Abstract
Background
Gastric cancer (GC) is a common malignant tumor of the digestive tract. Claudin-5 is upregulated in GC tumor tissues, but its effect on GC is imprecise. In this study, we analyzed the influence of claudin-5 on GC.
Objectives
To investigate the effect of Claudin-5 on Gastric cancer (GC).
Results
We confirmed that the content of claudin-5 in MKN45 and AGS cells was boosted by Claudin-5 transfection, but decreased by si-Claudin-5 transfection. Besides, claudin-5 overexpression expedited GC cells proliferation, migration and invasion, but repressed GC cells apoptosis. Silencing claudin-5 curbed tumor growth in vivo.
Conclusion
Claudin-5 overexpression expedited GC cells proliferation, migration and invasion, but repressed GC cells apoptosis.
Highlights
-
Claudin-5 expedited the proliferation of GC cells.
-
Claudin-5 repressed GC cells apoptosis.
-
Claudin-5 facilitated the migration and invasion of GC cells.
-
Silencing claudin-5 curbed tumor growth in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is the third leading cause of cancer-associated death globally (Gao and Wu 2019; Smyth et al. 2020). Despite great advances in surgical and adjuvant treatments for GC, 5-year survival rates for GC sufferers remain low due to cancer recurrence caused by metastasis (Seeneevassen et al. 2021; Xu et al. 2023). Therefore, it is crucial to elucidate the molecular mechanisms related to GC metastasis in order to improve patient prognosis (Hashimoto and Oshima 2022). Cancer metastasis is an intricate procedure involving epithelial-mesenchymal transformation (EMT) of GC cells, during which GC cells obtain mesenchymal capabilities that enable them to enter the bloodstream and invade other organs (Tian et al. 2020). EMT involves cellular reprogramming through alterations in gene transcription (Pan et al. 2021). Thus, research the molecular mechanism of EMT is necessary to ascertain a new strategy for treating GC.
Claudins are essential tight linking proteins that connect adjacent epithelial cells, mesothelial cells, or endothelial cells, and they play a role in blocking paracellular pathways (Hashimoto and Oshima 2022). There are at least 24 known types of claudins, which are involved in regulating epithelial cell permeability and forming different barriers (Hashimoto and Oshima 2022). Claudin-5 is primarily expressed in endothelial cells and is involved in the formation of the blood–brain barrier. It is highly expressed in vascular endothelial cells and vascular tumors (Turunen et al. 2009). Claudin-5 was first described by Morita et al. (1999), it was initially identified as a missing protein in patients with the genetic disorder velo-cardio-facial syndrome, and the gene was localized to chromosome 22q11. Claudin-5 is expressed in a variety of tumors, for example, it is highly expressed in breast cancer and has a possible role in the metastasis of breast cancer (Escudero-Esparza et al. 2012). Overexpression of claudin-5 has been found in ovarian epithelial tumors and is linked with tumor aggressive behavior (Turunen et al. 2009). Moreover, the level of claudin-5 was reduced in oral squamous cell carcinoma (Phattarataratip and Sappayatosok 2016). In addition, claudin-5 could modulate the colitis-associated tumorigenesis (Zhang et al. 2022). Besides, claudin-5 serves as a marker for angiosarcoma and hemangioendotheliomas (Miettinen et al. 2011). These studies indicate that the expression of claudin-5 is abnormal in a variety of cancers, indicating that claudin-5 may have an important regulatory role in a variety of cancers. Studies have shown that claudin-5 expression is increased in GC tumor tissues, and patients with positive claudin-5 protein expression have significantly shorter survival times than those with negative claudin-5 protein expression (Yang et al. 2018). Therefore, we speculate that claudin-5 is a key factor regulating GC progression and may be an important target for GC molecular therapy. However, the role of claudin-5 in GC has remained uncertain. Consequently, this study focuses on the effect of claudin-5 on GC, which is of great significance for the development of molecular targeting drugs with GC.
Herein, we investigated the effects of claudin-5 on the proliferation, apoptosis, migration and invasion of GC cells, providing potential targets for molecular therapy of GC.
Materials and methods
Cell culture
The human GC cell lines MKN45 and AGS (Cell Bank of Chinese Academy of Sciences, Shanghai, China) were employed for this examination. MKN45 and AGS cells were cultivated in RPMI-1640 (BIOSUN, Shanghai, China) complemented by 10% fetal bovine serum (FBS; BIOSUN) and antibiotics (100 U/mL penicillin and 100 µg/mL streptomycin; Solarbio, Beijing, China) at 37 °C with 5% CO2.
Tissue collection
In this research, 30 pairs tumor tissues and corresponding paracancer tissues were collected from 30 GC patients of the First Affiliated Hospital of Bengbu Medical College and stored at – 80 °C. All research was carried out in line with the Ethics Committee of The First Affiliated Hospital of Bengbu Medical College (Approval no. 2022-342). All participants signed informed consent forms. The clinicopathological characteristics of all recruited participants were collected and analysed.
Cell transfection
Claudin-5 over-expression vector (Claudin-5) and control (vector) were utilized to regulate claudin-5 expression. The small interfering RNA (siRNA) against claudin-5 (si-Claudin-5) and control (si-NC) were synthesized by GenePharma (Shanghai, China). Lipofectamine 2000 (Solarbio) was employed for transfection with plasmids.
Western blot
GC cells and tumor tissues lysates were extracted utilizing RIPA lysis buffer (Solarbio). The lysate was centrifuged, and the supernatant was collected. A BCA protein assay kit was utilized to evaluate the protein concentration. Proteins were separated by 10% SDS-PAGE and then transferred to a PVDF membrane (Solarbio). After blocking, the membrane was probed with primary antibodies against claudin-5 (ab131259; 1:1000; Abcam, Cambridge, MA, USA), Bax (ab32503; 1:1000; Abcam), cleaved-caspase3 (ab32042; 1:1000; Abcam), and β-actin (ab8226; 1:1000; Abcam) overnight at 4 °C. The membrane was then coped with goat anti-rabbit IgG (ab205718; 1:2500; Abcam) for 1 h. The immunoblots were visualized utilizing an ECL-Plus reagent (Solarbio). Image J software was employed to scrutinize the bands, which were normalized to β-actin.
Cell proliferation assay
MKN45 and AGS cells proliferation was evaluated utilizing the CCK-8 and colony formation assay. For the CCK-8 assay, MKN45 and AGS cells were planted in 96-well plates and hatched with 100 μL CCK-8 solution (10%; Solarbio) for 4 h. The absorbance was examined at 450 nm through a microplate reader (Tecan, Männedorf, Switzerland). For the colony formation assay, treated MKN45 and AGS cells (200 cells/dish) were seeded in 10 mL culture medium and cultured for 14 days. Whereafter, the cells were coped with 4% paraformaldehyde (Solarbio) for 15 min and then treated with GIEMSA staining solution (Solarbio) for 20 min. Finally, images were captured.
Flow cytometry assay
Treated MKN45 and AGS cells (2 × 105/mL) were planted in 96-well plates. After 48 h, the cells were resuspended in PBS (Solarbio), and analyzed according to the instructions of the Annexin V FITC/PI apoptosis kit (Solarbio). Following this, the data were assessed using Flow Jo software.
Wound healing assay
MKN45 and AGS cells with different treatment were planted in 6-well plates. The scratch wounds were created utilizing a sterile tip of 10-μL pipette tips. Following this, the medium was added and the images of migrated cells were captured using a microscope (Olympus, Tokyo, Japan) at 0 h and 24 h.
Transwell assay
The invasion assay was adopted using a transwell chamber (Corning Inc., Corning, NY, USA) precoated with 100 μL Matrigel (Corning). The treated MKN45 and AGS cells (1 × 105 cells) were resuspended in serum-free RPMI-1640 medium (BIOSUN) and seeded in the higher chambers of a 24-well chamber (Corning). Following this, RPMI-1640 medium (BIOSUN) comprising 20% FBS was supplemented to the inferior chambers as a chemoattractant. Whereafter, the cells were cultured for 48 h and the cells on the upper surface were coped with 100% methanol (Solarbio), exposed to 0.05% crystal violet (Solarbio) and imaged through a microscope (Olympus).
Xenograft mouse model
Animal research was adopted in line with the Guide for the Care and Use of Laboratory Animals (2011) and standed to the Ethics Committee of The First Affiliated Hospital of Bengbu Medical College (Approval no.2022-342). The BALB/c nude mice (five weeks old, female; Shanghai Laboratory Animal Company, Shanghai, China) were utilized for the xenograft tumor experiment. MKN45 cells (4 × 105) stably transfected with the short hairpin RNA (shRNA) against claudin-5 (sh-Claudin-5; GenePharma) and control (sh-NC; GenePharma) were respectively injected into the backs of the mice. Tumor volume was scrutinized every 5 days using a caliper, and the tumor volume was calculated as 1/2 × length × width2. After 5 weeks, the mice were euthanized, and the tumor weight was assessed.
Immunohistochemistry (IHC)
IHC was adopted utilizing the PV-9000 kit (ZSGB-BIO, Beijing, China) according to the instruction. Tumor tissues were prepared as paraffin sections and then exposed to primary antibodies against claudin-5 (ab131259; 1:1000; Abcam) overnight at 4 °C. Following this, the sections were coped with HRP goat anti-rabbit IgG H&L (ab97051; 1:500; Abcam) for 1 h. Finally, these paraffin sections were counter-stained with DAB and hematoxylin. Whereafter, the paraffin sections were observed through a microscope (Olympus).
Statistical assay
All tests were performed in triplicate, and the data were displayed as mean ± SD. Statistical analysis was adopting exploiting GraphPad Prism 7 (GraphPad Inc., La Jolla, CA, USA). Student’s t test was utilized for paired comparison. Analysis of variance (ANOVA) was adopted for multiple comparison. P < 0.05 was regarded as validate statistical significant.
Results
Claudin-5 expedited GC cells proliferation
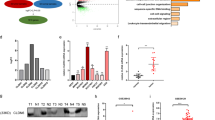
To observe the role of claudin-5 in GC, we synthesized the overexpression vector (Claudin-5) and knockdown vector (si-Claudin-5) for claudin-5, along with their respective controls (Vector and si-NC). We confirmed that the content of claudin-5 in MKN45 and AGS cells was boosted by Claudin-5 transfection, but decreased by si-Claudin-5 transfection (Fig. 1A). These results revealed the successful construction of the relevant vector for claudin-5. Additionally, we discovered that the MKN45 and AGS cells viability (Fig. 1B) and colony formation ability (Fig. 1C) were enhanced by Claudin-5 transfection, but declined by claudin-5 knockdown. Consequently, we concluded that claudin-5 expedited MKN45 and AGS cells proliferation.
Claudin-5 repressed GC cells apoptosis
In this part, we assessed the effect of claudin-5 on GC cells apoptosis. Flow cytometry analysis revealed that MKN45 and AGS cells apoptosis was repressed by claudin-5 upregulation, but elevated by silencing claudin-5 (Fig. 2A). Moreover, the Bax and cleaved-caspase3 protein levels were diminished by overexpression of claudin-5, but boosted after downregulated claudin-5 (Fig. 2B). Therefore, these results indicated that claudin-5 repressed MKN45 and AGS cells apoptosis.
Claudin-5 facilitated GC cells migration and invasion
Herein, we analyzed the influence of claudin-5 on GC cells metastasis. We confirmed that the MKN45 and AGS cells migration (Fig. 3A and B) and invasion (Fig. 3C and D) were heightened by Claudin-5 transfection, but lessened by silence of claudin-5. Thus, we demonstrated that claudin-5 facilitated MKN45 and AGS cells migration and invasion.
Claudin-5 facilitated GC cells migration and invasion. A and B Wound healing assay was adopted to assess MKN45 and AGS cells migration. C and D Transwell assay was carried out to evaluate MKN45 and AGS cells invasion. Compared with the Vector group: **P < 0.01; compared with the si-NC group: #P < 0.05, ##P < 0.01
Silencing claudin-5 curbed tumor growth in vivo
In this part, we evaluated the influence of claudin-5 on GC tumor growth. We built a xenograft tumor mouse model and revealed that the tumor volume and weight were reduced by sh-Claudin-5 transfection (Fig. 4A). Moreover, we adopted western blot and IHC to evaluate the abundance of claudin-5 in tumor tissues. We revealed that the content of claudin-5 was declined by sh-Claudin-5 transfection (Fig. 4B and C). Consequently, we revealed that silencing claudin-5 curbed tumor growth in vivo.
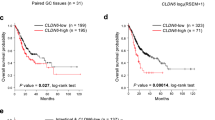
Claudin-5 was upregulated in GC
Finally, the tumor tissues and corresponding paracancer tissues of GC patients were used to analyse the level of claudin-5 in GC. We carried out western blot and IHC assay and found that the abundance of claudin-5 was enhanced in GC tumor tissues versus the paracancer normal tissues (Fig. 5A and B). Moreover, the clinicopathological characteristics of all recruited participants were presented in Table 1. We confirmed that GC patients with high claudin-5 expression level had a higher probability of lymph node metastasis than those with low claudin-5 expression level (Table 1). Therefore, we revealed that claudin-5 promoted the progression and metastasis of GC.
Discussion
In this research, we confirmed that the content of claudin-5 in MKN45 and AGS cells was boosted by Claudin-5 transfection, but decreased by si-Claudin-5 transfection. Besides, claudin-5 overexpression expedited GC cells proliferation, migration and invasion, but repressed GC cells apoptosis. Silencing claudin-5 curbed tumor growth in vivo. This results indicate that claudin-5 is a potential target for molecular therapy of GC.
GC is a type of tumor that originates from the gastric mucosa, the endothelial tissue of the stomach (Liu et al. 2019). GC often goes unnoticed in the early stages as it typically presents no obvious symptoms until it reaches an advanced stage (Machlowska et al. 2020). The exact causes of GC are not fully understood, but there are several potential risk factors, including Helicobacter pylori infection, long-term consumption of preserved, smoked, and pickled foods, high-salt diets, nutritional deficiencies, gastric polyps, gastric ulcers, family history of gastric cancer, and certain genetic mutations (Agullo-Garcia et al. 2020; Alipour 2021; Jin et al. 2020; Machlowska et al. 2020). Early-stage GC may not present noticeable symptoms. However, as the tumor grows and spreads, symptoms gradually appear, such as indigestion, stomach pain, weight loss, nausea and black stool (Waldum and Fossmark 2021). Advanced-stage GC may also cause anemia, fatigue, and jaundice (Waldum and Fossmark 2021). Diagnosis of GC typically involves physical examination, medical history inquiry, endoscopy, tissue biopsy, and imaging tests (Byun 2023; Weil et al. 2023; Yao et al. 2020; Zhang and Yu 2020). The treatment of GC depends on the tumor type, stage, and the overall condition of the patient (Seeneevassen et al. 2021; Tan 2019; Urs et al. 2022; Waldum and Fossmark 2021).
Metastasis of GC commonly occurs in the liver, peritoneum, lymph nodes, lungs, and bones. It can happen through direct extension to adjacent organs and tissues or through indirect dissemination via the bloodstream and lymphatic system (Rausei et al. 2016; Zhong et al. 2016). Lymph nodes are the most commonly involved areas, with GC typically spreading first to the surrounding lymph nodes and then to other sites (Ma et al. 2022). While curing metastatic GC is challenging, early diagnosis and comprehensive treatment can improve prognosis and quality of life for patients (Matsumoto et al. 2023). Regular follow-up and early intervention are important for optimal disease management in individuals diagnosed with GC (Conti et al. 2023).
Studies have found that claudin-5 is often dysregulated in cancer cells (Piontek et al. 2020). Yasuhiro Nakashima et al. (2020) illustrated that the sensitivity and specificity of claudin-5 expression could differentiate mesothelioma from angiosarcoma. Serge Paschoud et al. (2007) revealed that claudin-5 expression patterns could distinguish lung squamous cell carcinoma from adenocarcinoma. Zhang et al. (2022) confirmed that claudin-5 could against colitis-related tumorigenesis. These studies suggest that claudin-5 is crucial for tumor development. In this research, we found that claudin-5 overexpression expedited GC cells proliferation, but repressed GC cells apoptosis. Moreover, silencing claudin-5 curbed GC tumor growth in vivo. These results are similar to the findings of Zhang et al. (2022). In addition, Aikaterini Nanou et al. (2021) revealed that claudin-5 could promote neuroinflammation and tumor metastasis. Zheng et al. (2019) found that down-regulation of claudin-5 could inhibit migration and invasion of human non-small cell lung cancer cells. Herein, we found that claudin-5 overexpression expedited GC cells migration and invasion, which were alike with the description of Zheng et al. (2019). Moreover, we found for the first time that claudin-5 was upregulated in human GC tumor tissues and claudin-5 might linked with the lymph node metastasis of GC. This result confirmed again that claudin-5 promoted the progression and metastasis of GC. However, there are still some shortcomings in this paper. We only studied the role of claudin-5 in cell and mouse models. In the follow-up study, we will further verify the conclusions of this study in clinical practice.
Conclusion
Herein, we revealed that claudin-5 overexpression expedited GC cells proliferation, migration and invasion, but repressed GC cells apoptosis. These findings provide a relevant theoretical basis for the development of GC molecular diagnosis and further explain the molecular mechanism of GC cell metastasis.
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and any raw data can be obtained from the corresponding author upon request.
References
Agullo-Garcia A et al (2020) Clinical and anatomopathological features of eosinophilic oesophagitis in children and adults. Allergol Immunopathol (madr) 48:560–567
Alipour M (2021) Molecular mechanism of helicobacter pylori-induced gastric cancer. J Gastrointest Cancer 52:23–30
Byun S-H (2023) Multiple sinus pauses during suspension laryngoscopy with external laryngeal manipulation in hyperextended neck position in a patient with enlarged cervical lymph nodes: a case report. Signa Vitae 19(4):217–222
Conti CB et al (2023) Early gastric cancer: update on prevention, diagnosis and treatment. Int J Environ Res Public Health 20(3):2149
Escudero-Esparza A, Jiang WG, Martin TA (2012) Claudin-5 is involved in breast cancer cell motility through the N-WASP and ROCK signalling pathways. J Exp Clin Cancer Res 31:43
Gao K, Wu J (2019) National trend of gastric cancer mortality in China (2003–2015): a population-based study. Cancer Commun (lond) 39:24
Guide for the Care and Use of Laboratory Animals (2011) The National Academies collection: reports funded by National Institutes of health
Hashimoto I, Oshima T (2022) Claudins and gastric cancer: an overview. Cancers (basel) 14(2):290
Jin G et al (2020) Genetic risk, incident gastric cancer, and healthy lifestyle: a meta-analysis of genome-wide association studies and prospective cohort study. Lancet Oncol 21:1378–1386
Liu X et al (2019) Alterations of gastric mucosal microbiota across different stomach microhabitats in a cohort of 276 patients with gastric cancer. EBioMedicine 40:336–348
Ma D et al (2022) PET/CT for predicting occult lymph node metastasis in gastric cancer. Curr Oncol 29:6523–6539
Machlowska J et al (2020) Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci 21(11):4012
Matsumoto C et al (2023) Gastric cancer with a giant lymph node metastasis: a case report and review of the literature. Clin J Gastroenterol 16:336–343
Miettinen M, Sarlomo-Rikala M, Wang ZF (2011) Claudin-5 as an immunohistochemical marker for angiosarcoma and hemangioendotheliomas. Am J Surg Pathol 35:1848–1856
Morita K, Sasaki H, Furuse M, Tsukita S (1999) Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol 147:185–194
Nakashima Y et al (2020) Frequent expression of conventional endothelial markers in pleural mesothelioma: usefulness of claudin-5 as well as combined traditional markers to distinguish mesothelioma from angiosarcoma. Lung Cancer 148:20–27
Nanou A et al (2021) Endothelial Tpl2 regulates vascular barrier function via JNK-mediated degradation of claudin-5 promoting neuroinflammation or tumor metastasis. Cell Rep 35:109168
Pan G et al (2021) EMT-associated microRNAs and their roles in cancer stemness and drug resistance. Cancer Commun (lond) 41:199–217
Paschoud S, Bongiovanni M, Pache JC, Citi S (2007) Claudin-1 and claudin-5 expression patterns differentiate lung squamous cell carcinomas from adenocarcinomas. Mod Pathol 20:947–954
Phattarataratip E, Sappayatosok K (2016) Expression of claudin-5, claudin-7 and occludin in oral squamous cell carcinoma and their clinico-pathological significance. J Clin Exp Dent 8:e299-306
Piontek A et al (2020) Targeting claudin-overexpressing thyroid and lung cancer by modified Clostridium perfringens enterotoxin. Mol Oncol 14:261–276
Rausei S et al (2016) Seventh tumor-node-metastasis staging of gastric cancer: five-year follow-up. World J Gastroenterol 22:7748–7753
Seeneevassen L et al (2021) Gastric cancer: advances in carcinogenesis research and new therapeutic strategies. Int J Mol Sci 22(7):3418
Smyth EC et al (2020) Gastric cancer. Lancet 396:635–648
Tan Z (2019) Recent advances in the surgical treatment of advanced gastric cancer: a review. Med Sci Monit 25:3537–3541
Tian S et al (2020) SERPINH1 regulates EMT and gastric cancer metastasis via the Wnt/beta-catenin signaling pathway. Aging (albany NY) 12:3574–3593
Turunen M, Talvensaari-Mattila A, Soini Y, Santala M (2009) Claudin-5 overexpression correlates with aggressive behavior in serous ovarian adenocarcinoma. Anticancer Res 29:5185–5189
Urs AB et al (2022) Dentinogenic ghost cell tumor associated with odontoma: a unique histopathological entity and its surgical management. J Clin Pediatr Dent 46:148–151
Waldum H, Fossmark R (2021) Gastritis, gastric polyps and gastric cancer. Int J Mol Sci 22(12):6548
Weil CR et al (2023) Importance of radiographic tumor regression during radiotherapy in squamous cell versus adenocarcinoma of the uterine cervix as assessed by MRI and cone beam CT. Eur J Gynaecol Oncol 44:68–78
Xu T et al (2023) Effect of anlotinib combined with camrelizumab on clinical efficacy and short-term prognosis in male patients with advanced gastric cancer. J Men’s Health 19:52–57
Yang L, Sun X, Meng X (2018) Differences in the expression profiles of claudin proteins in human gastric carcinoma compared with non-neoplastic mucosa. Mol Med Rep 18:1271–1278
Yao K et al (2020) Guidelines for endoscopic diagnosis of early gastric cancer. Dig Endosc 32:663–698
Zhang Y, Yu J (2020) The role of MRI in the diagnosis and treatment of gastric cancer. Diagn Interv Radiol 26:176–182
Zhang Y et al (2022) Vitamin D receptor upregulates tight junction protein claudin-5 against colitis-associated tumorigenesis. Mucosal Immunol 15:683–697
Zheng Z et al (2019) Sulforaphane metabolites inhibit migration and invasion via microtubule-mediated Claudins dysfunction or inhibition of autolysosome formation in human non-small cell lung cancer cells. Cell Death Dis 10:259
Zhong J, Chen Y, Wang LJ (2016) Emerging molecular basis of hematogenous metastasis in gastric cancer. World J Gastroenterol 22:2434–2440
Acknowledgements
Not applicable.
Funding
This work was supported by Scientific Research Project of Bengbu Medical College (Grant No. 2021byzd045).
Author information
Authors and Affiliations
Contributions
SL and ZW designed the study and carried them out, SL, TZ, FX, ZD, JD supervised the data collection, analyzed the data, interpreted the data, SL and ZW prepare the manuscript for publication and reviewed the draft of the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Author Sandang Li declares that he/she has no conflict of interest; author Taizhe Zhang declares that he/she has no conflict of interest; author Fuchen Xie declares that he/she has no conflict of interest; author Zhaohui Du declares that he/she has no conflict of interest; author Jie Du declares that he/she has no conflict of interest; author Zhenjie Wang declares that he/she has no conflict of interest.
Ethical approval
Ethical approval was obtained from the Ethics Committee of The First Affiliated Hospital of Bengbu Medical College (Approval no.2022-342).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, S., Zhang, T., Xie, F. et al. Claudin-5 overexpression correlates with proliferation and migration in gastric cancer. Mol. Cell. Toxicol. (2023). https://doi.org/10.1007/s13273-023-00407-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s13273-023-00407-5