weight was stabilised, and re-weighed again to obtain the WHC. The particle size distribution was determined in one replicate of each soil type (FAOUN 1984). All samples were pre-treated with hydrogen peroxide to destroy OM, and then mixed with a sodium hexametaphosphate solution to enable particle desegregation. The different fractions were separated via mechanical shaking and the use of different pore sieves (2 mm, 1 mm, 500 μm, 250 μm, 125 μm and <63 μm), although only the clay/silt content (<63 μm) will be shown. The whole physico-chemical characterization procedure is further described by Pereira et al. (2008).
3.3 Chemicals
Mikado, marketed in Europe by Bayer CropScience, is a foliar-applied post-emergence herbicide mostly used in corn crops through terrestrial application, for the control of broadleaf weeds and annual grasses (ter Halle et al. 2006). Mikado is produced as a concentrated suspension containing 300 g a.i. L-1, being its recommended rate of application of 1.5–2 L ha-1. Its a.i. is sulcotrione, a 2-benzoylcyclohexanodione from the triketone class of compounds, whose mode of action relies on the inhibition of the enzyme p-hydroxyphenylpyruvate dioxygenase (HPPD) (Chaabane et al. 2007; Matringe et al. 2005). In plants, HPPD is involved in the biosynthesis of plastoquinones and vitamin E. Plastoquinones are important components of the chloroplastic electron-transfer chain at the photosystem II, being also critical cofactors for phytoene desaturase—an enzyme involved in the biosynthesis of carotenoid. As such, the inhibition of HPPD contributes to the bleaching of plants, due to carotenoid depletion and consequent destabilisation of the photosynthetic apparatus, followed by necrosis and death. Sulcotrione presents a water solubility of 165 mg L-1 (25°C) and its degradation rates in soil vary between 15–74 days in the laboratory and 1–11 days in the field (Tomlin 2000). Sulcotrione K oc values range between 44 (high pH, sandy clay loam soil type) to 940 (low pH, sandy soil type; Tomlin 2000). The analytical grade compound (CAS no. 99105-77-8) needed for the avoidance tests with the a.i. was provided by Bayer CropScience, Monheim, Germany. The WHO (World Health Organisation) classified sulcotrione as moderately hazardous (Bayer CropScience 2004).
Viper (Dow AgroSciences) is also a post-emergence herbicide, though it is applied in rice fields via terrestrial or aerial spraying, for the control of annual grasses, sedges, and broadleaf weeds (Roberts et al. 2003). Its formulation type is oil dispersible, containing 2.14% XDE-638 and 97.86% of other ingredients, including an adjuvant that has methanol (information provided by Dow AgroSciences fact sheet). Viper is applied at a rate of 2–2.5 L ha-1. The a.i. of Viper is penoxsulam ([20.4 g a.i. L-1), a triazolopyrimidine sulfonamide compound, which acts as an acetolactate synthase (ALS) inhibitor, targeting the biosynthesis of branch-chained amino acids (valine, leucine, isoleucine), a metabolic pathway found in fungi, microorganisms and plants (Roberts et al. 2003; Jabusch and Tjeerdema 2005). Thereby, ALS inhibition decreases protein and enzyme synthesis, resulting in a rapid cessation of organism growth. The solubility of penoxsulam in water is pH-dependent [0.0057 g L-1 at pH 5, 0.41 g L-1 at pH 7 and 1.46 g L-1 at pH 9 (all at 19°C)], and its K oc is 104 (Roberts et al. 2003). Penoxsulam soil half-life varies between 2–118 days, depending on the degradation pathway (U.S. EPA 2007). According to WHO (2005), penoxsulam is unlikely to present acute toxicological hazards under normal use. Analytical standard samples of penoxsulam (CAS no. 219714-96-2) were obtained from Dow AgroSciences LLC.
3.4 Avoidance tests
Following the procedures established by ISO (2005), the avoidance tests were carried out in two-chamber glass recipients (area = 0.026 m2), which were separated by a card divider, before the introduction of 200 g dry soil into the control (left side) and test (right side) sections (either contaminated with pesticide or not—as in the case of testing a natural soil for its habitat function quality, based on its intrinsic properties). Afterwards, soil water content was adjusted to 40% of the WHC (previously determined as described above) with distilled water for the standard soil L, and to 27 and 28% for R and C soils, respectively. The latter moisten percentages were lower, since the natural soils were too clayed to retain more water without compromising earthworm maintenance. Ten earthworms, previously washed and dried with absorbent paper were then placed in the line dividing the two sections, after withdrawing the card divider. Finally, the recipients were wrapped with a transparent and perforated plastic cover, being left for 48 h under the same conditions as the breeding cultures. After that period, the control and test soils were separated and the number of earthworms in each section was counted as described in ISO (2005). Two validity criteria were assured for the correct performance of the avoidance tests: (a) random distribution of earthworms on both sections of the recipient test when filled with the same uncontaminated soil, (b) no mortality (Hund-Rinke and Wiechering 2001).
3.4.1 Dual-control tests and habitat function of natural soils
The use of avoidance as an endpoint assumes that earthworms are randomly distributed in the two sections of the testing recipient containing the same soil type (Hund-Rinke and Wiechering 2001; Yeardley et al. 1996). Thereby, in an attempt to validate this criterion, dual-control tests were performed with ten replicates for each natural soils C and R, testing the same uncontaminated soil type in both sections. Since L is considered a reference soil, it was assumed that earthworms presented a random and homogeneous distribution under such conditions.
Additionally, the habitat function provided by the natural soils coming from the rice and corn fields was tested as well, against the standard soil L (i.e. L vs. C, and L vs. R), and against one another (i.e. R vs. C). Ten replicates were used as well for each test combination. The evaluation of natural soils’ habitat function will allow one to ascertain if their pedological characteristics per se constrain earthworm’s maintenance. Whenever a natural soil was significantly avoided by earthworms, it was not used for the subsequent ecotoxicological assessment of pesticides, as long as the reduced habitat function of the natural soil could mask earthworm behaviour to pesticide effects.
3.4.2 Toxicity of active ingredients and formulated herbicides
The soils used as substrates for the avoidance assays with pesticides were the standard soil L and the natural soil R, as neither of them was significantly avoided by earthworms (c.f. results’ section). Overall, their spiking was done by thoroughly mixing the test solution with one batch of soil, before introducing it into the test vessel (ISO 2005). Before placing the earthworms in the test vials, the testing solution was led to equilibrate in soil matrix for 1–2 h. Four replicates were carried out for each tested concentration.
The avoidance tests conducted with the a.i.s were only performed on L soil for the concentrations 100.0, 250.0, 500.0, 750.0, 1,000.0 mg sulcotrione kg-1 and 3.00, 15.0, 30.0, 60.0, 100 mg penoxsulam kg-1. Before the test started, test solutions were individually prepared for each concentration. The respective quantities of sulcotrione were dissolved in 4 ml acetone (99% purity), thereby enabling the contamination of four replicates per concentration. A negative control was run in our lab (i.e. an avoidance test with LUFA 2.2 contaminated by 1 ml acetone in the test section per replicate), and it was concluded that the solvent was not constraining the earthworms’ response (unpublished data). In turn, each penoxsulam test solution was obtained by dissolving the respective quantity of reagent in distilled water (at pH 9 and 19°C) followed by ultrasonic dispersion, before being mixed in a LUFA 2.2 soil batch.
The tested concentrations for each formulated herbicide were defined according to their recommended application rates, corresponding to 3.96 mg a.i. kg-1 for Mikado and 0.33 mg a.i. kg-1 for Viper. Since no avoidance behaviour was verified at that level, higher nominal concentrations, arranged in a geometric series, were tested: 126.60, 253.21, 506.42, 1012.84, 2025.68 mg a.i. kg-1 for Mikado, and 23.4, 35.1, 52.7, 79.0, 118.5 mg a.i. kg-1 for Viper, respectively. The test solutions were prepared with distilled water in the same way as aforementioned.
3.5 Data analysis
The results of dual-control and soil comparison tests were presented as the average number of earthworms on the test soil per test vessel, for each combination, according to ISO (2005) guidelines. However, a percentage of effect (% avoidance) could be calculated for the testing of chemical contaminants on uncontaminated soils, following the expression: % avoidance = ((E − T) / E) × 100, where E is the expected number of worms in the control soil assuming an homogeneous distribution of earthworms in the test recipient (if N = 10, than E = 5), and T is the average number of worms counted in the test soil per concentration (ISO 2005). Hence, the transformed data could then be used for subsequent statistical analyses, considering negative responses as 0% avoidance.
Notwithstanding, the calculation of an avoidance effect resulting from the testing of chemicals slightly differs in published works, which may cause misunderstandings, e.g. regarding the application of methods for data transformation and its respective interpretation. Some authors (e.g. Amorim et al. 2005; Garcia et al. 2008; Antunes et al. 2008) expressed the avoidance effect of chemical contaminants as the average percentage of net response [i.e. NR = ((C − T) / N) × 100, where C = sum of worms found in the control soil, T = sum of worms found in the test soil, N = total worms per replicate], while Loureiro et al. (2005) calculated avoidance as A = (N−2 × T) / N, where N = number of worms per replicate and T = number of worms in the test soil. In fact, the final outcome is similar to the one obtained with the equation suggested by the guideline (ISO 2005). However, the mathematical reasoning that sustains the ISO % avoidance expression is more coherent with the expected random migration of earthworms through both test sections, which corresponds to the no-avoidance or no-effect situation that is considered as null hypothesis when performing statistical comparison tests.
Two main approaches were used for data assessment: (a) application of a threshold value and (b) statistical analyses. The threshold value-method considers that a test soil presents limited habitat function when >80% of earthworms are in the control soil (or <20% are in the test soil) (Hund-Rinke and Wiechering 2001), which corresponds to >60% avoidance [from the expression suggested by ISO (2005) for the calculation of % avoidance, if N = 10, then (5 − 2) / 5 × 100 = 60%]. This evaluation criterion is a less sensitive approach, in comparison with the statistical one, and this is the reason why it was initially proposed to minimise the influence of different physico-chemical properties between the reference and site contaminated soils on earthworm behaviour (Hund-Rinke et al. 2005). Nevertheless, both methods are often used together, as a potential way of improving the robustness of data interpretation (e.g. Sousa et al. 2008).
Therefore, regarding the statistical approach, different analyses were made. First, a pairwise t-test was conducted in order to compare the number of earthworms in the control and test sections for the dual-control tests and those intended to compare the quality of different soils. Secondly, for the testing of herbicides, a one-way analysis of variance (one-way ANOVA) followed by the post hoc Dunnett’s test (Zar 1996) was used to assess significant differences of the % avoidance values between individual chemical concentrations and the control, for each treatment (the control was considered to be 0% avoidance for L soil and equal to the % avoidance value calculated for dual-control tests carried out with the C and R soils), thereby allowing the determination of NOEC (no-observed effect concentration) and LOEC (low-observed effect concentration) values. Third, and also just for the testing of herbicides, a Probit regression analysis was applied to the % avoidance data in order to determine the effect concentration at a 50% level (EC50) and its respective confidence limits at 95% probability (95%-CL) (Finney 1971). As described in the guideline (ISO 2005), the EC50 of an avoidance test represents 75% of the earthworms in the control section and 25% in the test soil. If the worms distribute randomly in the test vessel (no-effect situation), at the end of the exposure period there will be 50:50% (if N = 10, it will be 5:5 worms) in each side. However, if there is avoidance behaviour and half of the earthworms (i.e. 50% of the five earthworms in the test soil) move from the test soil to the control one, it means that 2.5% or 25% of them avoided staying in the test side, what corresponds to 50% of effect [i.e. ((5 − 2.5)/5) × 100 = 50%].
4 Results
The physico-chemical properties of the standard and natural soils used as substrate tests are described in Table 1. Natural soils C and R presented slightly higher pH (H2O) than L soil, while the latter had the highest conductivity value (57.2 μS cm-1). Since both natural soils are characterised as very clayed soils (53.3 and 43.1% clay/silt for C and R soils, respectively), the recorded OM content (5.33 and 4.52% for C and R soils, respectively) and WHC (107.25 and 109.91% for C and R soils, respectively) are more elevated than those in L soil (4.06 and 48.0%, respectively).
In a general view, both validity criteria were fulfilled for the avoidance tests once no earthworms died and their distribution between the two chambers was approximately 50:50% in the dual-control tests carried out with the natural soils C and R (Fig. 1). As such, there was no significant avoidance behaviour (Table 2) when the same uncontaminated natural soil was placed in each side of the test recipient. However, when assessing the natural soils’ habitat function, the pairwise t-test (see Table 2) pointed out that earthworms had significantly avoided the C soil when tested against L and R soil (i.e. for L-C and R-C combinations), whilst they preferred R soil relatively to the L one (i.e. for L-R combination; see Fig. 1). Since the habitat function of C soil is impacted concerning earthworm maintenance (18.9 and 19.4% earthworms were in the soil C when tested against L and R soils, respectively), it was not considered for further testing of the formulated herbicide applied in corn fields, Mikado, as it could mislead the interpretation of the response of earthworms.
Average number of earthworms in the test soil (the one on the right side of hyphen) for dual-control tests (combinations C-C and R-R) and the comparison of different soils (combinations L-C, L-R and R-C). L (LUFA 2.2), C (corn field soil), R (rice field soil). Error bars represent standard error. Asterisk (*) indicates a significant difference on earthworm distribution between the two sections for each combination, pairwise t-test, P ≤ 0.05
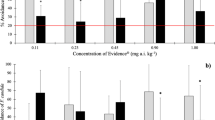
Avoidance tests conducted for the testing of herbicides had generally depicted a positive concentration−effect relationship (Fig. 2 and Fig. 3). In doing so, the LOEC (>1,000 mg a.i. kg-1) and EC50 (1,263.3 mg a.i. kg-1) values determined for the behaviour of earthworms when exposed to sulcotrione were slightly lower relative to those calculated for Mikado exposures (1,012.8 and 1,301.3 mg a.i. kg-1, respectively; Table 3), using the soil L as substrate. Accordingly, the habitat function limit criterion of 60% avoidance was surpassed under the two highest Mikado concentrations (1,012.84 and 2,025.68 mg a.i. kg-1), whereas the % avoidance for sulcotrione was always below that limit.
Average percentage of E. andrei avoidance response under different concentrations of the (a) active ingredient sulcotrione and the (b) formulated herbicide Mikado, on standard soil LUFA 2.2. Error bars represent standard error. Asterisk (*) indicates a significant avoidance response, one-way ANOVA, P ≤ 0.05
Average percentage of E. andrei avoidance response under different concentrations of the (a) active ingredient penoxsulam and the (b) formulated herbicide Viper on LUFA 2.2, and of the (c) formulated herbicide Viper on the natural rice field soil. Error bars represent standard error. Asterisk (*) indicates a significant avoidance response, one-way ANOVA, P ≤ 0.05
Avoidance tests with penoxsulam on L soil resulted in a significant % avoidance under the highest tested concentration (see Table 3, Fig. 3a), being the LOEC of 100 mg a.i. kg-1 and the EC50 of 80.6 mg a.i. kg-1, which were higher than those point estimates calculated for Viper (LOEC = 52.7 and EC50 = 51.5 mg a.i. kg-1), although the EC50s were within the same range (see Table 3). The concentrations of penoxsulam and Viper that had induced significant avoidance response coincided with the ones inducing a limited habitat function of the respective soils, according to the Hund-Rinke and Wiechering (2001) criterion. Regarding the testing of Viper in R soil (see Fig. 3c), the % avoidance was significantly enhanced when earthworms were exposed to the two highest concentrations (see Table 3, Fig. 3c; LOEC = 79.0 mg a.i. kg-1 and EC50 = 56.9 mg a.i. kg-1; see Table 3), being the habitat function of the test soil impaired (i.e. % avoidance was >60%) for the same concentrations. Thereby, though within the same range, the toxicity of Viper on L soil was slightly higher than that verified in the avoidance tests conducted with R soil as substrate (see Fig. 3b vs. c). This was supported by the lower values calculated for the point estimates in the former set-up—L soil contaminated with Viper (see Table 3). Overall, earthworms depicted more elevated % avoidance under lower concentrations of penoxsulam or Viper than those of sulcotrione or Mikado (see Figs. 2 and 3, Table 3).
5 Discussion
The first part of this study attempted on the evaluation of the role of intrinsic physical and chemical properties of natural soils on their habitat function. In fact, the pedological properties such as texture, pH, and OM content can present a wide range between different natural soils (Jänsch et al. 2005). Therefore, the individual soil properties must be considered when natural soils are used, as well as their suitability as a habitat by earthworms, must be ascertained prior to testing (Edwards and Bohlen 1996).
At the light of the obtained results, the dual-control avoidance tests evidenced a random distribution of organisms either for C or R soils. However, when the habitat function of both natural soils was tested against that of L soil, dissimilar responses were shown by earthworms. While the R soil was significantly preferred by them, the C soil was significantly avoided (see Fig. 1), evidencing the limited habitat function of the latter. Considering that the pH measured in the three soils is within the preferred range for E. andrei and that this species optimally choose soils with very high OM content (Jänsch et al. 2005) as is seemingly the case of C and R soils, the dissimilar response of earthworms could be related to different intrinsic pedological properties of soils. Some authors (e.g. Natal-da-Luz et al. 2004) had already pointed out that the quality of organic and inorganic fractions of soil may constrain the avoidance behaviour of earthworms. Along with the OM levels, the extremely high silt/clay content of natural soil samples may also compromise the response of earthworms (Jänsch et al. 2005), albeit only in C soil could it act as a combined effect contributing for the decrease of its habitat function. As a result, the C soil was not used for further testing with chemicals to prevent masking effects of pesticides on the avoidance behaviour of earthworms with those constrained by soil properties.
As such, this study strengthens that L soil is obviously not representative of all conditions entailed by different natural soil characteristics, once earthworms preferred the natural soil R. Consequently, the use of a single standard natural soil per se, like L, though allowing data reproducibility and comparison between laboratories, it will somehow provide a rough and inaccurate assessment of soil contamination effects as far as it may not estimate overall field scenarios (Amorim et al. 2005; Jänsch et al. 2005). As aforementioned, in order to increase the ecological relevance of the performed avoidance assays with pesticides, the R soil was also used as a substrate to test the toxicity of the formulated compound Viper.
In general, earthworms showed an avoidance response to soils contaminated with a.i. and formulated herbicides, which trend assumed a positive concentration−effect relationship. Besides, it was noticeable that the a.i.s were generally less repellent than the respective formulated compound (see Figs. 2 and 3). Notwithstanding, the tested concentrations were far above the recommended application rates of pesticide, meaning therefore, that they would not have a negative impact under realistic situations, while considering the avoidance response of this particular species. Actually, this was already expected considering that herbicides, though biologically active, they are not designed to affect animal species. Consequently, their impairments are likely to occur at higher concentrations than those corresponding to the prescribed spraying rate.
Mikado had significantly constrained the habitat function of L soil for earthworms’ at the two highest tested concentrations (see Fig. 2b). As a matter of fact, these concentrations are beyond the range suggested for the testing of chemicals (ISO 2005), thus the risk represented by this formulated herbicide on E. andrei avoidance behaviour is quite low. Similarly, sulcotrione did not represent a risk for earthworm maintenance under concentrations up to the test limit of 1,000 mg a.i. kg-1 (see Fig. 2a, Table 3). Available data indicate a LC50 of 1,000 mg a.i. kg-1 (FOOTPRINT PPDB 2008) for acute exposures of earthworms to sulcotrione. Nevertheless, the soil used as substrate in the tests was not specified. As so, in this situation, the avoidance test was apparently as sensitive as the acute assay with earthworms.
Penoxsulam and its respective formulated product, Viper, induced stronger avoidance behaviour on E. andrei than sulcotrione and Mikado, since their avoidance-EC50s were remarkably lower (see Table 3). E. andrei was able to detect the presence of penoxsulam and avoid the contaminated L soil at an EC50 (see Table 3) that was at least one order of magnitude lower than the acute-LC50 value (>1,000 mg a.i. kg-1) determined for E. fetida (Dow AgroSciences—Penoxsulam Technical Herbicide Safety Data Sheet), when exposed to the same chemical (the substrate was not specified). On these grounds, the avoidance response seemed to be more sensitive than the acute toxicity endpoint for penoxsulam, although such interpretations should be cautiously taken, since different species and soils were used. Anyway, the apparently higher sensitivity of avoidance tests relatively to the acute ones has been extensively supported by other authors, along with the reduced ecological relevance of acute earthworm test, and its limited ability to predict or give an early warning of contaminant effects with low costs and effort evolved (Vermeulen et al. 2001; Hund-Rinke et al. 2005; Garcia et al. 2008).
Comparing the effects induced by a.i. vs. formulated product, Mikado and sulcotrione showed similar effects on earthworm behaviour. However, Viper constrained the habitat function of the test soil at lower LOEC (52.7 and 79.0 mg a.i. kg-1, for L and R soils, correspondingly) than penoxsulam (LOEC = 100 mg a.i. kg-1). Thereby, the behaviour of earthworms was slightly less affected when subjected to the a.i. penoxsulam (EC50 = 80.6 mg a.i. kg-1) than to the formulated herbicide—Viper—applied on rice fields (EC50: 51.5 and 56.9 mg a.i. kg-1 on L and R soil, respectively). Indeed, there are published studies indicating that adjuvants, which are added to pesticide formulations as a way to enhance their efficacy, may be responsible for the increased toxicity of the a.i. to certain non-target species (e.g. Tsui and Chu 2003; Cox and Surgan 2006). Although adjuvants are usually omitted from product labels or simply identified as ‘inert ingredients’, they are biologically or chemically active, and hence able to affect ecological receptors per se (Cox and Surgan 2006). Thus, focusing the ecotoxicological profile of new or existing agrochemicals on their a.i. may underestimate the actual toxicity of the formulated product. Our results reinforced the need for a careful assessment of the impacts of formulated products, as it is already established by the regulatory European documents (e.g. EEC 1991). Moreover, this is especially required for the terrestrial compartment so as to fulfil the huge lack of information available for this ecosystem.
Comparing the behaviour of E. andrei under different soils contaminated with Viper, a smooth sensitivity difference was observed. Smaller LOEC and EC50 values were depicted under L soil than under R soil (see Fig. 3a and b, Table 3). This outcome could be related with the higher OM and clay/silt content determined in R soil, what may constrain pesticide bioavailability (Ying and Williams 2000). Accordingly, Jabusch and Tjeerdema (2005) observed that the soil sorption of penoxsulam occurs both to OM and clay mineral sorption sites. In fact, it is likely that the pedological properties of natural soils may reduce the bioavailability of pesticides (e.g. EC 2003; Römbke et al. 2005; Farenhorst 2006; Garcia et al. 2008), though the properties of some reference standard soils could also be responsible to even lower pesticide bioavailability levels (e.g. artificial soils with higher OM content like the OECD soil). As such, it involves strengthening the use of standard and natural soils in avoidance tests for the testing of chemicals, as a way to achieve robust and feasible responses more closely related with field conditions and its potential overwhelmed impacts (O’Halloran 2006).
Overall, while focusing on indirect effects of stress factors (e.g. chemicals), the avoidance behaviour of earthworms is very promising as a short-term sublethal predictor of detrimental effects on ecosystem functioning and structure associated to the disappearance of earthworms, which play a major role as soil engineers (Reinecke et al. 2002; Smith et al. 2006). Notwithstanding, the detected concentrations by earthworms were beyond the application rates, which suggests that the risk of these pesticides to edaphic fauna will be low, if application rates are respected. Hence, the avoidance test was seemingly a useful preliminary assessment tool for the tested pesticides. Although some authors refer that the avoidance tests are as sensitive as the reproduction one, the latter should still be performed for the studied herbicides, since a different outcome may be retrieved. Indeed, the reproduction response is based on physiological effects that are not addressed by a behavioural endpoint.
6 Conclusions
Although LUFA 2.2 is a standard reference soil that has been used for sake of reproducibility and interlaboratorial comparison of tests, this study reinforced that other natural soils should be added for the assessment of chemicals, as the former would never cover all properties entailed by different soils. Regarding the tested herbicides, sulcotrione and Mikado affected the behaviour of earthworms in much less extent than penoxsulam and Viper. On the other hand, the soil contaminated with penoxsulam was avoided in less extent than that contaminated with the formulated herbicide Viper. Such occurrence was possibly related to the increased toxicity represented by the adjuvants added to the commercial products. Additionally, E. andrei behaviour was more affected under L soil contaminated with Viper than under R soil, what could rely on the potentially lower bioavailability of the pesticide on the latter substrate probably due to its high OM and clay contents. The tested concentrations, however, were beyond the application rates, which suggests that the risk of these pesticides to edaphic fauna will be low if application rates are respected. Overall, avoidance tests provided a valuable response either to compare the habitat function of different standard and agricultural natural soils or to test the effect of herbicides.
7 Recommendations and perspectives
An effort should be taken to enlarge the terrestrial ecotoxicological database, namely through the use of different natural soils, as a way to fulfil the huge lack of information available for this ecosystem. Yet, it will also improve the ecological relevance of pesticide assessment on soil environmental compartment, as far as the bioavailability of chemicals would be additionally integrated. In this context, additional research congregating a potential linkage between physiological activities sustaining the regular metabolism of earthworms and their avoidance behaviour or even their reproductive effects would be welcomed, especially in what regards formulated pesticides. Such approach would provide a robust and comprehensive understanding of chemical effects and would enhance the knowledge behind the avoidance response.
References
Achazi RK (2002) Invertebrates in risk assessment: development of a test battery and of short term biotests for ecological risk assessment of soil. J Soils Sediments 2(4):174–178
Amorim MJB, Römbke J, Soares AMVM (2005) Avoidance behaviour of Enchytraeus albidus: effects of benomyl, carbendazim, phenmediphan and different soil types. Chemosphere 59:501–510
Antunes SC, Castro BB, Pereira R, Gonçalves F (2008) Contribution for tier 1 of the ecological risk assessment of Cunha Baixa uranium mine (Central Portugal): II. Soil ecotoxicological screening. Sci Total Environ 390(2–3):387–395
Bayer CropScience (2004) MIKADO Safety Data Sheet according to Directive EEC 2001/58. Online at http://www.bayercropscience.pt
Bird J, Beer A, Guthrie L (2006) Products: new product pipelines slim down. In: Jarvis P (ed) Agrow Magazine, Issue 1. Leverkusen, Germany, p 44
CEC (Commission of the European Communities) (2006) Proposal for a Directive of the European Parliament and of the Council Establishing a Framework for the Protection of Soil and Amending Directive 2004/35/EC. COM(2006) 232 final, 2006/0086 (COD), Brussels
Chaabane H, Vulliet E, Joux F, Lantoine F, Conan P, Cooper JF, Coste CM (2007) Photodegradation of sulcotrione in various aquatic environments and toxicity of its photoproducts for some marine micro-organisms. Water Res 41(8):1781–1789
Cox C, Surgan M (2006) Unidentified inert ingredients in pesticides: implications for human and environmental health. Environ Health Persp 114(12):1803–1806
EC (European Commission) (2002) Guidance Document on Terrestrial Ecotoxicology Under Council Directive 91/414/EEC. Draft Working Document. European Commission, Health & Consumer Protection Directorate-General. SANCO/10329/2002, rev. 2 final, 17 October 2002, 39 pp
EC (European Commission) (2003) Technical Guidance Document on Risk Assessment in support of Commission Directive 93/67/EEC on Risk Assessment for new notified substances, Commission Regulation (EC) No 1488/94 on Risk Assessment for existing substances, Directive 98/8/EC of the European Parliament and of the Council concerning the placing of biocidal products on the market. Part II., Joint research Centre, Ispra
ECPA (European Crop Protection Association) (2003) ECPA Statistical Review 2002. D/03/EJ/12560, Brussels, 31 pp
Edwards CA, Bohlen PJ (1996) Biology and ecology of earthworms. Chapman and Hall, London
EEC (European Environment Commission) (1991) Council directive no. 91/414/EEC concerning the placing of plant protection products on the market. Official Journal of the European Union L230 of 19.08.1991:1–32
FAOUN (Food and Agriculture Organization of the United Nations) (1984) Physical and chemical methods of soil and water analysis. Soils Bull 10:1–275
Farenhorst A (2006) Importance of soil organic matter fractions in soil-landscape and regional assessments of pesticide sorption and leaching in soil. Soil Sci Soc Am J 70:1005–1012
Finney DJ (1971) Probit analysis. Cambridge University Press, pp 333
FOOTPRINT PPDB (The FOOTPRINT Pesticide Properties Database) (2008) FOOTPRINT: creating tools for pesticide risk assessment and management in Europe. Research Project in the 6th Framework Program for Research and Technological Development. [Online]. Retrieved on August from: http://sitem.herts.ac.uk/aeru/footprint/en/index.htm
Garcia M, Römbke J, de Brito MT, Scheffczyk A (2008) Effects of three pesticides on the avoidance behavior of earthworms in laboratory tests performed under temperate and tropical conditions. Environ Pollut 153(2):450–456
Gerrard J (2000) Fundamentals of soils. Routledge Fundamentals of Physical Geography, Routledge, Taylor & Francis Group, London, Great Britain, 230 pp
Hund-Rinke K, Wiechering H (2001) Earthworm avoidance test for soil assessments—an alternative for acute and reproduction tests. J Soil Sediments 1(1):15–20
Hund-Rinke K, Lindemann M, Simon M (2005) Experiences with novel approaches in earthworm testing alternatives. J Soils Sediments 5(4):233–239
ISO (International Organization for Standardization) (2005) Draft ISO-17512: Soil Quality and Avoidance test for evaluating the quality of soils and the toxicity of chemicals. Test with earthworms (Eisenia fetida/andrei). Geneve, Switzerland
Jabusch TW, Tjeerdema RS (2005) Partitioning of penoxsulam, a new sulfonamide herbicide. J Agric Food Chem 53:7179–7183
Jänsch S, Amorim MJ, Römbke J (2005) Identification of the ecological requirements of important terrestrial ecotoxicological test species. Environ Rev 13:51–83
Lavelle P, Decaëns T, Aubert M, Barot S, Blouin M, Bureau F, Margerie P, Mora P, Rossi J-P (2006) Soil invertebrates and ecosystem services. Eur J Soil Biol 42:S3–S15
Løkke H, van Gestel CAM (1998) Handbook of soil invertebrate toxicity tests. Wiley, Chichester, UK
Loureiro S, Soares AMVM, Nogueira AJA (2005) Terrestrial avoidance behaviour tests as screening tool to assess soil contamination. Environ Pollut 138(1):121–131
Matringe M, Sailland A, Pelissier B, Rolland A, Zink O (2005) p-Hydroxyphenylpyruvate dioxygenase inhibitor-resistant plants. Pest Manag Sci 61:269–276
Meazza G, Scheffler BE, Tellez MR, Rimando AM, Romagni JG, Duke SO, Nanayakkara D, Khan IA, Abourashed EA, Dayan FE (2002) The inhibitory activity of natural products on plant p-hydroxyphenylpyruvate dioxygenase. Phytochemistry 59:281–288
Muthukaruppan G, Janardhanan S, Vijayalakshmi GS (2005) Sublethal toxicity of the Herbicide Butachlor on the earthworm Perionyx sansibaricus and its histological changes. J Soils Sediments 5(2):82–86
Natal-da-Luz T, Ribeiro R, Sousa JP (2004) Avoidance tests with Collembola and earthworms as early screening tools for site-specific assessment of polluted soils. Environ Toxicol Chem 23(9):2188–2193
Natal-da-Luz T, Amorim M, Römbke J, Sousa JP (2008) Avoidance tests with earthworms and springtails: Defining the minimum exposure time to observe a significant response. Ecotox Environ Saf, doi:10.1016/j.ecoenv.2007.09.005
O’Halloran K (2006) Toxicological considerations of contaminants in the terrestrial environment for ecological risk assessment. Hum Ecol Risk Assess 12:74–83
Pereira R, Antunes SC, Marques S, Gonçalves F (2008) Contribution for tier 1 of the ecological risk assessment of Cunha Baixa uranium mine (Central Portugal): I Soil chemical characterization. Sci Total Environ 390:377–386
Reinecke SA, Reinecke AJ (2007) The impact of organophosphate pesticides in orchards on earthworms in the Western Cape, South Africa. Ecotox Environ Saf 66:244–251
Reinecke AJ, Maboeta MS, Vermeulen LA, Reinecke SA (2002) Assessment of lead nitrate and mancozeb toxicity in earthworms using the avoidance response. Bull Environ Contam Toxicol 68:779–786
Roberts DW, Knuteson JA, Jackson R (2003) The dissipation of penoxsulam in flooded rice fields. In: Pesticides in Air, Plant, Soil & Water Systems: XII Symposium Pesticide Chemistry, Institute of Agricultural and Environmental Chemistry of the Catholic University, Piacenza, Italy, pp 349–357
Römbke J (2006) Tools and techniques for the assessment of ecotoxicological impacts of contaminants in the terrestrial environment. Hum Ecol Risk Assess 12:84–101
Römbke J, Jänsch S, Didden W (2005) The use of earthworms in ecological soil classification and assessment concepts. Ecotox Environ Saf 62:249–265
Smith R, Pollard SJT, Weeks JM, Nathanail CP (2006) Assessing significant harm to terrestrial ecosystems from contaminated land. Soil Use Manage 21:527–540
Sousa A, Pereira R, Antunes SC, Cachada A, Pereira E, Duarte AC, Gonçalves F (2008) Validation of avoidance assays for the screening assessment of soils under different anthropogenic disturbances. Ecotox Environ Saf 71:661–670
SPAC (Soil and Plant Analysis Council) (2000) Handbook of reference methods. CRC, Boca Raton, Florida
ter Halle A, Drncova D, Richard C (2006) Phototransformation of the herbicide sulcotrione on maize cuticular wax. Environ Sci Technol 40(9):2989–2995
Tominack RL (2000) Herbicide formulations. Clin Toxicol 38(2):129–135
Tomlin CDS (2000) The pesticide manual, 12th edn. British Crop Protection Council, Surrey
Tsui MTK, Chu LM (2003) Aquatic toxicity of glyphosate-based formulations: comparison between different organisms and the effects of environmental factors. Chemosphere 52(7):1189–1197
U.S. EPA (U.S. Environmental Protection Agency) (2007) Penoxsulam. Human Health Risk Assessment for Proposed Uses on Fish and Shellfish. PC Code: 119031, Petition no. 5F7012, DP Num: 325461. U.S.EPA Office of Prevention, Pesticides and Toxic Substances, Washington, DC 20460
Vermeulen LA, Reinecke AJ, Reinecke SA (2001) Evaluation of the fungicide manganese-zinc ethylene bis(dithiocarbamate) (Mancozeb) for sublethal and acute toxicity to Eisenia fetida (Oligochaeta). Ecotox Environ Saf 48:183–189
WHO (World Health Organization) (2005) The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification. International Program on Chemical Safety, NLM Classification: WA 240, WHO Library Cataloguing-in-Publication Data, Geneva, Switzerland
Yeardley RB Jr, Lazorchak JM, Gast LC (1996) The potential of an earthworm avoidance test for evaluation of hazardous waste sites. Environ Toxicol Chem 15(9):1532–1537
Ying G-G, Williams B (2000) Laboratory study on the interaction between herbicides and sediments in water systems. Environ Pollut 107:399–405
Zar JH (1996) Biostatistical Analysis, 3rd edn. Prentice-Hall, Inc., USA, p 662
Zhou S-P, C-q Duan, Fu H, Chen Y-H, Wang X-H, Yu Z-F (2007) Toxicity assessment for chlorpyrifos-contaminated soil with three different earthworm test methods. Journal of Environmental Sciences 19:854–858
Acknowledgments
We thank Bayer CropScience and Dow AgroSciences LLC for the free supply of the technical ingredients sulcotrione and penoxsulam, respectively. Furthermore, we still would like to thank Dow AgroSciences LLC for the provided formulated product Viper. CR Marques was supported by a PhD grant from FCT (Fundação para a Ciência e Tecnologia).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marques, C., Pereira, R. & Gonçalves, F. Using earthworm avoidance behaviour to assess the toxicity of formulated herbicides and their active ingredients on natural soils. J Soils Sediments 9, 137–147 (2009). https://doi.org/10.1007/s11368-009-0058-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-009-0058-0