Abstract
The purpose of this study was to determine whether transcatheter arterial chemoembolization (TACE) protocol affects the total volume of chemotherapy injected into the liver as well as subsequent arterial patency. A total of 160 patients with primary or secondary liver cancer were treated with 3 different chemoembolization protocols at a single institution. Data were analyzed retrospectively. Group 1 (n = 36) consisted of slurry of chemotherapy, oil and polyvinyl alcohol particles (PVA), group 2 (n = 91), chemotherapy and oil followed by PVA, and group 3 (n = 33), chemotherapy and oil followed by Gelfoam pledgets. The total volume of chemotherapy injected into the liver was recorded. Arterial patency was determined during subsequent chemoembolizations. The mean percentage of total intended chemotherapy dose administered was 54.6% for group 1, 75.3% for group 2, and 80.6% for group 3. Arterial patency at follow-up angiography was 56% for group 1, 74% for group 2, and 81% for group 3. The slurry protocol (group 1) significantly reduced arterial patency and injectable volume of chemotherapy during TACE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The management of patients with advanced primary or metastatic liver cancer remains extremely challenging. Despite the development of new treatment modalities, clinical outcomes are typically poor. Few therapeutic options are effective, especially since neither conventional systemic chemotherapy nor radiation therapy can halt the progression of disease [1 2 3]. Transcatheter arterial chemoembolization (TACE), which consists of delivering a mixture of ethiodized oil, chemotherapeutic agents and embolic particles directly to the tumor, is commonly performed to treat these types of liver cancer. TACE is particularly effective in providing symptomatic relief and prolonging the life of patients with neuroendocrine tumors, especially those of the carcinoid type [4]. In addition, multiple studies, through neither randomized nor prospective, have shown TACE to have a survival benefit in patients with hepatocellular carcinoma when compared to historical data [5 6 7 8 9 10 11]. This survival benefit was accompanied by significant tumor response, as demonstrated by a reduction in tumor volume on imaging studies and in tumor marker levels after TACE [8 9 10 11]. Several randomized controlled trials comparing TACE to supportive care have failed to demonstrate a statistically significant survival benefit of TACE [9 12 13 14]. As described in a forthcoming meta-analysis of these trials, it is likely that severe methodological deficiencies inherent to these studies underlie their failure to validate TACE [15]. Recently, two rigorous, randomized, controlled trials have demonstrated a survival benefit associated with chemoembolization [16 17]. The exacting nature of the protocols used in these trials underscores the importance of the chemoembolization procedure in achieving good outcomes.
Although many different chemoembolization protocols have been used in the past [8 9 10 11 12 13 14 18], the combination of some chemotherapeutic agents and a vehicle such as iodized oil constitutes the basis of most TACE procedures. An embolic material consisting of either polyvinyl alcohol (PVA) or Gelfoam particles is usually added next. These embolic agents produce different effects on vasculature, since PVA causes permanent or semi-permanent occlusion, whereas Gelfoam causes temporary occlusion with recanalization taking place within 2 weeks [19]. Given these properties of these embolic agents, use of a particular embolic agent may affect the actual amount of chemoembolization mixture administered into the tumor, potentially causing permanent arterial occlusion, thereby compromising arterial access for subsequent TACE. The choice of embolic material—PVA or Gelfoam particles—as well as the manner in which these agents are delivered—slurry of chemotherapy, oil and embolic particles or chemotherapeutic agents and oil followed by embolic material—may therefore play a critical role in the success of TACE. The purpose of this study was to assess whether the choice of TACE protocol and embolic material would affect 1) the amount of chemotherapy actually injected into the tumor and 2) subsequent arterial patency.
Materials and Methods
Patient Population
Between August 1995 and December 2001, 385 TACE procedures were performed at a single institution on 160 patients with unresectable primary or secondary liver cancer. Each patient provided informed consent prior to each TACE procedure; the data were analyzed retrospectively to assess changes in TACE protocol at our institution. The type of tumor was consistent among all groups (Table 1). Of these patients, 121 had at least one repeat hepatic arteriogram during a subsequent TACE procedure that demonstrated the status of arterial supply to the tumor. The 39 patients who underwent only one TACE procedure without further arteriograms were excluded from this study because the patency of the arterial supply to the tumor could not be assessed angiographically. Most patients with metastatic liver cancer treated with TACE failed to respond to conventional systemic chemotherapy, resulting in tumor progression. As described by Chung [19], we could safely perform selective (segmental or sub-segmental) TACE for patients with small tumors who have poor liver function (Child-Pugh class C). Main portal vein thrombosis was not considered an absolute contraindication to chemoembolization, but special precautions were taken to minimize the use of embolic particles in order to reduce the risk of hepatic necrosis or infarct. However, patients demonstrating the constellation of a massive tumor combined with severe portal vein invasion and poor liver function were not offered TACE. The characteristics of the patient population are given in Table 1.
Chemoembolization Protocol
After obtaining arterial access, a diagnostic visceral arteriogram was performed to delineate the arterial supply to the tumor, determine the presence of variant arterial anatomy, and confirm portal vein patency. As stated earlier, portal vein thrombosis did not necessarily constitute a contraindication to performing TACE. A catheter was then advanced selectively into the right or left hepatic artery, distal to the cystic artery. Patients with multiple or diffuse lesions received lobar embolizations. Using a microcatheter to select a second or third-order branch of the right or left hepatic artery, patients with unifocal tumors were treated with selective chemoembolization. The proportion of patients treated with super-selective chemoembolization is shown in Table 1.
After having safely positioned the catheter within the artery feeding the tumor, the chemoembolization mixture was infused into the artery. The same three chemotherapeutic agents were used consistently at our institution (based on the Hospital of the University of Pennsylvania protocol), regardless of tumor type. This regimen was comprised of cisplatin 100 mg (Bristol Myers Squibb, Princeton, NJ), doxorubicin 50 mg (Adriamycin; Pharmacia-Upjohn, Kalamazoo, MI) and mitomycin C 10 mg (Bedford Laboratories, Beford, OH) mixed in 10 ml of water-soluble contrast medium (Omnipaque; Winthrop Pharmaceuticals, New York, NY). After having received the chemotherapy from the pharmacy, it was consistently mixed with an equivalent volume of ethiodized oil.
Study Groups
Patients were treated with three different chemoembolization protocols. In group 1 (n = 36 patients), PVA particles (0.1–0.2 cc of 150–250 μm dry particles; Boston Scientific Medi-tech, Natick, MA) were mixed with the chemotherapy-oil mixture at the beginning of the procedure and injected as part of a slurry throughout the procedure. In group 2 (n = 91), PVA particles (150–250 μm) were administered after injection of the chemotherapy-oil mixture. In group 3 (n = 33), small pledgets of gelatin sponge (Gelfoam; Upjohn, Kalamazoo, MI) were used for particle embolization after administration of the total dose of the chemotherapy-oil mixture. In all groups, the chemotherapy was mixed in a 1:1 volume ratio with lipiodol. The only difference between groups 2 and 3 was the type of embolic agent used (PVA for group 2, Gelfoam for group 3). The mean volumes of chemotherapy, delivered by the pharmacy, were identical for all groups. The goal of therapy for all patients was to administer the entire intended dose (all 20 ml) of chemotherapy and oil mixture. The procedure was stopped either at the point of near stasis within the main artery feeding the tumor (approximately 90% reduction of flow) or after the entire amount of chemotherapy was administered, in which case the main vessel remained mostly patent. For groups 2 and 3, embolic agents were consistently delivered at the end of the procedure.
Nearly identical proportions of tumor types, tumor sizes, portal venous thrombosis and degrees of hypervascularity at angiography among the three groups (Table 1) ensure that these variables would not confound arterial patency data. Seven patients had colorectal metastases (2, 4, and 1 in groups 1–3, respectively), 29 patients had neuroendocrine tumors (6, 17, and 6 in groups 1–3), and 101 had hepatocellular carcinoma (24, 56, and 21 in groups 1–3). The mean greatest transverse tumor diameter measured by CT or MRI was 7.2, 8.3, and 7.9 cm for each group, respectively. Fourteen patients presented with portal vein thrombosis (3, 8, and 3 in groups 1–3, respectively). All three groups had comparable proportions of hypervascular tumors (89%, 93%, 94% for each group, respectively). The mean interval between consecutive arteriograms was 9 ± 0.4 weeks for group 1, 7 ± 0.2 weeks for group 2, and 7 ± 0.2 weeks for group 3. Note that differences in follow-up are largely due to scheduling issues and were not related to the patency of the vessels or the status of the liver.
In order to assess arterial patency, arteriograms obtained during each chemoembolization procedure were reviewed simultaneously by two experienced interventional radiologists blinded to both the type of particle embolization used during the procedure and the TACE protocol. Patency was assessed based on recanalization of previously embolized arteries. All patients underwent a celiac or common hepatic arteriogram if not superselective catheterization at follow-up to assess the patency of previously treated vessels. A vessel was considered patent or recanalized when forward flow extending into the hepatic parenchyma or the tumor itself was identified. Each treated vessel was considered patent or nonpatent at follow-up. The same artery or arteries feeding the tumor was assessed for patency after each chemoembolization; in this manner, baseline pre-TACE arterial flow could be compared to arterial flow at follow-up in the same patient. Using this method, we could assess both the flow of main feeding vessels and overall tumor vascularity. A tumor was considered hypervascular when an area of tumor blush uniformly hyperattenuating to liver parenchyma was identified. Areas of tumor blush corresponded to the known location of the tumor on cross-sectional imaging.
The actual volume of chemoembolization mixture (either slurry of chemotherapy, oil and particles or chemotherapy and oil alone) as well as the volume of chemotherapy administered during each procedure was obtained immediately following each TACE procedure from dictated procedural reports. The amount of chemotherapy actually injected into the tumor was then expressed as a percentage of the total volume of chemotherapy retrieved from the pharmacy. Occasionally, the embolic effects of ethiodol would preclude administration of a complete dose of chemotherapy for patients in groups 2 and 3; these patients still received PVA or Gelfoam particles following injection of chemotherapy, ensuring protocol consistency.
Data Analysis
Proportions from each of the three groups were compared to each other using 2-sample Z tests. All P values are two-tailed. Statistical significance was established when P ≤ 0.05. All data ranges shown indicate the standard deviation of a given data set. Chi-square cross-tabulation tests were used to evaluate differences between the groups in age, gender, race, proportion of patients with hepatocellular carcinoma, tumor vascularity, interval between procedures and tumor size. None of these variables approached statistical significance.
Results
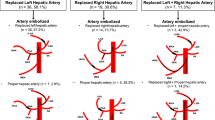
Patients treated with the slurry of chemotherapy, lipiodol, and PVA particles (group 1, n = 36) received a significantly lower dose of chemotherapy than those treated with either PVA or Gelfoam, administered at the end of the procedure (groups 2 (n = 91) and 3 (n = 33), respectively). The mean volume of chemotherapy administered to patients in group 1 was 55 ± 4% (5.0/9.0 ml) of the intended volume, whereas it was 75 ± 5% (6.4/8.5 ml) for patients in group 2 and 81 ± 7% (6.9/8.5 ml) for patients in group 3 (Table 1). Differences between groups 1 and 2 (P = 0.028) and groups 1 and 3 (P = 0.02) were statistically significant, whereas the difference between groups 2 and 3 (P = 0.52) was not.
The number of patients who received the entire intended dose of chemotherapy was lowest in group 1. Indeed, the entire dose of chemotherapy was given to 19% of the patients in group 1 (7/36 patients, 9 ml), 86% of the patients in group 2 (78/91, 8.5 ml), and 91% of the patients in group 3 (30/33, 8.5 ml). The number of patients that received the entire intended dose of chemotherapy was significantly greater both in groups 2 and 3 than in group 1 (P < 0.0001). However, no difference was found between groups 2 and 3 (P = 0.46).
Arterial patency was found to be greatest when the embolic agents (PVA or Gelfoam) were administered at the end of the procedure. In group 1, 56% of previously embolized arteries were found to be recanalized at follow-up (Fig. 1), whereas 74% and 81% (Fig. 2) of previously embolized vessels in groups 2 and 3 had recanalized at follow-up. The degree of recanalization was significantly greater in group 2 (Fig. 1) than in group 1 (P = 0.05). However, no statistically significant differences were observed between groups 2 and 3 (P = 0.42) and between groups 3 and 1 (P = 0.07). Despite the great difference in average recanalization between groups 1 and 3 (56% vs. 81%), the low number of patients in groups 1 and 3 is likely to account for the lack of statistical significance between the two groups.
Discussion
Transcatheter arterial chemoembolization was designed to achieve regional tumor control by delivering high doses of chemotherapeutic agents directly to the tumor site and slowing down the transit time of these agents within the tumor. The combination of intraarterial delivery of chemotherapy and some degree of arterial embolization allowed for greater drug concentration within the tumor, minimizing systemic toxicity in the process [19 20]. Despite its potency, chemoembolization has not been able to provide a cure for liver cancer. However, it has proven effective at prolonging survival, especially in patients with hepatocellular carcinoma and metastatic neuroendocrine tumors, and at providing symptomatic relief [21 22], justifying its usefulness as a palliative tool against liver cancer.
After having gained experience using the TACE protocol consisting of the slurry of chemotherapy, lipiodol and PVA, we noted that the complete dose of chemotherapy could not frequently be administered, often diminishing the efficacy of TACE. This observation constituted the basis of our study. Our results confirmed that a higher percentage of patients received the entire dose of chemotherapy and that the mean volume of injected chemotherapy was greater when the embolic particles were injected at the end of the procedure. Thus, if the goal of chemoembolization is to deliver the highest possible dose of chemotherapy to the tumor, patients treated with the slurry protocol may in fact have been deprived of the theoretical effects of the chemotherapeutic agents. It is likely that early injection of PVA as part of the slurry of chemotherapy, lipiodol and embolic material prevented the chemotherapy from being delivered to the tumor. On the other hand, when PVA particles were injected at the end of the procedure, they had no impact on the dose of chemotherapy delivered at the time of the chemoembolization or during subsequent chemoembolizations.
The type of embolic material used (PVA or Gelfoam) did not significantly affect the volume of chemotherapy administered since most patients received the intended dose of chemotherapy (86% with PVA and 90% with Gelfoam). Note that not all patients in these groups received the entire amount of chemotherapy. This is likely due to the embolization effects of the lipiodol, which can reduce arterial inflow to the tumor, especially further distally.
To account for these factors, Matsuo et al. [23] matched the volume of lipiodol used in chemoembolization to tumor size and vascularity. They found that tumor necrosis was maximized when the injected volume of lipiodol (in ml) was less than tumor diameter (in cm) for tumors larger than 5 cm. Our study supports Matsuo’s findings, since the embolic effects of lipiodol occasionally prevented administration of the complete dose of chemotherapy for some patients. Reduction of the lipiodol volume may have allowed increased delivery of chemotherapy, thereby facilitating a greater tumor response. The approach described by Matsuo may be warranted to ensure delivery of the entire dose of chemotherapy.
Some controversy continues to surround the relative amounts of embolization and chemotherapy that should be used to optimally treat liver cancer. In fact, the precise effects of embolization on tumor cells remain largely unknown. In a recent study, Kobayashi et al. [24] found that blood levels of vascular endothelial growth factor were markedly increased in patients who had been treated with embolization, suggesting a direct link among the degree of embolization, tumor hypoxia, and the stimulation of new blood vessels [24]. However, the impact of embolization on the patency of arteries directly responsible for feeding the tumor has not been evaluated. Whereas some studies have reported excellent results with embolization alone (mostly against neuroendocrine tumors), others support the use of chemotherapy as a part of a cocktail, containing an oily vehicle and embolic particles, delivered to the tumor [6 25 26].
Several reports have shown TACE to be much more effective when administered multiple times as opposed to a single treatment, since those patients demonstrate a greater degree of tumor necrosis [27 28]. Since recanalization of tumor vasculature is a prerequisite to subsequent TACE procedures, determination of the long-term effects of embolic agents is of critical importance. Our study suggests that the type of chemoembolization protocol rather than the type of embolic material had a significant impact on the rate of arterial recanalization or arterial patency. Indeed, when the slurry of chemotherapy, lipiodol and PVA particles was used, only 56% of previously embolized vessels were found to be patent at subsequent TACE procedures. However, when the PVA particles were administered separately at the end of the procedure, 74% of vessels remained patent. This was also true when Gelfoam pledgets were used (81% patency) in the same manner. These results are somewhat surprising since it has long been believed the embolization with PVA particles results in more permanent vessel occlusion than that with Gelfoam [29]. Because of their smaller size (150 μm), PVA particles are supposed to penetrate deeply into the vascular bed of the tumor, resulting in more distal and permanent vascular occlusion than encountered with Gelfoam pledgets. However, PVA particles do not appear to influence the patency of the main feeding vessel, as our study suggests.
The timing of embolic particle administration during chemoembolization has a significant impact on both the injectable volume of chemotherapy and subsequent arterial patency. This study does have limitations, since we did not randomize patients to various protocols, and we have not analyzed patient outcomes for each protocol. However, we believe these data show that the administration of PVA particles along with chemotherapy and lipiodol as part of a slurry effectively reduces the potential efficacy of chemoembolization, since less chemotherapy could be injected. This protocol also limits the benefits of subsequent chemoembolization therapy because of reduced arterial patency. Therefore, based on these results, we favor the use of embolic agents with the last dose of TACE.
(A) Selective digital subtraction arteriogram, via microcatheter, prior to chemoembolization with a slurry of PVA, chemotherapy, and ethiodol. This patient was treated for metastatic carcinoid tumor. (B) Selective digital subtraction arteriogram acquired 7 weeks later, showing minimal recanalization of the previously embolized arteries.
(A) Digital subtraction image of a diagnostic visceral arteriogram, obtained prior to the initial chemoembolization. Note that the hepatic arteries are widely patent. This patient was treated for a neuroendocrine tumor, metastatic to the liver. (B) Selective digital subtraction arteriogram, via microcatheter, immediately after chemoembolization using Gelfoam as the embolic material, administered at the end of the procedure. Note that the tumor vessels are occluded. (C) Selective digital subtraction arteriogram acquired 8 weeks after chemoembolization, shown in Figure 2b, showing complete recanalization of the treated arteries.
References
S Okada (1998) ArticleTitleChemotherapy in hepatocellular carcinoma. Hepatogastroenterology 45 IssueIDSuppl. 3 1259–1263
P Rougier E Mitry MC. Clavero-Fabri (1998) ArticleTitleChemotherapy and medical treatment of hepatocellular carcinoma. Hepatogastroenterology 45 IssueIDSuppl 3 1264–1266
ZY Tang (1998) ArticleTitleTreatment of hepatocellular carcinoma. Digestion 59 556–562 Occurrence Handle10.1159/000007531 Occurrence Handle1:STN:280:DyaK1czntV2nsw%3D%3D Occurrence Handle9705539
JG Drougas LB Anthony TK Blair et al. (1998) ArticleTitleHepatic artery chemoembolization for management of patients with advanced metastatic carcinoid tumors. Am J Surg 175 408–412 Occurrence Handle9600289
PM Sanz-Altamira LD Spence MS Huberman et al. (1997) ArticleTitleSelective chemoembolization in the management of hepatic metastases in refractory colorectal carcinoma: A phase II trial. Dis Colon Rectum 40 770–775
Y Hatanaka Y Yamashita M Takahashi et al. (1995) ArticleTitleUnresectable hepatocellular carcinoma: Analysis of prognostic factors in transcatheter management. Radiology 195 747–752 Occurrence Handle7754005
Y Bayraktar F Balkanci B Kayhan et al. (1996) ArticleTitleA comparison of chemoembolization with conventional chemotherapy and symptomatic treatment in cirrhotic patients with hepatocellular carcinoma. Hepatogastroenterology 43 681–687
B Sangro M Herráiz MA Martínez-González et al. (1998) ArticleTitlePrognosis of hepatocellular carcinoma in relation to treatment: A multivariate analysis of 178 patients from a single European institution. Surgery 124 575–583 Occurrence Handle10.1067/msy.1998.90359 Occurrence Handle1:STN:280:DyaK1cvgvVWiuw%3D%3D Occurrence Handle9736912
InstitutionalAuthorNameGroupe d’Etude et de Traitement du Carcinome Hepatocellulaire (1995) ArticleTitleA comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. N Engl J Med 332 1256–1261 Occurrence Handle7708069
JC Trinchet N Ganne-Carrie M Beaugrand (1998) ArticleTitleIntra-arterial chemoembolization in patients with hepatocellular carcinoma. Hepatogastroenterology 45 IssueIDSuppl 3 1242–1247 Occurrence Handle9730382
CL Liu ST Fan (1997) ArticleTitleNonresectional therapies for hepatocellular carcinoma. Am J Surg 173 358–363 Occurrence Handle10.1016/S0002-9610(96)00384-4 Occurrence Handle1:STN:280:ByiB1c3kvVw%3D Occurrence Handle9136797
J Bruix JM Llovet A Castells et al. (1998) ArticleTitleTransarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: Results of a randomized, controlled trial in a single institution. Hepatology 27 1578–1583
G Pelletier M Ducreux F Gay et al. (1998) ArticleTitleTreatment of unresectable hepatocellular carcinoma with lipiodol chemoembolization: A multicenter randomized trial. J Hepatol 29 129–134 Occurrence Handle10.1016/S0168-8278(98)80187-6 Occurrence Handle1:CAS:528:DyaK1cXmvVWhsLk%3D Occurrence Handle9696501
JL Raoul D Guyader JF Bretagne et al. (1997) ArticleTitleProspective randomized trial of chemoembolization versus intra-arterial injection of 131I-labeled-iodized oil in the treatment of hepatocellular carcinoma. Hepatology 26 1156–1161
JFH Geschwind DE Ramsey MA Choti et al. (2002) ArticleTitleChemoembolization of hepatocellular carcinoma: Results of a meta-analysis. Am J Clin Oncol Occurrence Handle11943901
CM Lo H Ngan WK Tso et al. (2002) ArticleTitleRandomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 35 1164–1171 Occurrence Handle10.1053/jhep.2002.33156
JM Llovet MI Real X Montana et al. (2002) ArticleTitleArterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet 359 1734–1739 Occurrence Handle10.1016/S0140-6736(02)08649-X Occurrence Handle12049862
ME Clouse KR Stokes JB Kruskal et al. (1993) ArticleTitleChemoembolization for hepatocellular carcinoma: Epinephrine followed by a doxorubicin-ethiodized oil emulsion and gelatin sponge powder. J Vasc Interv Radiol 4 717–725 Occurrence Handle7506597
JW Chung (1998) ArticleTitleTranscatheter arterial chemoembolization of hepatocellular carcinoma. Hepatogastroenterology 45 IssueIDSuppl. 3 1236–1241 Occurrence Handle9730381
J Isenberg R Fischbach I Krüger et al. (1996) ArticleTitleTreatment of liver metastases from colorectal cancer. Anticancer Res 16 1291–1295 Occurrence Handle8702252
JP Bronowicki K Boudjema L Chone et al. (1996) ArticleTitleComparison of resection, liver transplantation and transcatheter oily chemoembolization in the treatment of hepatocellular carcinoma. J Hepatol 24 293–300 Occurrence Handle10.1016/S0168-8278(96)80007-9 Occurrence Handle8778195
DH Berger CH Carrasco DC Hohn et al. (1995) ArticleTitleHepatic artery chemoembolization or embolization for primary and metastatic liver tumors: Post-treatment management and complications. J Surg Oncol 60 116–121 Occurrence Handle7564377
N Matsuo H Uchida H Sakaguchi et al. (1997) ArticleTitleOptimal lipiodol volume in transcatheter arterial chemoembolotherapy for hepatocellular carcinoma: Study based on lipiodol accumulation patterns and histopathologic findings. Semin Oncol 24 IssueIDSuppl 6 S6-61–S6-70
N Kobayashi M Ishii Y Ueno et al. (1999) ArticleTitleCo-expression of Bcl-2 protein and vascular endothelial growth factor in hepatocellular carcinomas treated by chemoembolization. Liver 19 25–31
GF Stefanini P Amorati M Biselli et al. (1995) ArticleTitleEfficacy of transarterial targeted treatments on survival of patients with hepatocellular carcinoma. An Italian experience. Cancer 75 2427–2434
C Tellez AB Benson III MT Lyster et al. (1998) ArticleTitlePhase II trial of chemoembolization for the treatment of metastatic colorectal carcinoma to the liver and review of the literature. Cancer 82 1250–1259 Occurrence Handle1:CAS:528:DyaK1cXitlWltbg%3D Occurrence Handle9529016
K Yamamoto M Masuzawa M Kato et al. (1997) ArticleTitleEvaluation of combined therapy with chemoembolization and ethanol injection for advanced hepatocellular carcinoma. Semin Oncol 24 IssueIDSuppl. 6 S6-50–S6-55
HJ Jaeger U-M Mehring F Castaneda et al. (1996) ArticleTitleSequential transarterial chemoembolization for unresectable advanced hepatocellular carcinoma. Cardiovasc Intervent Radiol 19 388–396 Occurrence Handle10.1007/s002709900085
L Guillais R Leroyer (1991) ArticleTitleTherapeutic embolization devices. J Pharm Clin 10 241–246
Acknowledgements
This study was presented in part at the Annual Meeting of the Cardiovascular and Interventional Radiology Society of Europe, in Prague, Czech Republic, from September 26–30, 1999 and at the Annual Scientific Meeting of the Society for Cardiovascular and Interventional Radiology in Baltimore, Maryland from April 6–11, 2002. JFG was supported by an American Cancer Society research grant (58-005-39).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Geschwind, JF., Ramsey, D., van der Wal, B. et al. Transcatheter Arterial Chemoembolization of Liver Tumors: Effects of Embolization Protocol on Injectable Volume of Chemotherapy and Subsequent Arterial Patency . CVIR 26, 111–117 (2003). https://doi.org/10.1007/s00270-002-2524-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-002-2524-6