Abstract
Background
Claudins are tight junction (TJ) proteins, and the relationship between the level of expression and localization of TJ protein, and tumor aggressiveness in early gastric cancer (GC) is still far from clear.
Aims
To investigate the expression of claudins and Ki-67 in early GC cells and surrounding normal gastric mucosa.
Methods
A total of 53 early GC lesions removed via endoscopic mucosal resection or endoscopic submucosal resection were evaluated. All of the GCs were characterized as well to moderately differentiated adenocarcinoma. The labeling index (LI) of Ki-67 was calculated for each sample. To assess the prevalence of epithelial TJs, immunofluorescent staining for claudin-3, claudin-4, and claudin-7 was performed. The immunoreactivity was graded according to the percentage of stained cells.
Results
Claudin-3, claudin-4, and claudin-7 expression at TJs in GC and intestinal metaplasia were significantly higher than that in gastric mucosa with no intestinal metaplasia. The Ki-67 LI of GC specimens was inversely correlated with claudin-3 expression, but not with claudin-4 or claudin-7 expression. Claudin-3 expression was significantly lower at the submucosal invasive front of GCs.
Conclusions
The down-regulation of claudin-3 was associated with the proliferative potential of GC cells, indicating that claudins may have a pivotal role in the progression of GC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is one of the most common malignant tumors worldwide [1]. Most intramucosal GCs and some submucosal GCs can be treated and controlled by endoscopic mucosal resection (EMR) or endoscopic submucosal resection (ESD). After ESD was introduced, en bloc resection of early GC improved the rate of curative resection and lead to more accurate diagnoses of invasiveness [2].
Tight junctions (TJs) are expressed on the apical end of the lateral membrane surface and form the epithelial barrier against paracellular transport; moreover, they maintain epithelial cell polarity via their fence function [3]. Changes in the expression of TJ proteins are characteristic of many human diseases, including cancer. Among the TJ proteins, claudins are the most important structural and functional components of TJ strands [3]. At present, at least 27 subtypes of claudins have been identified, and these subtypes are expressed in an organ-specific manner and regulate the tissue-specific physiological functions of TJs [3, 4]. Furthermore, claudins are involved in various pathological conditions, including abnormal inflammation [5] and tumor progression [6]. However, many claudins have not been examined in detail, and, therefore, functional differences in this family of proteins may await discovery.
Recent studies indicate that claudin expression is altered in various cancers, including GC. Claudin-3, claudin-4, and claudin-7 are up-regulated in GC [7–9], and the up-regulation of claudin-4 in GC cells is reportedly significantly related to poor prognosis [8]. However, loss of claudin-3 and claudin-4 is also reportedly associated with poor survival in GC [10]. These claudins are detected not only in GC cells, but also in gastric epithelial cells. Therefore, the expression of these proteins does not directly affect the development of GC, or claudins may have roles that are unrelated to TJ formation [9].
The claudin expression at TJs in GC has not been fully assessed, and its significance remains controversial. Differences in the methods used to assess claudin expression may be related to the contrasting results and conclusions of the published studies. Claudins are often found on the lateral side of epithelial cells and this aberrant localization is unlikely to be related to normal TJ function. Claudins are normally detected on the most apical side of epithelial cells when they function as TJ proteins. However, previous studies related to GCs did not consider the subcellular localization of claudins. Furthermore, the relationship between tumor proliferation, and claudin expression and localization has not been fully assessed. Here, we evaluated the expression level of claudins at TJs in GC and surrounding gastric mucosa, and we assessed the correlation between claudin expression and the Ki-67 labeling index (LI, an index of cell proliferation) in GC.
Methods
Patients
A consecutive series of 52 patients with early GC was studied (male/female: 38/14, mean age 71.5 years). GC lesions (n = 53) that had undergone curative EMR or ESD were evaluated. GCs with submucosal invasion (n = 18) were included in this study. Double cancers were detected only in one male patient. All of the GC were classified as well to moderately differentiated adenocarcinoma using the Japanese Classification of Gastric Carcinoma [11]. Patient anonymity was preserved. This study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the institute.
Immunohistochemistry
Samples were fixed with 10% formalin solution and embedded in paraffin. Sections were cut to 3-μm thickness and were stained with hematoxylin and eosin (HE). To assess epithelial proliferation, samples were incubated with Ki-67 monoclonal antibody (Invitrogen, Carlsbad, CA, USA), followed by incubation with biotinylated horse anti-mouse secondary antibody (Vector Laboratories, Burlingame, CA, USA). The streptavidin–biotin complex method (Vector Laboratories) was used to visualize the immunoreactivity. Nuclei were counterstained with hematoxylin.
Immunofluorescent Staining of Junctional Proteins
Rabbit anti-claudin-3 and anti-claudin-7 polyclonal antibodies and mouse anti-claudin-4 monoclonal antibody (Invitrogen) were used. Cy3-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and Alexa 488-conjugated goat anti-mouse IgG (Invitrogen) secondary antibodies were used to visualize immunoreactivity. The specificity of the reaction was tested by incubation with non-immune rabbit serum or mouse IgG (Dako, Glostrup, Denmark).
Scoring of Immunostaining of TJ Proteins and Ki-67
Immunoreactivity was evaluated by two independent observers (T.Ok., X.C.), and both were blinded to the clinicopathological features and clinical outcomes associated with each sample. Non-neoplastic gastric mucosa without intestinal metaplasia (Non-IM) and with intestinal metaplasia (IM) and cancer regions were assessed. Only apical, not lateral, membrane staining of claudins was recorded and analyzed. The immunoreactivity of each claudin was graded using the following criteria: 0, marginal or no staining in less than 5% of the cells; 1+, between 0 and 2+; 2+, moderate to intense staining in more than 50% of the cells. Five high-power fields were analyzed in each region of the cases and the mean score was calculated. All of the sections were scored twice to confirm the reproducibility of the results. The Ki-67 LI was calculated after counting 1,000 cancer cells.
Statistical Analysis
Statistical evaluation was performed using SPSS13.0 (SPSS, Chicago, IL, USA). Correlation analysis was performed by Spearman’s rank correlation coefficient (r s). Multiple comparisons were performed using the Kruskal–Wallis test followed by Scheffe’s test. Single comparisons were performed by the Mann–Whitney test. A P-value < 0.05 was considered to be statistically significant.
Results
Expression of Claudins in Gastric Mucosa and Carcinoma
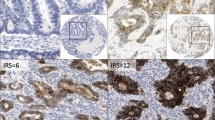
A representative GC case showed that dense claudin-3, claudin-4, and claudin-7 staining was evident at the border of the apical side of gastric epithelial cells in IM and differentiated adenocarcinoma cells, but not in Non-IM (Fig. 1). Claudins were also detected on the lateral side of the epithelial cells in GC, IM, and Non-IM, but this lateral staining was not taken in account for this analysis. The claudin-3, claudin-4, and claudin-7 expression was significantly higher in IM and cancer cells than in Non-IM (Fig. 2). The claudin expression was not related to the sex of the patients.
Immunofluorescent staining of claudin-3, claudin-4, and claudin-7 in gastric cancer (GC) lesions and surrounding gastric mucosa. Hematoxylin and eosin (HE) staining showed gastric mucosa in specimens of non-neoplastic gastric mucosa without intestinal metaplasia (Non-IM), with intestinal metaplasia (IM), and cancer (a, b, c). Claudin-3, claudin-4, and claudin-7 were detected in the gastric mucosa of IM (e, h, k) and cancer (f, i, l) specimens at the border of the apical surface of epithelial cells, but not in the gastric mucosa of Non-IM specimens (d, g, j). White bar = 100 μm, black bar = 50 μm
Correlation of Claudin Expression and Ki-67 LI
A representative case of GC showed that nuclear Ki-67 staining was frequent and intense, and that claudin-3 staining was largely absent (Fig. 3). There was a significant inverse correlation between claudin-3 expression and the Ki-67 LI (r s = −0.328, P = 0.014). However, claudin-4 or claudin-7 expression was not correlated with the Ki-67 LI r s = −0.002, P = 0.627; r s = −0.019, P = 0.779, respectively).
Detection of Claudins in GCs with Submucosal Invasion
The level of claudin expression was compared in mucosal regions and submucosal invasive fronts of GC specimens. In a representative submucosal GC specimen, claudin-3 expression was lower in the submucosal invasive front than in the mucosal regions of GC lesions (Fig. 4a, b). Claudin-3 and claudin-7 expression was significantly lower in the submucosal invasive front than in the mucosal regions of GC specimens (P = 0.003 and P = 0.021, respectively) (Fig. 4c). Claudin-4 appeared to be down-regulated in the submucosal part of GC, but the difference between staining in the submucosal and mucosal regions was not statistically significant (P = 0.072) (Fig. 4c). The claudin-3 expression and claudin-4 expression were significantly associated with one another (r s = 0.418, P = 0.034), but claudin-3 expression and claudin-7 expression were not associated (r s = 0.144, P = 0.773).
The expression of claudins in the mucosal and submucosal regions of GC lesions. a HE and b claudin-3 staining in a case of submucosal invasive gastric cancer. b The level of claudin-3 in the submucosal region (SM) was weaker than that in mucosal regions (M). Bar = 100 μm. c The levels of claudin-3 and claudin-7 was significantly lower in the SM region of GC than that in the M regions (##P = 0.003, #P = 0.021 vs. M, respectively). The level of claudin-4 was not significantly different in the SM and M regions
Discussion
Claudin proteins are thought to play an important role not only at TJs under normal physiological conditions, but also during tumorigenesis [12]. Here, we demonstrate that claudin-3, claudin-4, and claudin-7 expression was up-regulated not only in cancer cells, but also in IM. These findings were consistent with a previous report [13], and the investigators indicated that the expression of these claudins themselves was not the primary cause of GC development. We demonstrated that there is an inverse correlation between the proliferative activity of GC cells assessed by Ki-67 LI and claudin-3, but not claudin-4 or claudin-7, expression. Many studies have demonstrated that Ki-67 staining is positively correlated with more undifferentiated and metastatic cells in many malignancies [14]. Our data indicated that claudin-3 was a more reliable indicator of GC tumor proliferation in GC than claudin-4 or claudin-7. However, further studies on claudin-3 are necessary in order to fully elucidate the role of claudin-3 in GC progression.
We used GC samples resected by EMR and ESD to examine the early events associated with GC proliferation and invasion. Therefore, we focused on the differentiated type of GC that is the most frequent indication for endoscopic treatment. The type of claudins expressed in this type of GC is reportedly different from the types expressed in undifferentiated GC. Loss of claudin expression was found to be more frequently associated with diffuse GC than intestinal GC [6]. However, here, we propose that, even in the differentiated type of GC, claudins expression changes as lesions progress. Claudin-3 and claudin-7 expression at TJs at the invasive front of the regions of GC lesions were significantly lower than those in the mucosal regions. Claudin-4 expression was also lower at the invasive front than in the mucosal regions, but the decrease was not statistically significant. These data indicated that claudin-3, claudin-4, and claudin-7 might be decreased when cancer cells invade the submucosal layers and may be particularly important when considered along with the data showing that the down-regulation of claudin-3 and claudin-4 expression in the invasive front of advanced GC was correlated with more severe malignancy grades [15]. Alteration of the TJ protein expression at TJs may play an important role in the process of dedifferentiation [16] and cell dissociation, which are among the first steps in tumor invasion and metastasis [17]. Claudin-3 and claudin-4 are reportedly not expressed in cancer cells that invade the stroma individually or in clusters of cancer cells [18]. These observations indicate that a decrease in the expression of these proteins is involved in the initial step of cancer invasion of the stroma and vessels. Our data may also be consistent with previous reports which show that the presence of claudin-3 expression in GC is associated with better prognosis and lower incidence of lymphatic invasion [6, 19].
The overexpression of exogenous claudins was recently assessed in order to determine the functions of claudin proteins in cancer progression. Claudin-4 overexpression inhibited cell migration in AGS and Caco-2 cells [20]. Claudin-7 overexpression induced migration, invasion, and enhanced BrdU incorporation. However, the overexpressed claudin-7 did not localize on the cell surface in these experiments [21, 22]. These data indicate that the cytosolic expression of claudin-7 influences cells without affecting the TJ. To date, no study of claudin-3 overexpression has been published. Reduced expression of claudin-3 at TJs may affect the lymph node metastasis, but a limitation of this study is that we have not studied the relationship between lymph node metastasis or prognosis and claudin expression. Therefore, the exact regulation of claudin-3 at TJs in the submucosal invasive front of GC and the exact role of claudin-3 in the development and progression of GC must be elucidated in future studies examining submucosal invasive GC with or without lymph node metastasis or more advanced GC.
In conclusion, the down-regulation of claudin-3 was associated with the proliferative potential of GC, and this finding indicated that claudins may have a pivotal role in the progression of GC.
References
Parkin DM. Epidemiology of cancer: global patterns and trends. Toxicol Lett. 1998;102–103:227–234.
Park YM, Cho E, Kang HY, Kim JM. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc. 2011;25:2666–2677.
Tsukita S, Furuse M. Pores in the wall: claudins constitute tight junction strands containing aqueous pores. J Cell Biol. 2000;149:13–16.
Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Natl Rev Mol Cell Biol. 2001;2:285–293.
Oshima T, Miwa H, Joh T. Changes in the expression of claudins in active ulcerative colitis. J Gastroenterol Hepatol. 2008;23:S146–S150.
Soini Y, Tommola S, Helin H, Martikainen P. Claudins 1, 3, 4 and 5 in gastric carcinoma, loss of claudin expression associates with the diffuse subtype. Virchows Arch. 2006;448:52–58.
Johnson AH, Frierson HF, Zaika A, et al. Expression of tight-junction protein claudin-7 is an early event in gastric tumorigenesis. Am J Pathol. 2005;167:577–584.
Resnick MB, Gavilanez M, Newton E, et al. Claudin expression in gastric adenocarcinomas: a tissue microarray study with prognostic correlation. Hum Pathol. 2005;36:886–892.
Oliveira SS, Morgado-Díaz JA. Claudins: multifunctional players in epithelial tight junctions and their role in cancer. Cell Mol Life Sci. 2007;64:17–28.
Satake S, Semba S, Matsuda Y, et al. Cdx2 transcription factor regulates claudin-3 and claudin-4 expression during intestinal differentiation of gastric carcinoma. Pathol Int. 2008;58:156–163.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma—2nd English edition—response assessment of chemotherapy and radiotherapy for gastric carcinoma: clinical criteria. Gastric Cancer. 2001;4:1–8.
Ouban A, Ahmed AA. Claudins in human cancer: a review. Histol Histopathol. 2010;25:83–90.
Ohtani S, Terashima M, Satoh J, et al. Expression of tight-junction-associated proteins in human gastric cancer: downregulation of claudin-4 correlates with tumor aggressiveness and survival. Gastric Cancer. 2009;12:43–51.
Jalava P, Kuopio T, Juntti-Patinen L, Kotkansalo T, Kronqvist P, Collan Y. Ki67 immunohistochemistry: a valuable marker in prognostication but with a risk of misclassification: proliferation subgroups formed based on Ki67 immunoreactivity and standardized mitotic index. Histopathology. 2006;48:674–682.
Matsuda Y, Semba S, Ueda J, et al. Gastric and intestinal claudin expression at the invasive front of gastric carcinoma. Cancer Sci. 2007;98:1014–1019.
Lee SK, Moon J, Park SW, Song SY, Chung JB, Kang JK. Loss of the tight junction protein claudin 4 correlates with histological growth-pattern and differentiation in advanced gastric adenocarcinoma. Oncol Rep. 2005;13:193–199.
Tan X, Tamori Y, Egami H, et al. Analysis of invasion-metastasis mechanism in pancreatic cancer: involvement of tight junction transmembrane protein occludin and MEK/ERK signal transduction pathway in cancer cell dissociation. Oncol Rep. 2004;11:993–998.
Kamata I, Ishikawa Y, Akishima-Fukasawa Y, et al. Significance of lymphatic invasion and cancer invasion-related proteins on lymph node metastasis in gastric cancer. J Gastroenterol Hepatol. 2009;24:1527–1533.
Jung H, Jun KH, Jung JH, Chin HM, Park WB. The expression of claudin-1, claudin-2, claudin-3, and claudin-4 in gastric cancer tissue. J Surg Res. 2011;167:e185–e191.
Mima S, Tsutsumi S, Ushijima H, et al. Induction of claudin-4 by nonsteroidal anti-inflammatory drugs and its contribution to their chemopreventive effect. Cancer Res. 2005;65:1868–1876.
Zavala-Zendejas VE, Torres-Martinez AC, Salas-Morales B, Fortoul TI, Montaño LF, Rendon-Huerta EP. Claudin-6, 7, or 9 overexpression in the human gastric adenocarcinoma cell line AGS increases its invasiveness, migration, and proliferation rate. Cancer Invest. 2011;29:1–11.
Takehara M, Nishimura T, Mima S, Hoshino T, Mizushima T. Effect of claudin expression on paracellular permeability, migration and invasion of colonic cancer cells. Biol Pharm Bull. 2009;32:825–831.
Acknowledgments
We thank Ms. Noriko Kamiya for the technical assistance. This work was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan through a Grant-in-Aid for Scientific Research (C) 2010–2012 (no. 22590691).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okugawa, T., Oshima, T., Chen, X. et al. Down-Regulation of Claudin-3 Is Associated with Proliferative Potential in Early Gastric Cancers. Dig Dis Sci 57, 1562–1567 (2012). https://doi.org/10.1007/s10620-012-2043-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2043-5