Abstract
Purpose
Contrast material pooling on angiography within a tumor bed during embolization for hepatocellular carcinoma (HCC) presents as the vascular lake (VL) phenomenon. This retrospective study aimed to evaluate the frequency and management of VLs during chemoembolization with drug-eluting beads (DEBs) and the relationship between the VL and local response.
Materials and methods
A total of 123 HCC patients without vascular invasion or intrahepatic metastases who underwent chemoembolization with DEBs (50–100 µm superabsorbent polymer microspheres loaded with epirubicin) were enrolled. Gelatin sponge particles (GS) were injected for additional embolization in limited patients with steadily increasing size of VLs during DEB injection. Overall, 338 nodules were divided into three groups: lesions without a VL (non-VL; n = 250); lesions with a VL but without additional GS embolization [VL–GS (−), n = 58]; and lesions with both VL and additional GS embolization [VL–GS (+); n = 30]. The local responses were statistically compared by Fisher’s exact test with the Bonferroni correction.
Results
The frequency of VLs was 26.0 % (88/338). The response rate in the non-VL group (54.0 %) was significantly lower than those in the VL–GS (−) (91.4 %; P < 0.0001) and VL–GS (+) (96.7 %; P < 0.0001) groups.
Conclusions
VL is a common angiographic finding and might be associated with the local response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemoembolization is recommended for unrespectable intermediate-stage hepatocellular carcinoma (HCC) as the first-line palliative treatment because of its positive impact on survival [1, 2]. Drug-eluting beads (DEBs) are recently developed calibrated microspheres designed to bond with chemotherapeutic agents and release the drugs over time after chemoembolization. DEBs showed an improved side-effect profile, while maintaining effectiveness, compared with conventional iodized oil-based transcatheter arterial chemoembolization (c-TACE) for treatment of HCC [3]. Chemoembolization using DEBs is technically similar to c-TACE [4], but occasionally provides a characteristic angiographic finding different from those for c-TACE, which is demonstrated as contrast material pooling associated with an aberrant intratumoral space newly developed during embolization. This angiographic finding can persist late into the venous phase, presents as the vascular lake (VL) phenomenon, and can resemble the angiographic findings of contrast material extravasation in hepatic injuries or hepatic tumor ruptures [5, 6]. To the best of our knowledge, the pathophysiologic mechanism underlying VL formation has not been fully elucidated, and there is a lack of information on both the clinical significance and safe management of VLs during the procedure.
The purpose of the present retrospective study was to investigate the frequency, predictive factors, and management of VLs during chemoembolization with DEBs in patients with HCC and the relationship between the VL and local response.
Materials and methods
Patients and tumor selection
We reviewed the initial procedure for 323 consecutive HCC patients who received chemoembolization with DEBs loaded with epirubicin between May 2007 and December 2012 in our institution. The selection criteria required patients to have: (1) well-to-moderately preserved liver function (Child-Pugh classification A or B); (2) no tumor thrombi in the portal and hepatic veins; (3) no extrahepatic metastases; (4) no multiple spread lesions (satellite lesions) within a narrow area. The reason for the last criterion is that angiography is a two-dimensional imaging modality that does not provide information regarding the anteroposterior direction. Consequently, it is difficult to identify individual lesions by both contrast-enhanced computed tomography (CECT) and angiography, especially when multiple and small lesions are distributed around the main lesions within a narrow area. Therefore, we excluded patients with multiple satellite lesions that were diagnosed on pretreatment baseline CECT. Of the 323 patients, 123 met the indicated criteria. Because of the retrospective nature of the study involving the use of imaging data and clinical records with no change in patient care, institutional review board approval was not required at our institution. Written informed consent was obtained from all patients before the procedure.
The patients comprised 84 males and 39 females with a mean age of 74 years (range 51–91 years). All of the patients had underlying chronic liver disease, which was caused by hepatitis C virus infection in 99, hepatitis B virus infection in 11, hepatitis B and C virus infections in 9, and other in 4. According to the Child-Pugh classifications, 80 patients (65.0 %) were class A and 43 (35.0 %) were class B. Seventy-seven patients (62.6 %) had undergone c-TACE with iodized oil (Lipiodol Ultrafluide; Laboratoire Guerbet, Aulnay-Sous-Bois, France) and gelatin sponge particles (GS) before chemoembolization with DEBs, which was conducted as a treatment for local recurrence or residual lesions after c-TACE. Regarding the lesions, 12 patients (9.8 %) had a solitary lesion and 111 (90.2 %) had multiple lesions (range 2–5). In the 123 enrolled patients, a total of 430 HCCs were detected on baseline CECT in this study. Of the 430 lesions, 338 with a long axis of ≥1 cm were defined as the target lesions for nodule-based analysis because these lesions could be detected and identified by both CECT and angiography, and residual small lesions with a long axis of <1 cm were excluded from the study. The mean longest tumor diameter of the 338 target lesions was 3.0 ± 1.9 cm (range 1.0–8.0 cm).
Beads
The DEBs used in this study were superabsorbent polymer microspheres that are mainly available in Europe and the USA under the brand names HepaSphere® and QuadraSphere® (Merit Medical Systems, South Jordan, UT, USA), respectively, and have diameters ranging from 50 to 100 µm in the dry state. The method for loading the DEBs with epirubicin was described elsewhere [5]. Briefly, a vial containing 25 mg of beads was loaded with 25 mg of epirubicin diluted in 5 ml of nonionic contrast material (iomeprol 350 mgI/ml) or NaCl-containing contrast material (mixture of iomeprol 350 mgI/ml and 10 % NaCl at a 4:1 ratio). After a loading period of about 30 min, the beads had expanded by approximately two times with the NaCl-containing contrast material and six times with the nonionic contrast material [7, 8].
Procedure
All procedures were performed by experienced interventional radiologists at our institution using a 2.1-F microcatheter through a 4-F catheter, which was inserted through the femoral artery according to the Seldinger method. The catheter position and choice of contrast material (nonionic or NaCl-containing) at the time of epirubicin loading were determined according to the tumor size, distribution, and vascularity as well as vascular anatomical variation. After angiographic identification of the tumor blush and catheterization into the feeding arteries, the prepared DEBs were suspended in contrast material (iomeprol 350 mgI/ml) diluted with normal saline (50:50) and then injected slowly in frequent small doses (0.5–1.0 mg of DEBs per injection) through the microcatheter. When initial near stasis of each feeding artery was achieved, a waiting time of about 5 min was allowed to ensure deeper distribution of the DEBs into the tumor microvasculature by the blood flow (redistribution of DEBs) [9]. Subsequently, more DEBs were injected by the same method until secondary near or complete stasis had been seen fluoroscopically. After the embolization with DEBs alone, angiography was performed to evaluate the achievement of the DEB embolic endpoint, which was defined as the disappearance or considerable reduction of the tumor blush and considered to demonstrate technical success.
Selective embolization was performed in the segmental artery or more distally for 203 lesions (60.1 %), while nonselective embolization was performed in the lobar artery for 135 lesions (39.9 %). The contrast material for dissolving epirubicin powder was the nonionic contrast material for 169 lesions (50 %) and the NaCl-containing contrast material for 169 lesions (50 %). In 44 lesions (13.0 %), embolization was performed through extrahepatic collateral pathways coupled with hepatic arteries [right inferior phrenic artery, n = 36; right internal thoracic artery, n = 5; right adrenal artery, n = 5; left inferior phrenic artery, n = 2; right renal capsular artery, n = 1 (duplicated)].
No antibiotic prophylaxis was given before or after embolization. Patients were discharged from the hospital at 48 h after embolization. No type of next or parallel treatment was performed within 3 months after embolization to preserve the liver function.
Management of VL
VL was defined as focal, well-circumscribed, and persistent retention of contrast material in various forms on the venous phase of angiography and was distinguished from a tumor blush. A VL occurred at various times during the procedure and changed its size with continuous injection of DEBs. During the DEB procedure, arteriography was repeated to evaluate the onset and size of the VL. Even in the presence of a VL on the angiogram, slow injection of DEBs was repeated until closure of the neovascularity. The operation was finished after confirming the disappearance or remarkable shrinkage of the VL compared with the maximum size observed during the DEB procedure and achievement of the DEB embolic endpoint. However, when the size of the VL increased or remained unchanged despite continuous DEB injection, the embolic material was switched from DEBs to GS (Gelpart; Nippon Kayaku, Tokyo, Japan) with a diameter of 1 or 2 mm, which was additionally injected until the disappearance of the VL.
Assessment
Baseline and follow-up CECTs were performed by multidetector computed tomography with a bolus contrast injection. Lesions that showed hypervascularity in the arterial phase of CECT and had relatively low density on the portal venous phase of CECT were diagnosed as viable HCC. Complications associated with embolization and VLs were evaluated on the basis of physical and hematological findings obtained during 4 weeks of follow-up after the procedure as well as CECT at postoperative week 4. The local response of each target lesion was evaluated by CECT at 3 months after the procedure according to the modified RECIST criteria [10]. The response rate (RR) of the target lesions was defined as the percentage of responders [complete response (CR) or partial response (PR)] to the total number. In view of the nature of nodule-based analysis, the responses of non-target lesions and new lesions were not evaluated in this study.
To evaluate the frequency and predictive factors of VLs, the 338 nodules were retrospectively divided into two groups according to the onset of VL on angiography: lesions with VL (VL group, n = 88) and lesions without VL (non-VL group, n = 250). Comparisons were made between the two groups with regard to tumor and treatment characteristics. Tumor information on baseline CECT was categorized by maximum diameter (<3 vs. ≥3 cm) and location (proximal vs. peripheral). With regard to tumor location, lesions were labeled as proximal if they were within 4 cm of the portal vein bifurcation and peripheral if not. Treatment information was categorized by contrast material used for dissolving epirubicin powder (nonionic vs. NaCl-containing), embolization selectivity (selective vs. nonselective), need for extrahepatic collateral embolization (no vs. yes), and dose of beads used (<10 vs. ≥10 mg).
To evaluate the relationships among VLs, additional GS embolization, and local response, the 338 nodules were retrospectively divided into three groups according to the onset of VLs and the need for additional GS embolization to deal with non-shrinking VLs: lesions without a VL (non-VL group, n = 250); lesions with a VL but without an additional GS embolization [VL–GS (−) group, n = 58] (Fig. 1); and lesions with both VL and additional GS embolization following DEB injection [VL–GS (+) group, n = 30] (Fig. 2).
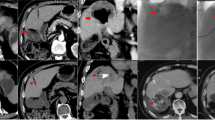
A lesion with a vascular lake (VL) that was not additionally injected with gelatin sponge particles (GS) in the VL–GS (−) group. The patient was a 68-year-old female with multiple recurrent hepatocellular carcinomas (HCCs) after repeated conventional transcatheter arterial chemoembolization. a Contrast-enhanced computed tomography (CECT) showed a 2.0-cm HCC at a peripheral site in the hepatic anterior segment (arrow). b Selective angiography of the anterior segmental branch of the right hepatic artery showed two tumor blushes, one of which was identified as a contrast-enhanced lesion in liver segment 5 on CECT (arrow). Chemoembolization was performed at this point. c Selective angiogram after chemoembolization with 7 mg of drug-eluting beads (DEBs) showed VL formation (arrow) with complete disappearance of the tumor blush. d CT during selective angiography just after chemoembolization showed pooling of the contrast material within the tumor (arrow), representing VL formation. e Selective angiography showed disappearance of the VL after additional injection of 1.0 mg of DEBs alone. f CECT at week 4 after chemoembolization revealed a lack of contrast enhancement in the majority of the tumor areas (arrow), suggesting a partial response
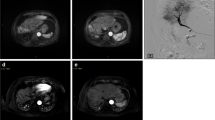
A lesion with a vascular lake (VL) that was additionally injected with gelatin sponge particles (GS) in the VL–GS (+) group. The patient was an 84-year-old male with a solitary 3.5-cm hepatocellular carcinoma (HCC) in the right hepatic lobe. a Selective angiography of the posterior superior subsegmental branch of the right hepatic artery showed a tumor blush. Chemoembolization was performed at this point. b After chemoembolization using 7 mg of drug-eluting beads (DEBs), selective angiography showed the onset of VL (arrow) and residual tumor blush. c After additional chemoembolization using 8 mg of DEBs, the majority of the tumor blush disappeared, and the size of the VL evidently increased. d Additional embolization using GS was performed until the VL disappeared completely
Statistical analysis
To investigate the correlations between the VL and tumor or treatment characteristics, the six variables for the VL and non-VL groups were analyzed by univariate analyses using the chi-square test. The variables significantly associated with VL (P < 0.05) in the univariate analyses were sequentially entered into a logistic regression model and subjected to multivariate analyses to assess their relative contributions.
To compare the local responses among the non-VL, VL–GS (−), and VL–GS (+) groups, the RR was compared between each set of two groups within the three groups by Fisher’s exact test. According to the Bonferroni correction for multiple comparisons, the results were considered significant for P < 0.017, representing an overall alpha level of <0.05.
Results
Frequency and predictive factors of VL
The frequency of VL was 26.0 % (88/338) by nodule-based analysis. The DEB embolic endpoint was achieved on angiography for all target lesions, regardless of the presence or absence of a VL, giving a technical success rate of 100 %.
Compared with the non-VL group, more lesions in the VL group had a maximum tumor diameter of ≥3 cm (67.0 vs. 22.8 %, P < 0.0001) and the dose of beads used ≥10 mg (67.0 vs. 48.0 %, P = 0.0021) (Table 1). In the multivariate logistic regression analyses, a maximum tumor diameter of ≥3 cm remained predictive for the appearance of VL (hazard ratio 6.98; 95 % CI 3.72–13.09; P < 0.0001) (Table 2). The frequencies of VL for lesions with maximum tumor diameters of <3 and ≥3 cm were 13.1 % (29/222) and 50.9 % (59/116), respectively.
Management of VL (Fig. 3)
Even after the onset of VL, DEBs continued to be injected into the 88 nodules with VL, resulting in the disappearance of the VL for 33 lesions and shrinkage of the VL for 25 lesions. Hence, the procedure was finished for these lesions [VL–GS (−) group]. Additional GS embolization was performed in the residual 30 lesions with stable or increased VL [VL–GS (+) group], resulting in complete disappearance of the VL on angiography for all 30 lesions. No embolization- or VL-associated serious complications, such as non-target organ embolization, bile duct damage (biloma and liver abscess), tumor rupture, and hemoperitoneum, were observed in any patients in the three groups.
Relationship between VL and local response
The local responses of the target lesions in the three groups, namely the non-VL, VL–GS (−), and VL–GS (+) groups, are listed in Table 3. The RR in the non-VL group (54.0 %) was significantly lower than those in the VL–GS (−) (91.4 %; P < 0.001) and VL–GS (+) (96.7 %; P < 0.001) groups.
Discussion
VL is a common angiographic finding during embolization using beads for patients with HCC. In our study, VL was noted in 26.0 % of nodules and occurred with a significantly higher frequency in patients with larger HCCs. However, there is no guideline for the management of VL during the procedure and insufficient information about VL-associated complications, including tumor rupture. In this study, no VL-associated complications were observed with additional GS embolization given exclusively to patients with non-shrinking VLs after DEB injection. This method for management of VLs might contribute to safety following chemoembolization with DEBs.
The etiology of VL during chemoembolization has not been well understood. Yamanaka et al. [11] discussed that VL formation as a histologic feature of the resected liver might reflect a blood space secondary to destroyed arterial vessels and tumor necrosis, which is demonstrated as contrast material pooling on preoperative angiography. However, they did not perform preoperative embolization in their patients. Therefore, the VL described in their paper resulted from the tumor nature and did not involve embolization. We hypothesized that VLs emerging during chemoembolization could arise through rupture of some of the tumor microvasculature, which may trigger partial disruption of the tumor architecture and result in a new blood space (Fig. 4). The tumor microvasculature formed by tumor angiogenesis is characterized by vessel dilation or an irregular shape [12] and is therefore vulnerable compared with normal vessel tissues. In the process of chemoembolization with DEBs, DEBs are first allowed to intensively flow into the part of the tumor with abundant blood flow, which can affect the intratumoral pressure gradient. As a result, the poorly developed tumor microvasculature may be partially subjected to a high inflow pressure and repeated arterial injection of contrast material, as well as physiological saline and beads during the procedure, and eventually become ruptured.
Hypothesis for vascular lake formation after chemoembolization using beads (black numbers indicate the virtual blood pressure). a Hepatocellular carcinoma seems to form an inhomogeneous tumor vasculature with a fragile microvasculature (arrow) through tumor neoangiogenesis. b Repeated arterial infusion of beads, contrast material, and normal saline during the procedure. The fragile microvasculature (arrow) receives increased vessel pressure because of the faster blockage of larger tumor vessels by the injected beads (arrowheads). c Vascular lake formation owing to rupture of the microvasculature (arrow). d Additional embolization using gelatin sponge particles (arrow) for a stable or increased vascular lake
This study showed that lesions with VLs were significantly associated with good tumor responses [local RR of 54.0 % in the non-VL group vs. 91.4 % in the VL–GS (−) group and 96.7 % in the VL–GS (+) group]. The appearance of VL may show the success of sufficient deep blockage of the feeding arteries, resulting in rupture of the tumor beds through the aforementioned mechanism. Therefore, a VL may be a favorable manifestation that is able to predict a good response.
We performed additional GS embolization for 34.1 % (30/88) of lesions with VL. This was because the injected DEBs were more likely to enter the larger VL cavity than the tumor vessels and also because no safety evidence was available for the presence or absence of associations between non-shrinking VL without additional GS injection and adverse events, including tumor rupture [4, 5]. In addition, there was no significant difference in RR between the VL–GS (−) and VL–GS (+) groups. This result further suggests that additional GS embolization for the management of VL may not meaningfully contribute to the local response. The GSs used in this study were considerably larger than the DEBs. Early switching of the embolic material from DEBs to GSs is considered a potential cause of incomplete blockage of deeper feeding arteries of the residual tumor stain. GS should be used after achievement of the DEB embolic endpoint exclusively for the purpose of avoiding the risk of post-embolic complications associated with VLs.
There are some limitations to the present study. First, the eligibility of the patients and the target lesions was restricted. This might lead to a biased assessment of the frequency of VL. Second, it remains unclear whether VL is actually a bead-specific finding, since an abnormal intratumoral space may also appear during c-TACE. However, it is difficult to radiographically distinguish between the iodized oil in VL and that accumulated in the entire tumor. Third, the observed differences in local responses between the VL group [including the VL–GS (−) group and VL–GS (+) group] and the non-VL group might be attributable to the differences found for some tumoral and procedural background factors. More lesions in the VL group had maximum tumor diameter of ≥3 cm and dose of beads used ≥10 mg; therefore, the tumor size and dose of beads used possibly influence the local response. However, it was impossible to evaluate the data with adjustment for such tumoral and procedural background factors because the dose of beads used could not be predicted prior to the procedure and also because this study had a retrospective nature. A further prospective study is necessary to evaluate the methods for management of VLs, including additional GS embolization, and the relationship between local responses and VL in chemoembolization with DEBs under appropriate control for various background factors.
In conclusion, VL is a relatively common angiographic finding without severe advanced events during chemoembolization with DEBs in patients with larger HCCs and might be associated with the local response.
References
Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–42.
Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–9.
Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41–52.
Kritzinger J, Klass D, Ho S, Lim H, Buczkowski A, Yoshida E, et al. Hepatic embolotherapy in interventional oncology: technology, techniques, and applications. Clin Radiol. 2013;68:1–15.
Seki A, Hori S, Kobayashi K, Narumiya S. Transcatheter arterial chemoembolization with epirubicin-loaded superabsorbent polymer microspheres for 135 hepatocellular carcinoma patients: single-center experience. Cardiovasc Intervent Radiol. 2011;34:557–65.
Osuga K, Hori S, Hiraishi K, Sugiura T, Hata Y, Higashihara H, et al. Bland embolization of hepatocellular carcinoma using superabsorbent polymer microspheres. Cardiovasc Intervent Radiol. 2008;31:1108–16.
Seki A, Hori S, Sueyoshi S, Hori A, Kono M, Murata S, et al. Transcatheter arterial embolization with spherical embolic agent for pulmonary metastases from renal cell carcinoma. Cardiovasc Intervent Radiol. 2013;36:1527–35.
Jiaqi Y, Hori S, Minamitani K, Hashimoto T, Yoshimura H, Nomura N, et al. A new embolic material: super absorbent polymer (SAP) microsphere and its embolic effects. Nihon Igaku Hoshasen Gakkai Zasshi. 1996;56:19–24.
Malagari K, Pomoni M, Moschouris H, Kelekis A, Charokopakis A, Bouma E, et al. Chemoembolization of hepatocellular carcinoma with HepaSphere 30–60 µm. Safety and efficacy study. Cardiovasc Intervent Radiol. 2014;37:165–75.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60.
Yamanaka J, Yamanaka N, Nakasho K, Tanaka T, Ando T, Yasui C, et al. Clinicopathologic analysis of stage II-III hepatocellular carcinoma showing early massive recurrence after liver resection. J Gastroenterol Hepatol. 2000;15:1192–8.
Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Seki, A., Hori, S. & Shimono, C. Management of vascular lake phenomenon on angiography during chemoembolization with superabsorbent polymer microspheres. Jpn J Radiol 33, 741–748 (2015). https://doi.org/10.1007/s11604-015-0486-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-015-0486-2