Abstract
The purpose of this study was to evaluate the size responses and vascular responses to three different sizes of Embosphere (EMBS) embolization particles used for chemo-embolization in patients with unresectable hepatocellular carcinoma (HCC). Forty-seven patients with biopsy proven HCC treated with TACE using EMBS (Biosphere Medical, Rockland, MA, USA) were included in this study. EMBS are non-resorbable tris-acryl gelatin defined-size microspheres. Sixteen patients were treated with 40–120 micron (40-μm), 13 patients with 100–300 (100-μm), and 18 patients with 300–500 (300-μm) EMBS particles. We measured the two-dimensional area and vascularity of the tumor index lesion on initial and subsequent CTs after treatment. Lesions were classified into four grades based on the degree of vascularity measured in 25% increments. Size of tumor after one treatment decreased by an average (avg) of 18% for 40–120-μm particles, 38% for 100–300-μm particles, and 17% for 300–500-μm particles. After three treatments, size decreased by an avg of 46% for 40–120-μm particles, 76% for 100–300-μm particles, and 46% for 300–500-μm particles. Vascularity decrease was also measured after the first and third treatments, and defined as a decrease of one or more grades in tumor vascularity. Results were as follows (% of patients with decrease). For 40–120-μm particles: 1 and 3 treatments, 53% and 88% of patients. For 100–300-μm particles: 1 and 3 treatments, 60% and 88% of patients. For 300–500-μm particles: 1 and 3 treatments, 50% and 57% of patients. It was concluded the 100–300-μm EMBS particles produce slightly higher responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary hepatic cancer and the fifth most common cancer worldwide. Almost a million new cases are diagnosed each year, with most cases presenting at advanced stages [1]. Unfortunately, it has been shown that there is an increasing incidence of HCC even in developed countries such as the USA [2]. Treatment options include local liver resection, liver transplantation, local ablative therapies and directed hepatic artery infusions [3–5]. Systemic chemotherapy results for HCC are usually disappointing [6]. Although liver transplant (LT) may offer the best treatment for patients who are not candidates for local resection, the availability of organs is limited. Furthermore, prolonged waiting times for LT may result in progression of disease even to the point of risking their candidacy [7–10]. Percutaneous treatment such as offered with trans-arterial chemo-embolization (TACE) is frequently employed to control tumor progression as primary therapy or while awaiting LT [11–13].
TACE for hepatic malignancies has been described in the medical literature for over two decades with various success rates [14–19]. This modality exploits the observation that 75–80% of the blood flow to the normal liver arises from the portal vein, but that the majority of the vascular flow to liver tumors is derived from the hepatic artery [20]. Intra-arterial chemotherapy infusion thus results in higher concentrations of the agents being delivered to the tumor bed while somewhat sparing normal hepatic parenchyma, assuming the portal vein is patent. Various combinations of chemotherapeutic and embolic agents have been used to try and decrease tumor burden and have been demonstrated to increase life expectancy as compared to systemic chemotherapy [21, 22]. However, there are no data on optimal particle size to be used. The recent introduction of defined-size particles permits the evaluation of useful particle size. The purpose of this study is to present our experience with TACE in patients with HCC using a combination of chemotherapy with three different size particles of Embogold Microspheres (EMBS, Biosphere Medical, Rockland, MA, USA).
Materials and methods
Forty-seven patients with biopsy-proven HCC recently underwent TACE with EMBS at our institution. On average they underwent TACE every 6–8 weeks. They were all referred to a single oncologist on staff at our institution (BIC) with extensive experience in treating patients with HCC.
TACE technique
All patients underwent baseline computed tomography (CT) scans for evaluation of tumor burden and location. Baseline liver-function tests were obtained in addition to renal function and coagulation studies. Appropriate pre-procedural intravenous antibiotics were administered to all patients. Vigorous hydration was initiated prior to the procedure. Most of the patients underwent TACE under conscious sedation. A combination of an anxiolytic agent, such as midazolam HCl, and pain medication such, as fentanyl citrate, was used. A minority of patients needed deeper sedation by the anesthesia service.
Complete baseline arteriograms of the superior mesenteric artery and celiac axis were obtained using standard selective percutaneous catheterization techniques to identify all hepatic arterial blood supply, including any aberrant vessels. Catheterizations were accomplished using standard percutaneous 5 French catheters such as the Cobra -2 and Sos Omni catheter (Angiodynamics, Queensbury, NY, USA). Angiograms were reviewed to ensure that there was complete visualization of all tumor vessels. If there was any discrepancy and all vessels could not be identified, aortograms were performed as needed to evaluate for any aberrant blood supply.
Individual treatment sessions were limited to unilobar or segmental therapy to minimize the risk of TACE-induced hepatic failure. Selective catheterization of the right or left hepatic artery was often achieved using a micro-catheter such as the Renegade catheter (Boston Scientific, Natick MA, USA), as needed.
About 5 mg of intra-arterial (IA) morphine and 20 mg of IA Decadron were infused into the vessel to be treated prior to administration of the chemotherapeutic agent to try and decrease chemo-toxicity to normal hepatocytes and to decrease the pain associated with embolization. If two vessels such as two left hepatic arteries were being treated then these pre-medications were divided in appropriate ratios between the vessels. In our experience the morphine appears to help with post-embolization pain.
The chemotherapeutic agent used was cisplatin at a dose of 125 mg m−2, infused over a period of approximately 30–45 min. Following chemotherapy infusion, embolization was performed with the predetermined size of EMBS. The EMBS were mixed with water-soluble contrast material (Optiray 320, Mallinckrodt, Hazelwood, MO, USA) as per the manufacturer’s directions and injected under fluoroscopic guidance. In most cases embolization was performed to the point of visibly stagnant blood flow. Only moderate antegrade slowing was used as the end point in those patients with elevated serum bilirubin levels and no embolization was performed in patients with a serum bilirubin level of greater than 2.0 mg dl−1 and they are excluded from this study.
Treatment was repeated every 6–8 weeks. Follow-up CT was obtained immediately prior to the next treatment. Thus, post cycle 1 CT was obtained immediately prior to treatment 2, and post cycle 3 CT was obtained immediately prior to treatment 4. Interestingly, even though EMBS are marketed as a permanent embolic agent, virtually all patients demonstrated a near baseline arteriogram on subsequent arteriograms despite being embolized to occlusion during the previous session.
Data analysis was obtained as under an IRB approved mechanism.
An honest broker collected all CT and angiographic data based on patients medical records. All patient data was de-identified and placed on a research Stentor server for independent review by the authors. Independent spreadsheets were then created based on randomly assigned patient serial numbers.
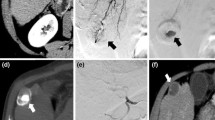
CT scans were evaluated for the largest lesion which was then classified as the index lesion. Data were collected for the index lesion. The two-dimensional size of the index lesion on initial and subsequent CT after TACE was recorded. Index lesions were also classified into four grades (A to D) based on the degree of vascularity measured in 25% increments with index lesions compared before and after TACE. Medical records were evaluated for any complications related to TACE by the oncologist since he had open access to the medical records based on patients signing an informed consent prior to the first treatment.
Results
After one treatment and based on the particle size used for embolization, tumor size decreased by an average of: 18, 38, and 17% after use of 40–120, 100–300, and 300–500-μm EMBS particles, respectively. After three treatments, size decreased by an average of: 46, 76, and 46% after use of for 40–120, 100–300, and 300–500-μm EMBS particles, respectively (Table 1).
Vascularity decreases were also measured after the 1st and 3rd treatments, and were defined as a percentage of patients with a decrease of 1 or more grades of tumor vascularity (Table 2). For 40–120-μm EMBS particles, decreases were found in 53% and 88% of patients, respectively, after the 1st and 3rd treatments. For 100–300-μm EMBS particles, decreases were found in 60% and 88% of patients, respectively, after the 1st and 3rd treatments. For 300–500-μm EMBS particles, decreases were found in 50% and 57% of patients, respectively, after the 1st and 3rd treatments (Table 3). One patient in the 300-μm group developed an intra-hepatic abscess that was treated with percutaneous drainage and went on to have further treatments.
Discussion
Unresectable hepatocellular carcinoma is a difficult cancer to manage due to its typically silent nature and advanced stage at clinical presentation. Those patients fortunate enough to be diagnosed early can be offered the potential for a cure with local resection and/or transplantation with resultant 5-year survival rates approaching 50%. Unfortunately less than a third of the patients present with such early stage, potentially curable disease. Patients with intermediate to advanced stage disease make up the bulk of patients seen clinically with median survival rates of less than 1 year [1, 3, 5]. Palliative and hospice care has traditionally been offered to these patients. With results of systemic treatment being dismal, newer treatment options developed over the last couple of decades bring the hope for increased survival.
Many of these newer treatment modalities rely on targeted delivery of the therapeutic agent, with a large bulk of them relying on trans-arterial delivery of different agents including chemotherapy, embolic agents, radio-immunotherapy, and gene vectors [23].
We have been treating HCC at our institution with a trans-arterial technique for several years, using cisplatin chemotherapy and either gelfoam or defined-size particles. Cisplatin has been observed to be more effective than doxorubicin as a single chemotherapeutic agent and has become our drug of choice [24]. Prior reports also indicated that hypoxia may result in increased uptake of chemotherapy into the hepatocytes, leading to the use of a combination of temporary embolic agents with intra-arterial chemotherapy [25]. Our initial experience was derived from the use of chemotherapy with Gelfoam (Upjohn, Kalamazoo, MI, USA), a temporary embolic agent. We chose a temporary agent to allow restoration of flow prior to the next treatment, since it is generally believed that multiple courses of TACE are more effective than a single administration [26]. Our protocol involved partial pre-embolization to slow antegrade flow followed by drug infusion over 30–45 min followed by a more aggressive final embolization. We felt that this slowed transit time of the chemotherapy agent through the vascular bed increased contact time. The Gelfoam particles were hand cut and then further broken down into smaller particles by running cut particles through a half open stopcock. Unfortunately, we ended up with particles of different non-reproducible sizes. In our experience there was no long-term permanent occlusion with Gelfoam.
EMBS were released in the USA in 1999 and marketed as defined-size embolic particles. Using predetermined sized particles seemed appealing. Furthermore, we were previously used to pre-embolizing the liver with gelfoam, and were concerned that a “permanent” agent such as EMBS might prevent the chemotherapy drug from reaching the target vessels during the chemotherapy infusion period. With various size particles of EMBS available, we were not sure what the optimal size of the embolic agent should be.
Our treatment practice evolved to administering the chemotherapy agent first followed by embolizing with EMBS only partially at the end. In fact, when chemotherapy is mixed with oil (Ethiodol or Lipiodol), others have found that if embolization was reserved till after administration of the mixture, more of the intended chemotherapeutic agent could be delivered and have thus advocated embolization at the end of the procedure [27].
With time we observed that when patients returned after 6–8 weeks for repeat treatment, follow-up arteriograms demonstrated that the vessels had completely returned to baseline appearance. We thus started performing more aggressive embolization to near complete stagnation often using several vials of EMBS, but still without any pre-embolization. We still noticed complete restoration of flow with resultant decrease in tumor vascularity, indicating that it did not seem to act as a permanent agent in the liver vasculature. Recent animal experiments elsewhere showed that 300–500-μm EMBS resulted in persistent occluded swine hepatic arteries at 1 week following embolization whereas, when smaller size particles (40–120 and 100–300) were used, the vessels were patent on 1 week follow-up angiograms [28]. Anecdotally, we have not seen any differences in the angiographic appearance of the vessels 6–8 weeks later on our follow-up studies amongst the different size EMBS. Vallee et al. [29] have demonstrated that EMBS can be safely mixed with various chemotherapy agents and are stable in vitro with virtually no change in their morphology, dimensions, or geometric characteristics.
With EMBS being pre-packaged in different sizes we were not sure what the optimal size particles would be. We thus decided to treat successive cohorts of HCC patients with different size particles. Due to our large Transplantation and Liver Cancer Center, we see several hundred patients annually with primary liver tumors. These patients are treated by nearly all described treatment modalities. Our primary liver tumor patients include patients with HCC, neuroendocrine tumors, and cholangiocarcinoma. For purposes of this paper we only evaluated those patients with primary HCC.
Our experience in HCC patients treated with EMBS has shown us that TACE with EMBS results in a decrease in tumor size and vascularity. Furthermore, despite aggressive embolization, patients demonstrated no significant long-term occlusion of normal hepatic vasculature. Patients treated with the 100–300-μm EMBS particles appeared to demonstrate a rapid and sustained decrease in size of the index lesion in this pilot study. Decrease in vascularity of the index lesion was seen with all three particle sizes and does not seem to differ much across the three sizes. The small patient population however, limited meaningful statistical analysis for concrete conclusions. Further follow-up data including liver enzyme levels, patient survival, and comparison with other embolic agents and analysis of response to additional treatments will be needed. We conclude that using EMBS for TACE, the 100–300-μm size particles appeared to produce higher response rates. Prospective comparison to other embolic agents will be needed in future.
References
Lau WY (2000) Primary liver tumors. Semin Surg Oncol 19:135–144
El-Serag HB, Mason AC (1999) Rising incidence of hepatocellular carcinoma in the United States. NEJM 340:745–750
Clark HP, Carson WF, Kavanagh PV, Ho CPH, Shen P, Zagoria RF (2005) Staging and current treatment of hepatocellular carcinoma. Radiographics 25:S3–S23
Llovet JM (2005) Updated treatment approach to hepatocellular carcinoma. J Gastroenterol 40:225–235
Varela M, Sala M, Llovet JM, Bruix J (2003) Treatment of hepatocellular carcinoma: is there an optimal strategy? Cancer Treat Rev 29:99–104
Ganne-Carrie N, Trinchet JC (2004) Systemic treatment of hepatocellular carcinoma. Eur J Gastroenterol Hepatol 16:275–281
Yao FY, Bass NM, Nikolai B, Davern TJ, Kerlan R, Wu V, Ascher NL, Roberts JP (2002) Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transplant 8(10):873–883
Yao FY, Bass NM, Nikolai B, Merriman R, Davern TJ, Kerlan R, Ascher NL, Roberts JP (2003) A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma: implications for the current organ allocation policy. Liver Transplant 9(7):684–692
Mazzaferro V, Regalia E, Doci R et al (1996) Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 334:693–699
Yao FY, Ferrell L, Bass NM, Bacchetti P, Ascher NL, Roberts JP (2002) Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transplant 8(9):765–774
Johnson EW, Holck PS, Levy AE, Yeh MM, Yeung RS (2004) The role of tumor ablation in bridging patients to liver transplantation. Arch Surg 139(8):825–9; discussion 829–30
Graziadei IW, Sandmueller H, Waldenberger P, Koenigsrainer A, Nachbaur K, Jaschke W, Margreiter R, Vogel W (2003) Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transplant 9(6):557–563
Hayashi PH, Ludkowski M, Forman LM, Osgood M, Johnson S, Kugelmas M, Trotter JF, Bak T, Wachs M, Kam I, Durham J, Everson GT (2004) Hepatic artery chemoembolization for hepatocellular carcinoma in patients listed for liver transplantation. Am J Transplant 4(5):782–787
Goldstein HM, Wallace S, Anderson JH, Bree RL, Gianturco C (1976) Transcatheter occlusion of abdominal tumors. Radiology 120:539–545
Chuang VP, Wallace S (1981) Hepatic artery embolization in the treatment of hepatic neoplasms. Radiology 140:51–58
Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S (1983) Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology 148:397–401
Lee KH, Sung KB, Lee DY, Park SJ, Kim KW, Yu JS (2002) Transcatheter arterial chemoembolization for hepatocellular carcinoma: anatomic and hemodynamic considerations in the hepatic artery and portal vein. Radiographics 22(5):1077–1091
Camma C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxi A, Cottone M (2002) Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 224(1):47–54
Gates J, Hartnell GG, Stuart KE, Clouse ME (1999) Chemoembolization of hepatic neoplasms: safety, complications, and when to worry. Radiographics 19(2):399–414
Breedis C, Young G (1954) The blood supply of neoplasm of the liver. Am J Pathol 30:969–985
Kamada K, Nakanishi T, Kitamoto M, Aikata H, Kawakami Y, Ito K, Asahara T, Kajiyama G. (2001) Long-term prognosis of patients undergoing transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: comparison of cisplatin lipiodol suspension and doxorubicin hydrochloride emulsion. Vasc Interv Radiol 12:847–854
Ebied OM, Federle MP, Carr BI, Pealer KM, Li W, Amesur N, Zajko A (2003) Evaluation of responses to chemoembolization in patients with unresectable hepatocellular carcinoma. Cancer 97:1042–1050
Kruskal JB, Goldberg N (2002) Emerging therapies for hepatocellular carcinoma: opportunities for radiologists. J Vasc Interv Radiol 13:S253–S258
Ono Y, Yoshimasu T, Ashikaga R et al (2000) Long-term results of lipiodol-transcatheter arterial embolization with cisplatin or doxorubicin for unresectable hepatocellular carcinoma. Am J Clin Oncol 23:564–568
Kruskal JB, Hlatky L, Hahnfeldt P, Teramoto K, Stokes KR, Clouse ME (1993) In vivo and in vitro analysis of the effectiveness of doxorubicin combined with temporary arterial occlusion in liver tumors. JVIR 4:741–747
Jaeger HJ, Mehring UM, Castaneda F et al (1996) Sequential transarterial chemoembolization for unresectable advanced hepatocellular carcinoma. Cardiovasc Intervent Radiol 19:388–396
Geschwind JF, Ramsey DE, van der Wal BC, Kobeiter H, Juluru K, Hartnell GG, Choti MA (2003) Transcatheter arterial chemoembolization of liver tumors: effects of embolization protocol on injectable volume of chemotherapy and subsequent arterial patency. Cardiovas Intervent Radiol 26(2):111–117
Soulen MC, Hoffman TW, Clark CM et al (2004) Abstract #110 Presented at the 29th Annual Scientific Meeting of the Society of Cardiovascular & Interventional Radiology, Phoenix, Arizona March 25–30
Vallee JN, Lo D, Guillevin R, Reb P, Adem C, Chiras J (2003) In vitro study of the compatibility of tris-acryl gelatin microspheres with various chemotherapeutic agents. J Vasc Intervent Radiol 14(5):621–628
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amesur, N.B., Zajko, A.B. & Carr, B.I. Chemo-embolization for Unresectable Hepatocellular Carcinoma with Different Sizes of Embolization Particles. Dig Dis Sci 53, 1400–1404 (2008). https://doi.org/10.1007/s10620-007-9995-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-007-9995-x