Abstract
Background
Endoscopic submucosal dissection (ESD) allows en bloc resection of the entire lesion, permitting a higher curative resection rate and increased quality of life by minimizing the resection size compared with that of endoscopic mucosal resection (EMR). Although ESD has been implemented at most university hospitals in Korea, potential complications of ESD such as bleeding and perforation raise doubts in the therapeutic decision on use of the ESD procedure for early gastric cancer patients and in reimbursement decision making. This systematic review aimed to address both the effectiveness and safety outcomes of ESD versus EMR for early gastric cancer.
Methods
MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), and Koreamed were searched using the primary keywords “stomach neoplasm” AND “endoscopic submucosal dissection” AND “endoscopic mucosal resection.” To assess the quality of selected studies, the methodologic approach of the Scottish Intercollegiate Guidelines Network was used. Five effectiveness-relevant and three safety-relevant outcome measures were extracted. Bibliography management and metaanalysis for each outcome were conducted using Review Manager 5.0.
Results
Three nonconcurrent cohort studies and nine retrospective cohort studies were identified. Metaanalyses showed ESD to be significantly more effective than EMR for en bloc resection (odds ratio [OR], 8.43; 95% confidence interval [CI], 5.20–13.67), complete resection (OR, 14.11; 95% CI, 10.85–18.35), curative resection (OR, 3.28; 95% CI, 1.95–5.54), and local recurrence (risk ratio [RR], 0.13; 95% CI, 0.04–0.41). Whereas intraoperative bleeding (RR, 2.16; 95% CI, 1.14–4.09), perforation risk (RR, 3.58; 95% CI, 1.95–6.55), and operation time (standard mean difference [SMD], 1.55; 95% CI, 0.74–2.37) were significantly greater for ESD, overall bleeding risk (RR, 1.22; 95% CI, 0.76–1.98) and all-cause mortality (RR, 0.65; 95% CI, 0.08–5.38) did not differ significantly between ESD and EMR.
Conclusions
Considering that bleeding risk did not differ significantly between ESD and EMR and that perforation risk usually does not lead to life-threatening disease, the effectiveness benefit of ESD can outweigh the overall harm compared with EMR on the condition that ESD is performed by experienced practitioners.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Early gastric cancer (EGC) is a malignant tumor confined to the mucosa or the submucosa regardless of lymph node metastasis, and its proportion of all gastric cancer patients surgically treated in Korea rose from 33% in 1999 to 50% in 2004 [1, 2]. As the diagnosis rate of EGC increased, endoscopic treatment came to the fore as a radical cure for EGC.
Since the 1980s, endoscopic mucosal resection (EMR) has been proposed as a replacement for invasive surgery because of the favorable long-term outcomes and improved quality of life for patients [3, 4]. The EMR procedure has been included in Korea’s National Health Insurance coverage since November 1999. Currently, the number of patients treated with EMR in Korea is increasing [4].
Meanwhile, endoscopic submucosal dissection (ESD), a new technique developed in the late 1990s in Japan, enables en bloc resection regardless of lesion size, and precise pathologic diagnosis becomes practical [5]. Besides its positive outcomes, ESD has controversial risks such as perforation, bleeding, and other technical difficulties [6]. In 2004, ESD was introduced in Korea. However, in 2008, the National Health Insurance Review and Assessment Service Agency of Korea temporarily halted reimbursement for ESD procedures due to its unclear outcomes per indication. This reimbursement decision currently is under review.
Although case reports and cohort studies show the efficacy of ESD [7, 8], longitudinal clinical trials presenting long-term outcomes identifying indications for which ESD might be efficacious are limited. A recent metaanalysis showed that ESD had higher en bloc and curative resection rates than EMR but had a longer operation time, with higher risk of perforation and bleeding than EMR [9]. However, the diseases targeted for ESD encompassed the entire range of gastrointestinal tract neoplasms including premalignant and malignant lesions of esophageal, gastric, and colorectal origin. This is opposed to a focus on EGC, and half the studies selected in that review were abstracts with limited information rather than full-text publications.
Accordingly, to facilitate determining the treatment territory of ESD for EGC, the current study aimed to address the benefits and harms of ESD compared with EMR in early gastric cancer by compiling existing clinical evidence for systematic review and metaanalysis.
Materials and methods
Search strategy
We searched for all studies comparing ESD and EMR for EGC patients published between January 1990 and April 2010 through MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials in addition to Koreamed (http://www.koreamed.org), a local electronic database providing information on Korean medical published research without language restriction. Given the lack of randomized clinical trials (RCTs) on ESD, controlled clinical trials and comparative observation studies of ESD also were selected in the search. The following key words were used: “stomach neoplasms” OR “gastric neoplasms” OR “stomach carcinoma” OR “gastric carcinoma” OR “stomach cancer” OR “gastric cancer” OR “gastric adenocarcinoma” OR “stomach adenocarcinoma” OR “mucosal gastric neoplasm” AND “ESD” OR “endoscopic submucosal dissection” AND “EMR” OR “endoscopic mucosal resection.” The latest date for updating the search was 30 April 2010.
Study selection
In the first stage of the study selection, the titles and abstracts of papers searched by keywords were examined to exclude irrelevant articles. Next, the full text of all selected studies was screened according to inclusion and exclusion criteria. The inclusion criteria specified (1) studies about EGC or gastric adenoma, (2) studies comparing ESD with EMR, and (3) studies reporting at least one of the appropriate clinical outcomes including en bloc resection, complete resection, curative resection, local recurrence, mortality, bleeding, perforation, and resection time. The exclusion criteria ruled out (1) nonoriginal research, (2) animal testing or preclinical trials, (3) abstract-only publication, (4) effectiveness not specific to ESD, (5) publication in a language other than English or Korean, and (6) case and ad hoc reports. Two investigators independently evaluated studies for eligibility and subsequently resolved any disagreements by discussion together with clinical expert consultation.
Data extraction
Using a data extraction form developed in advance, two reviewers independently extracted the following information: first author, publication year, country, research design, number of individuals in the ESD and EMR procedure groups, age and sex of patients, intervention types, observation period, results of major outcomes, and number of engaged research centers. Outcomes measures consisted of five effectiveness-relevant measures (rates of en bloc resection, complete resection, curative resection, local recurrence, and mortality) and three safety-relevant measures (bleeding, perforation, and resection time).
Complete resection was defined as an en bloc resection of tumor with the horizontal and vertical margins free of neoplasm [10, 11]. The definitions of curative resection were a bit different among the studies, but in general, a lesion within the histologic criteria for endoscopic resection with tumor-free margins was presented as the standard of curative resection and thus used in the metaanalysis [11–16]. A study was not included in the metaanalysis if there was evidence of severe bias or heterogeneity.
Statistical analysis
Metaanalyses were conducted using Review Manager 5.0 software (RevMan 5.0). For the outcomes data of en bloc resection, complete resection, and curative resection, we calculated the odds ratio (OR), and for local recurrence, mortality, bleeding, and perforation, we calculated the risk ratio (RR) as a summary statistic. For the continuous outcomes such as resection time, the standard mean difference (SMD) was calculated. All differences calculated were expressed as 95% confidence intervals (CI).
Taking a conservative approach, we used a random-effects model [17], assuming heterogeneity between studies included in the metaanalysis and consequently producing wider confidence intervals than a fixed-effects model would produce. In all metaanalyses, we assessed heterogeneity with Chi-square (p < 0.01) and I2 (I2 > 50%) [18], and if heterogeneity was found, we conducted sensitivity analyses.
Using software Stata 10 (Stata Corporation, College Station, TX, USA), a funnel plot was first used to detect any publication bias, and then the symmetry of the funnel plot was confirmed by Egger’s test with a p value level of 0.05 [19].
Assessment of study quality
The quality of the selected studies was evaluated with a methodology checklist developed by the Scottish Intercollegiate Guidelines Network (SIGN: http://www.sign.ac.uk/pdf/sign50.pdf). Because all the selected studies were nonrandomized, we used the SIGN guideline for quality assessment of nonrandomized studies, which consists of the following judgments: focused questions, selection bias of subjects, assessment of outcomes and exposure status, handling confounders, and provision of confidence intervals.
The criterion for the assessment was based on the index of “well covered,” “adequately addressed,” “poorly addressed,” “not addressed,” “not reported,” and “not applicable.” The overall evidence grade for nonrandomized studies was stated according to the evidence levels of SIGN as follows: 2++ (high-quality systematic reviews of case-control or cohort studies or high-quality case-control or cohort studies), 2+ (well-conducted case-control or cohort studies, and 2− (case-control or cohort studies with high risk of confounding or bias).
Results
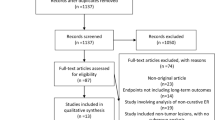
The search first identified a total of 473 studies through our search strategy (Fig. 1). After scanning titles and abstracts, we discarded 115 identical articles retrieved through multiple search engines. Then, a review with the inclusion and exclusion criteria excluded 345 articles because they were about neither EGC nor gastric adenoma (n = 120), ESD was not used (n = 17), EMR was not used (n = 31), none of clinical outcomes for ESD were reported (n = 20), the articles were nonoriginal research (n = 78), the articles were case reports or ad hoc reports (n = 67), the articles were animal testing or preclinical trials publishing only abstracts (n = 6), or the articles were published in a language other than English or Korean (n = 6). Among the remaining 13 papers [10–16, 20–25], 1 article [25] was additionally excluded due to selection bias in allocation of interventions to participants by assigning patients with lesion sizes larger than 15 mm to the ESD group and the others to the EMR group.
Finally, 12 studies were appropriate for the metaanalyses. These studies described a total of 3,806 gastric lesions: 1,734 lesions the ESD group and 2,072 lesions in the EMR group. All the studies had been published in the past 5 years, from 2006 to 2010. Among the 12 studies, 3 were nonconcurrent cohort studies comparing a prospective treatment group with a comparison group in the past [14, 22, 23], and the others were retrospective cohort studies [10–13, 15, 16, 20, 21, 24]. Although all the studies were about EGC or gastric adenoma, three studies had subjects with recurrent EGC after gastrectomy [10, 11, 15]. Of the 12 studies, 9 had been conducted in Japan [10–12, 15, 16, 20, 21, 23, 24], 2 in Korea [13, 22], and 1 in Italy [14]. All were published in English. Five or six outcomes were reported in each study among eight major outcomes. The characteristics of the studies included in the metaanalysis are summarized in Table 1.
Quality assessment using the checklist of SIGN resulted in a 2+ for the all studies included in the metaanalysis. Because ESD was developed and introduced after EMR, most of the studies had patients in the ESD group from an earlier period than the EMR group except for three studies performed using ESD and EMR during the same study period [12, 13, 16]. In addition, conditions such as tumor size, tumor location, and macroscopic type were not alike between the ESD and the EMR procedure groups in most studies. In these studies, subject selection was assessed as “poorly addressed,” whereas the subject selection in three studies was assessed as “well covered” [10, 23, 24]. However, because tumors of larger size or difficult locations tended to be allocated to the ESD group, the effectiveness of ESD was less likely to be overestimated than that of EMR. Eight studies examined the breakdown of key clinical outcomes according to a couple of factors such as tumor size and location [12, 13, 16, 20–24].
En bloc resection
The en bloc resection rate for either primary or recurrent EGC was reported in 10 studies [10, 12–15, 20–24]. The pooled analysis using the random-effects model demonstrated a significantly higher en bloc resection rate for the ESD group (1055/1150, 91.7%) than for the EMR group (882/1694, 52.1%) (OR, 8.43; 95% CI, 5.20–13.67) (Fig. 2). Because a moderate heterogeneity of existence was found (p = 0.007; I2 = 60%), several subgroup analyses were conducted. The ESD group showed higher en bloc resection rates for both primary EGC [12–14, 20–24] (OR, 7.60; 95% CI, 4.86–11.88) and recurrent EGC [10, 15] (OR, 62.93; 95% CI, 3.28–1208.40) (Fig. 2).
Five studies [13, 20, 21, 23, 24] reported en bloc resection rates according to tumor size. In the subgroup analyses of both tumors smaller than 10 mm and tumors larger than 10 mm, the en bloc resection rates in the ESD group (OR, 3.84; 95% CI, 1.62–9.12) were significantly higher than in the EMR group (OR, 13.40; 95% CI, 9.08–19.77), and there was no heterogeneity among the studies (p = 0.21, I2 = 32%; p = 0.34, I2 = 11%, respectively).
Another subgroup analysis of the en bloc resection rate by tumor location from three studies [12, 21, 23] also showed significantly higher en bloc resection rates for ESD versus EMR for upper (OR, 8.54; 95% CI, 1.07–67.97), middle (OR, 13.14; 95% CI, 6.24–27.70), and lower (OR, 19.42; 95% CI, 1.17–323.63) locations.
Four studies [10, 14, 15, 24] used an insulation-tipped diathermic knife (IT knife), and another four studies [13, 20, 21, 23] used a mixed knife for ESD. The pooled analyses presented higher en bloc resection rates for the ESD group for both the IT knife (OR, 16.80; 95% CI, 4.41–63.96) and the mixed knife (OR, 6.10; 95% CI, 3.10–12.01) than for the EMR group, and the difference was statistically significant.
Complete resection
Nine studies [10, 11, 13, 16, 20–24] reported the complete resection rate, incorporating 1,344 lesions for the ESD group and 1,542 lesions for the EMR group. In the pooled analysis with the random-effects model, ESD (1287/1401, 91.9%) showed a significantly higher complete resection rate than EMR (679/1579, 43.0%) (OR, 8.54; 95% CI, 4.44–16.45). A significant heterogeneity was noted (p < 0.00001, I2 = 83%), and thus two studies [13, 21] with less rigorous definitions for complete resection than the others were excluded based on the results of the sensitivity analysis. The subsequent pooled analysis still showed significantly better complete resection rates for the ESD group (956/1038, 92.1%) than for the EMR group (496/1351, 36.7%) in both primary EGC (OR, 14.14; 95% CI, 10.80–18.51) and recurrent EGC (OR, 13.70; 95% CI, 3.59–52.29). There was no heterogeneity across the studies (p = 0.63, I2 = 0%) (Fig. 3).
Subgroup analyses also showed better complete resection rates for ESD than for EMR across tumor sizes including tumors smaller than 10 mm [13, 16, 20, 21, 24] (OR, 10.62; 95% CI, 6.00–18.80), tumors 10–20 mm in size [13, 16, 20, 21, 24] (OR, 11.04; 95% CI, 4.20–29.00), and tumors larger than 20 mm [13, 16, 20, 21] (OR, 20.91; 95% CI, 5.12–85.40).
Subgroup analyses with respect to the knife type used during ESD showed that the complete resection rate was significantly higher with the IT knife [10, 24] (OR, 15.27; 95% CI, 5.78–40.35), the mixed knife [13, 20, 21, 23] (OR, 5.73; 95% CI, 1.69–19.43), and the hook and flex knife [11, 16] (OR, 12.08; 95% CI, 6.87–21.24) compared with the EMR group. However, one study [22] using the needleknife reported insignificant improvement in the complete resection rate of the ESD group over the EMR group (OR, 5.32; 95% CI, 0.25–115.13).
Curative resection
Five studies reported the curative resection rate [11, 12, 14–16]. The curative resection rate was significantly superior for the ESD group (774/973, 79.5%) compared with the EMR group (481/815, 59.0%) (OR, 3.28; 95% CI, 1.95–5.54) (Fig. 4). Because moderate heterogeneity was found (p = 0.01, I2 = 68%), one study [12] was removed from the primary pooled metaanalysis on the basis of sensitivity analyses. In the subsequent analysis, the superior curative resection of the ESD group compared with the EMR group did not change (OR, 3.57; 95% CI, 2.68–4.76), and there was no heterogeneity among the four studies (p = 0.46, I2 = 0%).
Local recurrence
Local recurrence rates were examined in nine studies [10–13, 15, 16, 20, 23, 24], and the pooled analysis showed a significantly lower local recurrence rate for the ESD group (13/1592, 0.82%) than for the EMR group (93/1850, 5.03%) (RR, 0.13; 95% CI, 0.04–0.41) (Fig. 5). No heterogeneity was identified with the random-effects model (p = 0.06, I2 = 47%). Because the follow-up period for the local recurrence varied across studies, the subgroup analyses were conducted for an observation period of less than 1 year [11, 13, 16], of 1–4 years [12, 15, 20, 23], and longer than 5 years [10, 24]. The ESD group had a significantly lower local recurrence rate than the EMR group during the 2- to 4-year follow-up period (RR, 0.13; 95% CI, 0.04–0.40). However, the significance vanished when observation was less than 1 year (RR, 0.27; 95% CI, 0.01–9.19) or more than 5 years (RR, 0.08; 95% CI, 0.00–1.30) (Fig. 5).
In one study [13], the nine local recurrence cases meant metachronous recurrence at a site other than the resection area, so sensitivity analysis was conducted excluding it. As a result, the recurrence rate in the ESD group became significantly lower compared with the EMR group during less than 1-year follow-up period (RR, 0.05; 95% CI, 0.01–0.36).
Two studies [12, 23] reported local recurrence rates based on tumor size. The ESD group (1/197, 0.51%) had a significantly lower local recurrence rate than the EMR group (37/438, 8.45%) for tumors larger than 10 mm (RR, 0.05; 95% CI, 0.01–0.27). For tumors smaller than 10 mm, the local recurrence rate was not significantly different between the ESD group (1/57, 1.7%) and the EMR group (11/435, 2.5%) (RR, 0.49; 95% CI, 0.08–2.85).
Mortality
Two studies [12, 15] reported the all-cause mortality rate at 3 years and at a median follow-up period of 43 months (range, 15–63 months), respectively. The pooled metaanalysis with the random-effects model showed that the ESD group had lower a mortality rate (3/349, 0.86%) than the EMR group (4/429, 0.93%), but the difference was not statistically significant (RR, 0.65; 95% CI, 0.08–5.38). No heterogeneity was found among the studies (p = 0.20, I2 = 39%).
Bleeding
All the studies [10–16, 20–24] reported the bleeding risk based on the analysis of 3,806 lesions. The pooled analysis showed the bleeding rate to be higher in the ESD group (116/1642, 7.06%) than in the EMR group (136/1919, or 7.09%), but the difference was not statistically significant (RR, 1.22; 95% CI, 0.76–1.98) (Fig. 6). There was no heterogeneity among the studies (p = 0.04, I2 = 48%).
Three studies [20, 22, 23] examined bleeding rates during and after the procedure. The metaanalysis showed that the intraoperative bleeding risk was 2.16 times higher for the ESD group (54/287, 18.82%) than for the EMR group (70/906, 7.73%), and the difference was statistically significant (RR, 2.16; 95% CI, 1.14–4.09). However, the postoperative bleeding risk was slightly lower in the ESD group (13/287, 4.53%) than in the EMR group (36/906, 3.97%), but the difference was not statistically significant (RR, 0.94; 95% CI, 0.29–3.02).
Subgroup analyses were conducted according to the knife type used in ESD. The metaanalyses of four studies [10, 14, 15, 24] using the IT knife only (RR, 0.89; 95% CI, 0.31–2.57) and two studies [11, 16] using the hook and flex knife (RR, 0.95; 95% CI, 0.54–1.68) showed lower bleeding risks in the ESD group than in the EMR group, but the relative risks were not statistically significant. However, ESD with the mixed knife [13, 20, 23] had 77 (15.5%) bleeds in 497 cases, which was significantly higher than the 105 bleeds (10.8%) in 976 cases in the EMR group (RR, 1.86; 95% CI, 1.07–3.24).
Perforation
The perforation risk was reported in all 12 studies [10–16, 20–24], and the pooled analysis showed a significantly higher perforation rate in the ESD group (80/1762, 4.54%) than in the EMR group (21/2,044, 1.03%; RR, 3.58; 95% CI, 1.95–6.55) (Fig. 7). No study heterogeneity was found (p = 0.16, I2 = 30%). In the subgroup analysis for the primary EGC [12–14, 16, 20–24], the perforation risk was significantly higher in the ESD group (RR, 3.67; 95% CI, 1.84–7.29), but the difference was not statistically significant for recurrent EGC [10, 11, 15] (RR, 2.57; 95% CI, 0.31–21.50) (Fig. 7).
To examine the effect of the knife type on perforation in ESD, subgroup analyses were conducted. The ESD group had nonsignificantly higher perforation rates than the EMR group across four different knife types, namely, the IT knife [10, 14, 15, 24] (RR, 5.10; 95% CI, 0.91–28.49), the hook and flex knife [11, 16] (RR, 2.23; 95% CI, 0.88–5.65), the mixed knife [13, 20, 21, 23] (RR, 4.13; 95% CI, 0.92–18.61), and the needleknife [22] (RR, 3.00; 95% CI, 0.33–27.38).
The information on whether surgical intervention was needed for perforation or not was available in all studies except one [16]. In the subgroup analysis, 7 (11.67%) of 60 patients with perforation in the ESD group and 1 (6.25%) of 16 patients in the EMR group were treated surgically, but there was no significant difference between them (RR, 1.87; 95% CI, 0.25–14.09).
Resection time
Nine studies [10, 11, 13, 14, 20–24] reported the resection time. Among them, two studies [14, 23] reported only the mean value without the standard deviation value, so they were excluded from the pooled analysis. The metaanalysis with the random-effects model showed that the operation time for the ESD group was significantly longer than for the EMR group (SMD = 1.55; 95% CI, 0.74–2.37), which was consistent with pooled analyses for both primary EGC (SMD = 1.45; 95% CI, 0.44–2.45) and recurrent EGC (SMD = 1.87; 95% CI, 1.09–2.65) (Fig. 8). There was significant heterogeneity in the pooled analysis (p < 0.00001, I2 = 97%). The different definitions of resection time may explain this heterogeneity between studies. Funnel plot analysis and Egger’s test showed no publication bias in the main metaanalysis for any of eight outcomes.
Discussion
This systematic review and metaanalysis found a distinct advantage of ESD over EMR in terms of clinical effectiveness and safety outcomes. The en bloc resection rate was significantly higher in the ESD group. All the subgroup analyses demonstrated the same superiority of ESD in the en bloc resection for primary or recurrent EGC; tumor smaller or larger than 10 mm; upper, lower, or middle tumor location; and IT knife or mixed knife type for ESD. Our pooled data indicated a significantly higher complete resection rate in the ESD group than in the EMR group. Commensurate with the en bloc resection rate, the complete resection rate in the ESD group was significantly higher across subgroup analyses than in the EMR group. The curative resection rate was significantly higher and the local recurrence rate significantly lower in the ESD group.
The mortality risk was lower in the ESD group, but the difference was not statistically significant. Our pooled data indicated no significant difference in the bleeding risk between ESD and EMR. On the other hand, ESD had a significantly higher perforation rate and a significantly longer resection time than EMR.
The superior outcomes of ESD in en bloc resection, curative resection, and local recurrence compared with EMR were consistent with the previous metaanalysis study [9], which covered all cancers in the gastrointestinal tract, whereas the current study focused on EGC. Mortality and complete resection data were first combined in the current metaanalysis, and indeed, complete resection was more frequently reported than curative resection among the studies included in this systematic review.
Although bleeding risk was significantly higher in the previous metaanalysis [9] including 9 studies, it was not statistically significant in the current metaanalysis including 12 studies. This may have been the case because the current study had almost twice the pooled ESD lesions as the previous metaanalysis [9] for the bleeding data, put together only EGC-focused studies, and used a random-effects model for the main pooled analysis. Although the definitions of bleeding in ESD and EMR varied across studies, most of the studies included in the metaanalysis reported no surgical treatment due to bleeding except for one study [13], in which one patient in the EMR group underwent surgery.
Perforation is the most serious complication in the ESD procedure. Consequently, several techniques such as endoknives have been developed to reduce perforation occurrence during endoscopic resection [5]. The IT knife, a representative example developed to reduce this disadvantage, has a powerful cutting wave to prevent electric leakage in the gastric wall [26]. In most studies, perforation could be recovered with conservative management such as endoscopic clipping, fasting, nasogastric aspiration, and broad-spectrum antibiotics [13, 24]. Because no mortality related to perforation was found, perforation of ESD was not considered a life-threatening complication. Also, because the perforation cases seemed to occur more frequently during the initial ESD operations, perforations are expected to decrease with accumulated experience of operators with ESD [20].
The ESD technique is difficult because a combination procedure of cutting and coagulation by electrosurgical current is performed simultaneously [20]. Consequently, the ESD procedure time is longer than EMR. In addition, because ESD tends to have expanded indications such as large tumors, submucosal invasion, and ulcerative tumors, more time is required for resection with ESD than with the conventional EMR technique. Although ESD took almost two times longer than EMR, for tumors larger than 20 mm, the procedure time was not significantly different between the two procedure types [22]. The procedure time for ESD during the experience period was shorter than during the introduction period of ESD implementation [21]. This information suggests the need for skilled and experienced operators.
As with any metaanalysis, the current study has possible limitations because evidence was combined from available studies. First, the studies available for the metaanalysis were limited by its nonrandomized design. Second, 75% of the studies had a nonconcurrent comparison group, in which probable selection bias might arise. Third, considering that ESD has been developed and performed actively in Japan, articles in the Japanese language should have been searched and included. However, among the six studies falling under the exclusion criterion of publication in a language other than English or Korean, no publication in the Japanese language was found. Fourth, although the superior advantage of endoscopic treatment lies in improving the quality of life for the patients together with equal clinical effectiveness, the outcome in terms of quality-adjusted life years was not considered in any of the included studies. In addition, long-term outcome data were lacking in the studies. Fifth, heterogeneity existed in the metaanalyses for curative resection rate and resection time. Finally, although the success of ESD has a stake in the operator’s competency, data available to reveal the validity in the outcome for ESD according to operator’s skill or experience are limited.
In conclusion, in the metaanalyses, the ESD group showed great advantages of achieving en bloc resection, complete resection, curative resection, and lower local recurrence for EGC compared with the EMR group. The disadvantages such as higher perforation rates and longer resection time in the ESD group might be overcome with the acquisition of skills and experience by operators. Considering all the outcome benefits of ESD over EMR in the current study, ESD should be the first choice for EGC treatment based on improved clinical outcomes and quality of life for EGC patients, provided the procedure is performed carefully by skilled operators. Further studies are needed to develop new measures for reducing the residual limitations with ESD and for validating the long-term outcomes with ESD.
References
Nakamura K, Ueyama T, Yao T, Xuan ZX, Ambe K, Adachi Y, Yakeishi Y, Matsukuma A, Enjoji M (1992) Pathology and prognosis of gastric carcinoma: findings in 10,000 patients who underwent primary gastrectomy. Cancer 70:1030–1037
Kang KJ, Lee JH (2010) Characteristics of gastric cancer in Korea—with an emphasis on the increase of the early gastric cancer (EGC). J Korean Med Assoc 53:283–289
Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S (2001) Endoscopic mucosal resection for treatment of early gastric cancer. Gut 48:225–229
Jung HY (2008) Extended approach of EMR/ESD in stomach cancer. J Korean Gastric Cancer Assoc 8:5–8
Hotta K, Oyama T, Akamatsu T, Tomori A, Hasebe O, Nakamura N, Kojima E, Suga T, Miyabayashi H, Ohta H (2010) A comparison of outcomes of endoscopic submucosal dissection (ESD) for early gastric neoplasms between high-volume and low-volume centers: multicenter retrospective questionnaire study conducted by the Nagano ESD study group. Intern Med 49:253–259
Sumiyama K, Kaise M, Nakayoshi T, Kato M, Mashiko T, Uchiyama Y, Goda K, Hino S, Nakamura Y, Matsuda K, Mochizuki K, Kawamura M, Tajiri H (2004) Combined use of a magnifying endoscope with a narrow band imaging system and a multibending endoscope for en bloc EMR of early-stage gastric cancer. Gastrointest Endosc 60:79–84
Jang JS, Lee EJ, Lee SW, Lee JH, Roh MH, Han SY, Choi SR, Jeong JS (2007) Endoscopic submucosal dissection for early gastric cancer and gastric adenoma. Korean J Gastroenterol 49:356–363
Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ, Kim HJ, Kim JJ, Ji SR, Seol SY (2009) Therapeutic outcomes in 1,000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD study group multicenter study. Gastrointest Endosc 69:1228–1235
Cao Y, Liao C, Tan A, Gao Y, Mo Z, Gao F (2009) Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy 41:751–757
Hirasaki S, Kanzaki H, Matsubara M, Fujita K, Matsumura S, Suzuki S (2008) Treatment of gastric remnant cancer postdistal gastrectomy by endoscopic submucosal dissection using an insulation-tipped diathermic knife. World J Gastroenterol 14:2550–2555
Hoteya S, Iizuka T, Kikuchi D, Yahagi N (2010) Clinical advantages of endoscopic submucosal dissection for gastric cancers in remnant stomach surpass conventional endoscopic mucosal resection. Dig Endosc 22:17–20
Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, Doi T, Otani Y, Fujisaki J, Ajioka Y, Hamada T, Inoue H, Gotoda T, Yoshida S (2006) A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer 9:262–270
Min BH, Lee JH, Kim JJ, Shim SG, Chang DK, Kim YH, Rhee PL, Kim KM, Park CK, Rhee JC (2009) Clinical outcomes of endoscopic submucosal dissection (ESD) for treating early gastric cancer: comparison with endoscopic mucosal resection after circumferential precutting (EMR-P). Dig Liver Dis 41:201–209
Catalano F, Trecca A, Rodella L, Lombardo F, Tomezzoli A, Battista S, Silano M, Gaj F, de Manzoni G (2009) The modern treatment of early gastric cancer: our experience in an Italian cohort. Surg Endosc 23:1581–1586
Yokoi C, Gotoda T, Hamanaka H, Oda I (2006) Endoscopic submucosal dissection allows curative resection of locally recurrent early gastric cancer after prior endoscopic mucosal resection. Gastrointest Endosc 64:212–218
Hoteya S, Iizuka T, Kikuchi D, Yahagi N (2009) Benefits of endoscopic submucosal dissection according to size and location of gastric neoplasm, compared with conventional mucosal resection. J Gastroenterol Hepatol 24:1102–1106
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K (2006) Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc 64:877–883
Watanabe K, Ogata S, Kawazoe S, Koyama T, Kajiwara T, Shimoda Y, Takase Y, Irie K, Mizuguchi M, Tsunada S, Iwakiri R, Fujimoto K (2006) Clinical outcomes of EMR for gastric tumors: historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc 63:776–782
Choi KS, Jung HY, Choi KD, Chung JW, Oh TH, Jo JY, Song HJ, Lee GH, Byeon JS, Myung SJ, Yang SK, Kim JH (2006) Endoscopic submucosal dissection for gastric tumors: complete resection rate, resection time, and complications in comparison with endoscopic mucosal resection after circumferential mucosal incision with a needle knife. Korean J Gastrointest Endosc 32:326–332
Shimura T, Sasaki M, Kataoka H, Tanida S, Oshima T, Ogasawara N, Wada T, Kubota E, Yamada T, Mori Y, Fujita F, Nakao H, Ohara H, Inukai M, Kasugai K, Joh T (2007) Advantages of endoscopic submucosal dissection over conventional endoscopic mucosal resection. J Gastroenterol Hepatol 22:821–826
Nakamoto S, Sakai Y, Kasanuki J, Kondo F, Ooka Y, Kato K, Arai M, Suzuki T, Matsumura T, Bekku D, Ito K, Tanaka T, Yokosuka O (2009) Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy 41:746–750
Yamaguchi Y, Katusmi N, Aoki K, Toki M, Nakamura K, Abe N, Morozumi K, Sugiyama M, Ishida H, Takahashi S (2007) Resection area of 15 mm as dividing line for choosing strip biopsy or endoscopic submucosal dissection for mucosal gastric neoplasm. J Clin Gastroenterol 41:472–476
Ohkuwa M, Hosokawa K, Boku N, Ohtu A, Tajiri H, Yoshida S (2001) New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy 33:221–226
Disclosures
Young-Mi Park, Eun Cho, Hye-Young Kang, Jong-Mann Kim have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, YM., Cho, E., Kang, HY. et al. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc 25, 2666–2677 (2011). https://doi.org/10.1007/s00464-011-1627-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-011-1627-z