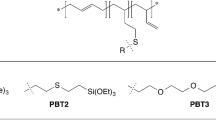
Abstract
In this paper, several methods of hydrophobization of cotton fabrics using the thio-ene click reaction were compared. Durable, superhydrophobic textiles were obtained in an easy way. Various variants of functionalized silsesquioxanes were used for the hydrophobization of fabrics. The synthesis of bifunctional silsesquioxanes (RSiMe2O)4(ViSiMe2O)4Si8O12 and (RSiMe2O)4(R’SiMe2O)4Si8O12 were performed via hydrothiolation of silsesquioxane derivative (ViSiMe2O)8Si8O12. Alkoxysilyl, alkyl and fluoroalkyl moieties were introduced as functional groups. Samples were prepared using four methods, differing in the modification method and the number of stages. During the research, fabrics were modified via (a) the dip-coating process, (b) carrying out thiol-ene click reactions directly on the surface of the fabric and (c) using both of these methods. The hydrophobicity of the fabric was evaluated by measuring the Water contact angle (WCA). The obtained samples were also examined using infrared analysis (FT-IR), Scanning electron microscope (SEM), and Elemental analysis (SEM–EDS). All analyses were performed before and after the washing process in order to verify the stability of the performed modifications.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thiol-ene click chemistry is a powerful tool for creating carbon–sulfur bonds. This type of reaction is characterized by mild process conditions. It can take place at ambient temperatures and atmospheric pressure and it is also insensitive to the presence of air and moisture. In addition, the reaction between thiols and unsaturated carbon–carbon bonds takes place with high yields and very often with high selectivity. Another advantage of hydrothiolation is the possibility to obtain a reaction using a wide range of solvents or reaction may even proceed without solvents. The thiol-ene click reaction also has the advantage of not requiring the use of expensive transition metal catalysts and the range of reactants is wide (Hoyle and Bowman 2010; Rissing and Son 2009; Sinha and Equbal 2019; Wendeln et al. 2010). Due to these considerable advantages, thiol-ene click chemistry is becoming more and more popular in many areas of science, such as chemical synthesis, the modification of polymers or material surface modification (Resetco et al. 2017; Lowe 2014; Lin and Lee 2021).
Thiol-ene chemistry has also found application in the modification of natural fabrics. Fabrics have many unique properties, such as biodegradability, breathability, pleasant touch, and being hypoallergenic, but their hydrophilicity limits their applications. The fabric hydrophobization process makes it possible to use them in such areas as outdoor, technical or medical clothing, upholstery fabrics, and interior finishing elements. One of interesting modification method for natural textiles is performing thiol-ene reaction direct on the surface of fibers. For this purpose the surface should be grafted by reactive thiol groups (or unsaturated groups), after which, a thiol-ene click reaction can be performed using UV radiation and a photoinitiator. In order to introduce SH groups onto the surface of fabrics, they have most often been modified by 3-mercaptopropyltrimethoxysilane (MPTMS) at hydrolysis-condensation reaction. In the next step, the SH-functionalized cotton was modified with various unsaturated derivatives as a result of the thiol-ene click reaction. To obtain hydrophobicity of the SH grafted surface, unsaturated Polyhedral oligomeric silsesquioxanes (POSS) with different additives were used (Zeng et al. 2019; Yang et al. 2020a, b; Xue et al.2019; Hou et al. 2018; Fang et al. 2020; Yang et al. 2019). There are also some reports of different approaches to the thiol-ene click reaction of fabric surface. For example spray deposition of a castor oil-based thiolated oligomer, octavinyl-POSS and hydrophobic SiO2 and then performing thiol-ene click reaction by UV curing (Shang et al. 2020). On the other hand, Chen et al. prepared vinyl-functionalized cotton and then performed photoinduced thiol-ene click reactions using alkylthiols containing from 6 to 10 carbon atoms (Chen et al. 2020).
POSS can be successfully used to modify fabrics due to the possibility of regulating surface energy and roughness. Silsesquioxanes are characterized by a cage nano-structure with inorganic Si–O-Si core and an organic substituent described with the general formula (RSiO1.5)n where the most popular is n = 8 functional groups. Their wide application also results from the fact that it is possible to introduce different numbers and types of functional groups in one molecule in order to obtain suitable properties (Fang et al. 2020; Maciejewski et al. 2015; Duszczak et al.2019). Therefore, octavinyl- (Rozga-Wijas and Chojnowski 2012; Li et al. 2013, 2012, 2014; Marra et al. 2013; Gao et al. 2004; Ni et al. 2014; Liu et al. 2016; Chen et al. 2015; Yang et al. 2020a, b) or octamercaptopropyl- (Li et al. 2019, Wang et al. 2018; Alvarado-Tenorio et al. 2013; Yu et al. 2009; Karuppasamy et al. 2017; Sun et al. 2017) POSS can be easily and effective functionalized or crosslinked via thiol-ene reactions. In the case of cotton fabric, it is important to introduce compounds with a group capable of bonding to the fabric surface, most often alkoxysilyl, and another for imparting special properties like hydrophobicity. Our earlier study showed that silsesquioxanes substituted with alkoxysilyl groups and fluorofunctionalized chains in combination with octakis(tetramethylammonium)octasilsesquioxane can make fabric superhydrophobic (Przybylak et al. 2016).
As already mentioned, the thiol-ene click reaction has been carried out in different ways to hydrophobize fabrics. Various silsesquioxanes and their derivatives have also been used. However, the influence of the method of carrying out the thio-ene click reaction on the hydrophobic effect of the modified surface has not been investigated so far. In our latest study, fabrics were hydrophobized with polysiloxanes using the thiol-ene click reaction. Research has shown that the method of introducing the modifier using the thiol-ene click reaction has a significant impact on the hydrophobic effect (Przybylak et al. 2020). As part of the subsequent research, the experience gained during the modification of fabrics with silsesquioxanes in the dip-coating process and polysiloxanes in the thiol-ene click reaction were combined. The aim of this current study was a comparison of different methods of hydrophobization of cotton fabric using thiol-ene click reaction and POSS derivatives. For this purpose novel, functionalized POSS derivatives via thiol-ene reaction were obtained. Moreover, analogous modifiers were synthesized directly on cotton fabric surface using thiol-ene click reaction. The key aim of this research was to investigate the influence of the type of modification on the hydrophobic effect of cotton fabrics.
Experimental
Materials
3-mercaptopropyltrimethoxysilane (MPTMS) and lithium aluminium hydride were purchased from ABCR. 1-octanethiol, 2,2-dimethoxy-2-phenylacetophenone (DMPA) and anhydrous toluene were obtained from Aldrich. Thioacetic acid was obtained from Acros Organic. Anhydrous magnesium sulfate was purchased from Fluorochem. Ethyl acetate and anhydrous THF were purchased from VWR. Anhydrous ethanol and 2-propanol were purchased from POCH. 1,1,2,2,3,3,4,4-Octafluoropentyl allyl ether was synthesized according to the literature (Maciejewski et al. 2012). A bleached cotton fabric was supplied by the Textile Factory in Łódź (Poland). The cotton fabric had the following parameters: mass per unit area 145 g/m2, plain weave, number of threads weft yarn 228 1/dm warp yarn 254 1/dm, linear mass of weft yarn 26,7 and warp yarn 28,9. Octakis(tetramethylammonium)octasilsesquioxane and, subsequently, octakis(dimethylvinylsiloxy)octa-silsesquioxane were synthesized using a previously described method (Dias Filho et al. 2006).
Preparation of functional silsesquioxanes
Two-stage synthesis of fluoro-functionalized thiol—3-(2,2,3,3,4,4,5,5-octafluoropentoxy)propane-1-thiol
A round-bottom flask equipped with a magnetic stirrer was loaded with 5-(allyloxy)-1,1,2,2,3,3,4,4-octafluoropentane 10.0 g (36.75 mmol) and thioacetic acid 3.08 g (40.46 mmol). Thiol-ene click reaction was carried out for1hr. The excess of thioacetic acid was removed under reduced pressure. The product was purified by trap to trap distillation to 12.55 g (98.5% purity) (3-(2,2,3,3,4,4,5,5-octafluoropentoxy)propyl)ethanethioate (including 2.5% of α-isomer, GC). The isolation yield of the synthesis was 96.5%. (Scheme 1.)
During the next stage, a three-necked, round-bottom flask equipped with a magnetic stirrer, reflux condenser and thermometer was loaded with LiAlH4 1.2 g (31.62 mmol) in a neutral gas atmosphere (argon). Next, anhydrous THF (140 ml) was added dropwise to a cooled (10 °C) suspension compound (3-(2,2,3,3,4,4,5,5-octafluoropentoxy)propyl)ethanethioate 12.28 g (35.27 mmol) and the reaction mixture was stirred continuously for 1 h at room temperature. The reaction mixture was carefully quenched with H2O at 0 °C, and then extracted with ethyl acetate. The organic extract was dried over anhydrous MgSO4 and filtered. Solvents were removed under reduced pressure. The product was purified by trap to trap distillation to 10.13 g (99% purity) 3-(2,2,3,3,4,4,5,5-octafluoropentoxy)propane-1-thiol (including 2.5% of α-isomer). The isolation yield of the synthesis was 93%. (Scheme 1.)
The reaction was monitored by FT-IR analyses (Fig. 1). The disappearance of a band at 1690 cm−1 was observed, corresponding to the stretching vibration of the carbonyl group from thioester group.
Spectroscopic characterization of (3-(2,2,3,3,4,4,5,5-octafluoropentoxy)propyl) ethanethioate
1H NMR (400 MHz, CDCl3): 6.05 (tt, 1H, J = 52.0, 5.6 Hz, CHF2); 3.91 (tt, 2H, J = 14.0, 1.7 Hz, OCH2CF2); 3.63 (t, 2H, J = 6.0 Hz, OCH2); 2.94 (t, 2H, J = 7.1 Hz, SCH2); 2.32 (s, 3H, CH3); 1.87 (p, 2H, J = 6.8 Hz, CH2CH2CH2).
13C NMR (101 MHz, CDCl3): 195.87 (C=O); 118.45–104.94 (CF2, CF2H); 71.43 (CH2O); 67.79 (t, 2JCF = 25.6 Hz, OCH2CF2); 30.69, 29.69, 25.68 (CH3, CH2).
MS (EI, 75 eV) m/z (%): 45.2 (11.0); 51.2 (19.6); 69.2 (11.4); 73.2 (11.8); 74.2 (10.8); 75.2 (14.9); 90.2 (18.0); 95.2 (16.2); 145.1 (11.9); 259.1 (28.2); 271.2 (31.8); 272.1 (33.8); 288.1 (100.0); 348.1 (10.6).
Spectroscopic characterization of 3-(2,2,3,3,4,4,5,5-octafluoropentoxy)propane-1-thiol
1H NMR (400 MHz, CDCl3): 6.04 (tt, J = 52.0, 5.5 Hz, 1H, CHF2); 3.91 (tt, J = 13.9, 1.7 Hz, 2H, OCH2CF2); 3.70 (t, J = 5.9 Hz, 2H, OCH2); 2.61 (dt, J = 8.2, 7.0 Hz, 2H, CH2SH); 1.88 (p, J = 6.5 Hz, 2H, CH2CH2CH2); 1.34 (t, J = 8.1 Hz, 1H, SH).
13C NMR (101 MHz, CDCl3): 118.46–104.95 (CF2, CF2H); 70.90 (CH2O); 67.81 (t, 2JCF = 25.6 Hz, OCH2CF2); 33.65 (CH2CH2CH2); 21.03 (CH2SH).
MS (EI, 75 eV) m/z (%): 47.2 (25.5); 51.2 (18.2); 71.2 (19.4); 74.2 (28.5); 75.2 (15.7); 272.1 (100.0).
Synthesis of octakis(vinyldimethylsiloxy)octasilsesquioxane (ViSi(Me) 2 O) 8 Si 8 O 12 ) (1)
Octakis(tetramethylammonium)octasilsesquioxane and, subsequently, octakis(vinyldimethylsiloxy)octasilsesquioxane were synthesized using a previously described method [Error! Bookmark not defined.]. The product was obtained as white powder, with the yield of 73%.
1H NMR (400 MHz, CDCl3):6.17–5.76 (m, 24H, SiCHCH2); 0.21 (48H, SiCH3).
13C NMR (101 MHz, CDCl3): 138.09 (=CH2); 132.62 (SiCH); − 0.06 (SiCH3).
29Si NMR (79 MHz, CDCl3): 0.52 (OSi(CH3)2CH=CH2); − 109.13 (SiO4).
General synthesis of (RSi(Me) 2 O) ∼4 (ViSi(Me) 2 O) ∼4 Si 8 O 12
R = (CH 2 ) 3 OCH 2 (CF 2 ) 3 CF 2 H (2a)
R = (CH2)7CH3 (2b)
R = (CH 2 ) 3 Si(OCH 3 ) 3 (2c)
A round-bottomed flask equipped with a magnetic stirrer was loaded with octakis(vinyldimethylsiloxy)octasilsesquioxane ((ViSi(Me)2O)8Si8O12) (1), anhydrous toluene (to obtain 50 wt.% final solution), 1-octanethiol, fluoro-functionalized thiol or 3-mercaptopropyltrimethoxysilane (4 mol per 1 mol of spherosilicate), and DMPA (0.01 mol per 1 mol of spherosilicate). The reaction mixture was stirred for 2 min and irradiated for 60 min. After a few minutes of irradiation, slight heating of the reaction mixture was observed. Toluene was removed under reduced pressure at 30 °C. The product was dissolved in anhydrous methanol or hexane and filtered off. The solvent was evaporated under reduced pressure at room temperature (Scheme 2).
2a, high-viscosity liquid, yield 99%
1H NMR (400 MHz, CDCl3): 6.19–5.76 (m, 16H, CH=CH2, CHF2); 3.92 (m, 8H, OCH2CF2); 3.68 (t, J = 6.8 Hz, 8H, OCH2); 2.58 (m, 16H, CH2SCH2); 1.87 (q, 8H, J = 6.5 Hz, CH2CH2CH2); 0.96 (m, 8H, SiCH2); 0.21, 017 (48H, SiCH3).
13C NMR (101 MHz, CDCl3): 137.99 (=CH2); 132.71 (SiCH); 104.94–118.17 (CF2, CF2H); 71.62 (CH2O); 67.83 (t, 2JCF = 25.5 Hz, OCH2CF2); 18.48, 26.58, 28.12, 29.45 (CH2); − 0.20, − 0.09 (SiCH3).
29Si NMR (79 MHz, CDCl3): 11.55 (OSi(CH3)2CH2); 0.84 (Si(CH3)2CH = CH2); -109.01 (SiO4).
2b, high-viscosity liquid, yield 95%
1H NMR (400 MHz, CDCl3): 6.17–5.75 (m, 12H, CH = CH2); 2.57 (m, 8H, SCH2); 2.50 (t, 8H, SCH2); 1.56 (p, 8H), 1.37 (m, 8H), 1.30 (s, 32H) (CH2); 0.97 (m, 8H, SiCH2); 0.87 (m, 12H, CH3); 0.21, 0.18 (48H, SiCH3).
13C NMR (101 MHz, CDCl3): 138.00 (=CH2); 132.68 (SiCH); 32.09, 31.98, 29.69, 29.38, 29.18, 29.16, 26.66, 22.80, 18.60 (CH2); 14.23 (CH3); − 0.07 (SiCH3).
29Si NMR (79 MHz, CDCl3): 11.50 (Si(CH3)2CH2); 0.68 (OSi(CH3)2CH=CH2); -109.10 (SiO4).
2c, high-viscosity liquid, yield 92%
1H NMR (400 MHz, CDCl3): 6.16–5.74 (m, 12H, CH=CH2); 3.55 (s), 3.47 (s) (36H, Si(OCH3)3); 2.55 (m, 16H, CH2SCH2); 1.68 (m, 8H, CH2CH2CH2); 0.95 (m, 8H, CH2Si); 0.75 (m, 8H, CH2Si(OCH3)3); 0.20, 0.17 (48H, SiCH3).
13C NMR (101 MHz, CDCl3): 137.98 (=CH2); 132.69 (SiCH); 50.91, 50.63 (Si(OCH3)3); 34.96 (CH2S); 26.48 (CH2); 22.93 (CH2CH2CH2); 18.56 (CH2); 8.74 (CH2Si(OCH3)3); -0.08 (SiCH3).
29Si NMR (79 MHz, CDCl3): 11.52 (OSi(CH3)2); 0.70 (Si(CH3)2CH=CH2); − 42.27 (Si(OCH3)3); − 109.10 (SiO4).
General synthesis of (RSi(Me)2O)∼4(R′Si(Me)2O)∼4Si8O12
R = (CH2)3OCH2(CF2)3CF2H, R’ = (CH2)3Si(OCH3)3 (3a)
R = (CH 2 ) 7 CH 3 , R’ = (CH 2 ) 3 Si(OCH 3 ) 3 (3b)
Compounds 2a and 2b were obtained according to the preparation described in Sect. 2.2.3. Next, 3-mercaptopropyltrimethoxysilane and DMPA (the second portion of DMPA) were added (4.1 mol per 1 mol of spherosilicate) to both of them. The reaction mixture was stirred 2 min and irradiated for 60 min. Toluene and excess of thiol was removed at high vacuum. The product was dissolved in anhydrous methanol or hexane and filtered off. The solvent was evaporated under reduced pressure at room temperature (Scheme 2.).
3a, high-viscosity liquid, yield 99%
1H NMR: (400 MHz, CDCl3): 6.03 (tt, J = 52.0, 5.8 Hz, 4H, CHF2); 3.89 (t, J = 14.0 Hz, 8H, OCH2CF2); 3.66 (t, J = 6.2 Hz, 8H, OCH2); 3.53 (s), 3.43 (s) (36H, Si(OCH3)3); 2.54 (m, 32H, CH2SCH2); 1.84 (p, J = 6.5 Hz, 8H, OCH2CH2CH2S); 1.66 (dt, 15.5 Hz, 7.4 Hz, 8H, SCH2CH2CH2Si); 0.94 (m, 16H, SiCH2); 0.72 (m, 8H, CH2Si(OCH3)3); 0.16 (s, 48H, SiCH3).
13C NMR: 118.42–104.91 (CF2, CF2H); 71.56 (CH2O); 67.75 (t, 2JCF = 25.5 Hz, OCH2CF2); 50.55, 50.73 (SiO(CH3)3); 34.92, 29.76, 29.40, 28.06, 26.51, 26.41, 22.91, 18.52, 18.41 (CH2); 8.71 (SiCH2); -0.20 (SiCH3).
29Si NMR (79 MHz, CDCl3): 11.69 (OSi(CH3)2); − 42.32 (Si(OCH3)3); − 109.14 (SiO4).
3b, high-viscosity liquid, yield (not isolated) 98%
1H NMR: (400 MHz, CDCl3): 3.54 (s), 3.44 (s) (36H, Si(OCH3)3); 2.52 (m, 32H, CH2SCH2); 1.66 (p, J = 7.5 Hz, 8H, SCH2CH2CH2Si); 1.54 (p, J = 7.2 Hz, 8H, CH2); 1.36–1.24 (m, 40H, CH2CH2CH2CH2CH3); 0.94 (m, 16H, Si(CH3)2CH2); 0.84 (m, 12H, CH3); 0.73 (m, 8H, CH2Si(OCH3)3); 0.16 (s, 48H, SiCH3).
13C NMR: 50.58, 50.80 (Si(OCH3)3); 34.95, 32.05, 31.92, 29.65, 29.34, 29.32, 29.12, 26.60, 26.44, 22.91, 22.74, 18.53, 14.18, 8.73 (CH2); − 0.11 (SiCH3).
29Si NMR (79 MHz, CDCl3): 11.63 (OSi(CH3)2); − 42.31 (Si(OCH3)3); − 109.14 (SiO4).
Modification of cotton textiles
Grafting of –SH groups on the surface of textiles
A 5% solution of 3-mercaptopropyltrimethoxysilane was prepared in a mixture of ethanol and water in the ratio 8: 2 (v/v). A 5% acetic acid was added to the solution and then a 1 h hydrolysis was performed. After that, the fabrics were immersedfor 15 min in the resulting solution. The excess of the modifier solution was removed by squeezing, and the fabrics were fixed for 15 min at 120 °C in order to obtain SH-functionalized cotton fabrics.
Textile modification by POSS via dip-coating process
A mixture of 5 vol. % of POSS (2c, 3a, 3b), 5 vol. % of acetic acid and 1% of H2O in isopropanol was hydrolyzed for 1 h at room temperature. The fabrics were then immersed in the prepared coating solution for 30 min. Subsequently, the excess of the modifier solution was removed by squeezing in a paper towel between the two rollers always with the same pressure. A next step the samples were dried for 1 h at 80 °C and fixed for 3 min at 130 °C.
Thiol-ene click reaction on the cotton fabrics surface
A typical procedure for thiol-ene modification is as follows: A mixture of POSS (1, 2a, 2b) (5 wt. %) and the photoinitiator DMPA (0.01 wt. %) in toluene was stirred for 10 min. Then, cotton fabrics with SH groups on the surface were placed into the obtained solution for 15 min. The treated samples were removed from the solution and irradiated for 15 min (7.5 min each site). The sample was exposed to a 280−600 nm light source (LQ-400 mercury lamp, Gröbel UV-Elektronik GmbH, 400 W, irradiation distance ~ 5 cm). After the above stages, the samples were rinsed with distilled water, the excess of the modifier solution was wiped with a paper towel and the fabrics were dried for 2 h at 60 °C.
The thiol-ene click reaction was carried out in a analogus way on the surface of fabrics previously modified with silsesquioxanes with vinyl bonds (2c and 1) and 1-octanethiol or fluoro-functionalized thiol.
Methods of modifying natural fabrics
In the first method, samples were modified by POSS substituted by 4 alkoxysilyl groups and four hydrocarbon or fluorofunctionalized chains (3a and 3b) via a dip-coating process. In the second method, cotton textiles were grafted with SH groups by modification by 3-mercaptopropyltrimethoxysilane (M) via a dip-coating process. Next, POSS, with four unsubsituted vinyl groups and four hydrocarbon or fluorofunctionalized chains (2a and 2b) were introduced on a fiber surface by thiol-ene click reaction. The third method was a three-stage process. First, textiles were modified by 3-mercaptopropyltrimethoxysilane (M) via a dip-coating process. Next, POSS with 8 unsubsituted vinyl groups (1) were introduced on a fiber surface by thiol-ene click reaction. Finally an thiol-ene click reaction was performed for a second time directly on to a cotton surface between vinyl groups from POSS and 1-octanethiol or fluoro-functionalized thiol. In the fourth method, fabrics were modified with silsesquioxanes with four unsubsituted vinyl groups and four alkoxysilyl groups (2c) via the dip-coating process, and then the thiol-ene click reaction was carried out directly on the surface of the fibers with 1-octanethiol or fluoro-functionalized thiol. All described methods are presented at Fig. 2.
Washing process
The durability of the modifications was determined by measuring the water contact angle immediately after the modification, as well as after the washing process. The washing process of modified fabric samples was carried out according to the standard PN-EN ISO 105-C06:2010 Textiles—Tests for color fastness—Color fastness to domestic and commercial laundering. The process was performed at 40 °C for 60 min. using a detergent and this was followed by rinsing. The washing was repeated five and fifteen times.
Characterization of modified samples
Determination of the amount of modifiers applied on the fabrics (add-on)
The total dry solid add-on in cotton fabric samples (A) was determined by weighing a fabric sample before (Wi) and after (Wf) its modification with a composition and thermal fixing. The Ohaus analytical balance was used for the measurements. Uptake (Table 1) was calculated according to the following equation:
FT-IR analysis
FTIR spectra of the modified fabrics were taken with a Bruker spectrometer, model Tensor 27, with a Specac Golden Gate single-reflection diamond ATR accessory.
NMR
NMR spectra were recorded with a Bruker Ultrashield 400 and 500 MHz spectrometer in commercially available CDCl3.
Analysis of the elemental composition of the applied coatings
The analysis was carried out by employing the SEM–EDS technique to determine the ultimate elements (Si, O, C, F and S) present in the modifying mixtures at weight percentage. A Hitachi S-3500 N electron scanning microscope (SEM) equipped with an EDS (energy-dispersive X-ray) detector (Ultra Dry Silicon Drift X-ray Detector made by Thermo Scientific) was used for the measurements. Each result is the average of 9 measurements. Image Resolution: 1024 by 768, Image Pixel Size:2.09 µm, Acc. Voltage:15.0 kV.
Determination of hydrophobic properties
The water contact angles were measured using an automatic video contact-angle testing apparatus (Krüss, model DSA 100 Expert). A 5 μl volume of water was applied to the treated cotton fabrics, and the contact angle was determined from the video camera images of the drop in the course of its formation. Each measurement is an average of five drops.
Capacity of absorption of a water drop. Water absorption on the fabric surface was tested according to the JIS 1090:1990 Standard.
Studies of surface topography
Microscopic evaluation of surface changes of modified and unmodified samples of the cotton fabric was carried out using a Hitachi S-3400 N electron scanning microscope; the samples were coated with a thin layer of gold before observations. SEM images were also taken, using an FEI Quanta 250 FEG instrument, equipped with a Large field detector (LFD) that records the Secondary electrons (SEs). For the LFD SE measurements the samples were not covered with any layers. The microscope was operated at low vacuum mode (70 Pa), and the accelerating voltage was 10 kV.
Results and discussion
Synthesis of (3-(2,2,3,3,4,4,5,5-octafluoropentoxy)propyl)ethanethioate via hydrothiolation of 1,1,2,2,3,3,4,4-octafluoropentyl allyl ether with thioacetic acid and then reduction by LiAlH4 were performed at the beginning of the research (Scheme 1.).
In the next stage, the synthesis of silsesquioxane derivatives containing an average of four unsubsituted vinyl groups and four functional groups (alkyl-, fluoroalkyl- or alkoxysilyl groups) were obtained by partial addition of 1-octanethiol, fluoro-functionalized thiol or 3-mercaptopropyltrimethoxysilane to (ViSi(Me)2O)8Si8O12 with a molar ratio of thiol to spherosilicate of 4:1. The obtained products (2a, 2b, 2 c) are mixtures of POSSs because the arrangement of functional groups in silsesquioxanes is statistical. However, the average numbers of functional groups is four. As with the hydrosilylation reaction during the radical addition of thiol, a statistical mixture of products is formed (Marra et al. 2013; Walczak et al. 2019), which was confirmed by MALDI-TOF–MS analyses (Scheme 2.).
The course of the POSS functionalization reaction is most preferably analyzed by NMR spectroscopy. Introduction of four functional groups into the POSS derivative took no longer than 1 h, since NMR analysis after 1 h showed no unreacted thiol. The substitution of all vinyl groups with two types of functional groups was carried out in two stages, in the second stage 10% excess of thiol was used to fully convert remaining vinyl groups. NMR analysis after synthesis reaction confirmed the complete disappearance of vinyl bonds in the silsesquioxanes (6.17–5.76 ppm at 1H NMR, 138.09 and 132.62 ppm at 13C NMR and 0.52 ppm at 29Si NMR), and CH2SCH2 bond formation (about at 2.5–2.6 ppm at 1H NMR), which confirmed successful incorporation of thiols into the silsesquioxane structure.
In summary, the synthesis of the functionalized silsesquioxane derivatives proceeds under mild conditions, with high yields and in a short time.
Synthesized compounds were used for the hydrophobization of cotton textiles. Samples were modified in four different ways (Fig. 2.).
The results of hydrophobization of natural fabrics were investigated by measuring the Water contact angle (WCA). The measurement was performed directly after the modification of the samples and after the five-time washing process. Figure 3 shows the water contact angle values for fabrics modified via the dip coating process by silsesquioxanes substituted with alkoxysilyl groups and with fluorofunctionelized chains (3a) or alkyl chains (3b). The same figure also presents samples modified by 3-mercaptopropyltrimethoxysilane (M) via the dip-coating process and subsequently by POSS with vinyl groups and fluorinated chains (2a) and by POSS with vinyl groups and hydrocarbon chains (2b) at thiol-ene click reaction.
The water contact angles value of all modified samples was quite similar. It can be seen that slightly higher hydrophobicity was achieved for textiles modified by POSS with alkyl chains. This result is quite surprising because, as is known from the literature, fluorinated substituents increase the hydrophobicity of compounds. However this may be due to the high electronegativity of the fluorine atoms and the interaction between them. During synthesis of functionalized POSS, the alkyl or fluorinated chains are substituted first, then in the case of (3a and 3b), the alkoxysilyl groups are introduced second. During POSS functionalization, the fluorinated chains can repel each other due to their electronegativity. This may result in their substitution at opposite corners of the POSS. At the same time, the alkyl chains had a greater chance of substituting on one side of the POSS cube due to the lower interaction of electronegativity (Fig. 4.)
Such an arrangement of the chains in the POSS cube may interfere in the effective hydrophobization of the fiber surface. Long chains can disturb covalent bond formation because both alkoxysilyl groups and vinyl groups have more difficult access to the cellulose hydroxyl groups, or SH groups in the case of fabric modified first by 3-mercaptopropyltrimethoxysilane. This structure of POSS derivatives also promotes the cross-linking of silsesquioxanes at the expense of fiber binding in the case of samples 3a and 3b. This can cause a worse orientation of the fluorinated chains on the surface. Fluorinated chains may be entangled in the siloxane network instead of creating a hydrophobic layer. Hydrocarbon chains do not interact so strongly with each other, which increases the chances of their better orientation on the surface. Therefore, these may be a reason for higher water contact angles of fabrics modified POSS-substituted alkyl chains.
The results presented in Fig. 3 show that the WCA of washed samples are the same, or slightly higher, than samples before the washing process, which confirms the durability of the modifications as well as resistance to leaching. The increase of WCA after washing can be caused by washing away unbound modifier particles. Moreover, the washing process can improve the orientation of chains on the fiber surface.
In the next part of study the influence of hydrocarbon or fluorofunctionalized chains on the hydrophobic effect of cotton fabrics was investigated. To this end, fluorofunctionalized thiol or octenothiol were introduced directly to the cotton surface via thiol-ene click reaction. Modifications were performed by two ways. In the first, a three-stage modification of the fiber surface was carried out. First, the fabric was modified with 3-mercaptopropyltrimethoxysilane via the dip-coating process (M). Then, thiol-ene click reactions were carried out between SH bonds on the fiber surface and vinyl bonds from unsaturated POSS (1). Finally, fluoro-functionalized thiol (F) or octanethiol (C) were attached to the remaining unsubstituted vinyl bonds in a thiol-ene click reaction on the fabric surface (method 3 in Fig. 2). In the second method, fabrics were modified with silsesquioxanes substituted with 4 alkoxysilyl groups and with 4 free vinyl groups (2c) via the dip coating process. The long-chain thiols were then bonded to the vinyl groups present on the surface of the fibers by a thiol-ene click reaction (method 4 at Fig. 2). The results of the modification are shown in Fig. 5.

modified by 3-mercaptopropyltrimethoxysilane (M) via dip-coating process, then by POSS with vinyl groups (thiol-ene click reaction) (1) and finally by fluoro-funcionalized thiol (F) or octanethiol (C) (thiol-ene click reaction) and values of samples modified by POSS with 4 vinyl groups and substituted by 4 alkoksysilyl group (2c) va dip coating and subsequently modified by fluoro-funcionalized thiol (F) or octanethiol (C) via thiol-ene click reaction
WCA values of samples
The results presented in Fig. 5 show that all modifications provided a high hydrophobic effect. Moreover WCA are even higher after the washing process. In the case of samples M + 1 + F, superhydrophibicity was obtained both before and after washing. Thus, high hydrophobicity both before and after washing can be caused by effective covalent bond formation at all stages of modification. To ensure that the modified fabrics were fully resistant to washing, the samples obtained with methods 3 and 4 were also washed 15 times. The presented results confirmed the hydrophobicity of the obtained fabrics at a similar level, even after fifteen washing times. The results presented in Fig. 4 are generally much better in comparison to the previous ones (Fig. 3). It may be surprising that the modification with the unsubstituted POSS (M + 1) (Fig. 5.) led to higher hydrophobicity than the modification with the silsesquioxanes substituted with the fluorinated (M + 2a) or hydrocarbon (M + 2b) chains (Fig. 3). Silsesquioxane (1) contains eight vinyl groups and no long-chain substituted in their corners, as in the case of silsesquioxanes 2a and 2b. During the second stage of modification of SH grafted cotton fibers, thiol-ene click reaction with vinyl POSS (1) was performed. There were no long chains in the vinyl POSS (1) structure that would interfere with the attachment of the silsesquioxane to the fabric. This is why the modifier could successfully attach itself to the fibers. Moreover, bonding of POSS was possible via four corners because of no spherical hindrance. We noticed a similar phenomenon in our earlier research, where modification with unsubstituted polysiloxanes gave a higher hydrophobicity than analogous polysiloxanes with alkyl chain polysiloxanes (Przybylak et al. 2020). A similar situation can be observed in the case of sample 2c. Fabric modified by silsesquioxanes substituted with 4 alkoxysilyl groups and with 4 free vinyl bonds (2c) has higher contact angles compared to silsesquioxanes with 4 alkoxysilyl groups and 4 fluorofunctionalized (3a) or hydrocarbon (3b) chains.
However, the values of the water contact angle of the samples M + 1 + F, M + 1 + C and 2c + F, 2c + C are much higher than samples M + 1 and 2c. In these instances, the modification was carried out via thiol-ene click reaction directly on the fabric surface between the vinyl bonds originating from POSS (1 or 2c) and fluoro-functionalized thiol (F) or 1-octanethiol (C). The samples treated with 3-mercaptopropyltrimethoxysilane and POSS (1) or by POSS 2c, had a large amount of vinyl bonds on their surface. Moreover, thanks to this, vinyl groups were placed on top of the modifier layer and well exposed. For these reasons, the attached thiols had easy access to the vinyl groups during the thiol-ene click reaction and could easily create bonds with POSS (1 and 2c). Good exposure of vinyl groups, as well as carrying out the thiol-ene reaction between thiols and silsesquioxanes directly on the surface of the fibers, allowed for a better orientation of the hydrocarbon and fluorinated chains. All the chains were on the surface of the modifying layer and were easy to bind to POSS. These factors contributed to the very high hydrophobicity of the obtained fabrics. In contrast to the previous results, the highest contact angle was obtained for the sample modified with fluorinated chains (M + 1 + F and 2c + F). In earlier modification methods, fluorinated chains could not fully expose their hydrophobic properties because they were entangled in a network of siloxane. This, in turn, resulted in the poor orientation of the fluorinated chains on the surface of the fibers. The hydrocarbon chains had slightly better orientations due to the lower electronegativity interactions with each other, which resulted in higher water contact angles. As a result of modification via method 3 and 4, all chains were on the surface of the modifier layer and had good orientation. Thus, the fluorofunctionalised chains can fully expose their hydrophobic properties.
Figure 6 depicts a photo of a water drop on sample M + 1 + F before the washing process (a) and after the washing process (b).
The hydrophobic properties of the modified samples were determined by measurement of the capacity of absorption of a water drop. This method involves determining the time (in seconds) of complete absorption of a water drop on the textile surface. The conducted tests showed that both before and after the washing process, water drops are stable for over 1800s on all modified fabrics. This is the maximum time allowed by the standard. This is a very good result, especially given that a high WCA value does not always go hand in hand with water droplet stability. Such an example of this can be found in the work of Ferrero and Periolatto (Ferrero and Perioletto 2013) where, despite high WCA values, the absorption time of water droplet after washing was lower than 1 min.
To confirm the effectiveness of the modification, the sample was subjected to FT-IR analysis (Fig. 7).
Bands at 1254 cm−1 can be observed on all spectra of fabrics modified by POSS, characteristic for Si–C(H3) and/or Si–C(H2) vibrations, which confirms the presence of the siloxanes on the cotton. At 789 cm−1 and 840 cm−1 rocking vibrations of C-H methylene groups is observed (more intensive on b and c spectra) which confirm the presence of hydrocarbon chains on silsesquioxane structures. The low intensity peak at 3070 cm−1 is observed on the (c) spectrum, which is the characteristic peak related to the stretching vibration of = CH bonds related to the vinyl groups in POSS, as well as a peak at 1600 cm−1 related to C=C bond stretching vibration. These bands are derived from incompletely reacted SH groups present on the surface of cotton and vinyl groups of POSS. This phenomenon may be due to the steric hindrance structure of the POSS cage. The presence of these bands in the sample confirms the earlier observations that the presence of long hydrocarbon and fluorofunctional chains hinders the thiol-ene click reaction between SH and vinyl bonds. The band expected at about 1070 cm−1 characteristic to Si–O–Si stretching vibration of bond in structure of POSS cage is overlapped with a broad band between 950 and 1100 cm−1 attributed to characteristic peaks of cellulose. The (d) and (e) spectra from the M + 1 + C and 2c + M samples, respectively, are less intense, which may indicate a smaller amount of modifiers attached. Moreover, the presence of characteristic bands in all spectra of modified fabrics proves that the modifications are resistant to washing.
The elemental composition of the impregnated fabrics was also tested in order to confirm the modification. Table 1 shows the results of the SEM–EDS analysis of the modified samples before and after the washing process.
The results of the SEM–EDS analysis and the add-on collected in Table 1 allows confirm of the previous observations. Fabrics modified with silsesquioxanes substituted by hydrocarbon or fluorofunctionalized chains (3a, 3b, M + 2a, M + 2b) had a larger add-on value compared to the other samples. This correlates with the results obtained in the FT-IR analysis, where the bands from samples 3b and M + 2b were more intense. The samples containing the fluorofunctionalized chains on their surface showed a slightly higher add-on value compared to the analogous samples containing the hydrocarbon chains. This difference is due to the higher molecular weight of the fluorofunctionalized chains. Fabrics functionalized with 3-mercaptopropyltrimethoxysilane (M) during the first stage have a higher add-on value compared to analogous samples modified by POSS derivatives This is due to the additional siloxane layer formed by the cross-linking of 3-mercaptopropyltrimethoxysilane. The results of the SEM–EDS analysis correlate with the add-on values. All the samples have a very similar elemental composition before and after the washing process. This confirms the durability of the modifications and the resistance of the obtained hydrophobic fabrics to the washing process. Fabrics modified by silsesquioxanes substituted by fluorofunctionalized or hydrocarbon chains (3a and 3b) contain more F, Si and S elements compared to analogous samples, where silsquioxanes were substituted with long-chain thiols directly on the fabric surface (2c + F, 2c + C). The comparison of the elemental composition of the samples M + 2a, M + 2b with M + 1 + F, M + 1 + C shows similar results. This confirms the previous observations that silsesquioxanes substituted by long hydrocarbon or fluorofunctionalised chain can attach to the fiber surface via a smaller amount of corners because of steric hindrance. Moreover, it promotes the formation of a chaotic siloxane network where only some of the POSS is occluded instead of bonding with the fabric. Moreover, during modification by POSS substituted only by 4 alkoxysilyl groups (2) or by unsubstituted vinylPOSS (1) there is no steric hindrance and silsesquioxanes can attach to the fabric by 4 corners. This causes less POSS to attach to the fibers but at the same time they create an ordered, homogeneous surface. Moreover, the remaining 4 vinyl bonds are well exposed to thiol-ene click reactions with long-chain thiols. Therefore, despite the lower amount of the modifier, good orientation of the fluorofunctional or hydrocarbon chains allows for better hydrophobicity.
Scanning electron microscopy was performed to analyze the surface of the modified fabric (Fig. 9).
SEM photos show clear differences between the raw fabric and all modified samples. The first photo (pure cotton) shows the structure of the unmodified fiber. The diversified morphology of the surface of the fibers, typical of cotton, is visible. In all cases, the presented photos of the modified samples show a homogenous layer of modifier. The modifications made resulted in the creation of a tight siloxane layer surrounding each fiber. The SEM photos show no agglomerates or modifier in the inter-fiber space. In pictures 3a and M + 2a the siloxane layer is slightly thicker compared to samples M + 1 + F and 2c + F. This correlates with the higher add-on and SEM–EDS values for these samples. The presence of the siloxane layer on all photos of the modified fibers confirms the resistance of the modifications to the washing process and their durability.
Summary
Four methods of cotton hydrophobization based on the thiol-click reaction were investigated in the course of the research. All the developed modification methods resulted in obtaining highly hydrophobic fabrics. The conducted research allowed for the selection of the most effective modification method, which was the attachment of long-chain thiols in the thiol-ene click reaction to silsesquioxanes present on the fiber surface. The introduction of an alkylthiol or a fluorofunctional thiol through a thiol-ene click reaction directly on the surface of the fibers allows for their very good orientation, which translates into a high hydrophobic effect. This method of modification avoids problems related to steric hindrance or undesirable cross-linking of modifiers. Moreover, the three-step modification developed minimizes the need to synthesize new compounds, as the reaction takes place directly on the fabric surface and the majority of the starting materials are commercially available. Moreover superhydrophobic fabrics were obtained by easy modification. These advantages contribute to the low cost of the developed modifications, which has an impact on the high application and commercial potential. Factors such as low price and ease of technology are crucial from an economic and technological point of view. All modifications gave a permanent hydrophobic effect. The obtained samples showed complete resistance to washing, which is one of the main goals in the hydrophobic treatment of textiles. Moreover, the fabrics were hydrophobized with 5% modifier solutions and the add-on values did not exceed 10% (method 2 and method 3), which proves the high efficiency and effectiveness of the developed modification methods. The obtained superhydrophobic fabrics retained their elasticity, soft touch, the impregnation process did not stiffen or change the color of the cotton. The conducted FT-IR analysis confirmed the effectiveness of the modification and its durability due to the presence of bands characteristic for modifiers in the spectra. SEM–EDS analysis indicated the presence of the same amount of modifying elements on the surface of the fibers both before and after washing. In the SEM pictures, a homogeneous and tight modifier layer surrounding each fiber was observed.
Data availability
Data available on request due to privacy restrictions. The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the lack of availability of a suitable repository.
References
Alvarado-Tenorio B, Romo-Uribe A, Mather PT (2013) Nanoscale anisotropic orientation in shape memory random POSS/Polycaprolactone nanocomposites. In MRS symposium proceedings. https://doi.org/10.1557/opl.2013.1117
Chen S, Li X, Li Y, Sun J (2015) Intumescent flame-retardant and self-healing superhydrophobic coatings on cotton fabric. ACS Nano 9:4070–4076. https://doi.org/10.1021/acsnano.5b00121
Chen X, Zhou Q, Zhang Y, Zhao J, Yan B, Tang S, Xing T, Chen G (2020) Fabrication of superhydrophobic cotton fabric based on reaction of thiol-ene click chemistry. Colloids Surf A Physicochem Eng Asp 586:124175. https://doi.org/10.1016/j.colsurfa.2019.124175
Dias Filho NL, Aquino HAD, Pires G, Caetano L (2006) Relationship between the dielectric and mechanical properties and the ratio of epoxy resin to hardener of the hybrid thermosetting polymers. J Braz Chem Soc 17:533–541. https://doi.org/10.1590/S0103-50532006000300016
Duszczak J, Mituła K, Januszewski R, Żak P, Dudziec B, Marciniec B (2019) Highly efficient route for the synthesis of a novel generation of tetraorganofunctional double-decker type of silsesquioxanes. ChemCatChem 11:1086–1091. https://doi.org/10.1002/cctc.201801609
Fang Y, Liu C, Li M, Miao X, Pei Y, Yan Y, Xiao W, Wu L (2020) Facile generation of durable superhydrophobic fabrics toward oil/water separation via thiol-ene click chemistry. Ind Eng Chem Res 59:6130–6140. https://doi.org/10.1021/acs.iecr.9b06761
Ferrero F, Periolatto M (2013) Application of fluorinated compounds to cotton fabrics via sol-gel. Appl Surf Sci 275:201–207. https://doi.org/10.1016/j.apsusc.2013.01.001
Gao Y, Eguchi A, Kakehi K, Lee YC (2004) Efficient preparation of glycoclusters from silsesquioxanes. Org Lett 6:3457–3460. https://doi.org/10.1021/ol040043a
Hou K, Zeng Y, Zhou C, Chen J, Wen X, Xu S, Cheng J, Pi P (2018) Facile generation of robust POSS-based superhydrophobic fabrics via thiol-ene click chemistry. Chem Eng J 332:150–159. https://doi.org/10.1016/j.cej.2017.09.074
Hoyle CE, Bowman CN (2010) Thiol–ene click chemistry. Angew Chem Int Ed 49:1540–1573. https://doi.org/10.1002/anie.200903924
Karuppasamy K, Prasanna K, Vikraman D, Kim HS, Kathalingam A, Mitu L, Rhee HW (2017) A rapid one-pot synthesis of novel high-purity methacrylic phosphonic acid (PA)-based polyhedral oligomeric silsesquioxane (POSS) frameworks via thiol-ene click reaction. Polymers 9:192. https://doi.org/10.3390/polym9060192
Li Y, Dong XH, Guo K, Wang Z, Chen Z, Wesdemiotis C, Quirk RP, Zhang WB, ChengSZD, (2012) Synthesis of shape amphiphiles based on POSS tethered with two symmetric/asymmetric polymer tails via sequential “grafting-from” and thiol–ene “click” chemistry. ACS Macro Lett 1:834–839. https://doi.org/10.1021/mz300196x
Li L, Xue L, Feng S, Liu H (2013) Functionalization of monovinyl substituted octasilsesquioxane via photochemical thiol-ene reaction. Inorganica Chim Acta 407:269–273. https://doi.org/10.1016/j.ica.2013.08.009
Li Y, Guo K, Su H, Li X, Feng X, Wang Z, Zhang W, Zhu S, Wesdemiotis C, Cheng SZD, Zhang WB (2014) Tuning “thiol-ene” reactions toward controlled symmetry breaking in polyhedral oligomeric silsesquioxanes. Chem Sci 5:1046–1053. https://doi.org/10.1039/C3SC52718B
Li H, Dai J, Xu Q, Lu C, Yang G, Wang F, Nie J, Hu X, Dong N, Shi J (2019) Synthesis of thiol-terminated PEG-functionalized POSS cross-linkers and fabrication of high-strength and hydrolytic degradable hybrid hydrogels in aqueous phase. Eur Polym J 116:74–83. https://doi.org/10.1016/j.eurpolymj.2019.03.062
Lin TC, Lee DJ (2021) Cotton fabrics modified for use in oil/water separation: a perspective review. Cellulose. https://doi.org/10.1007/s10570-021-03850-6
Liu L, Liu Z, Huang P (2016) Novel POSS based nanohybrids for improving tribological properties of liquid paraffin. RSC Adv 6:94876–94883. https://doi.org/10.1039/C6RA21618H
Lowe AB (2014) Thiol–ene “click” reactions and recent applications in polymer and materials synthesis: a first update. Polym Chem 5:4820–4870. https://doi.org/10.1039/C4PY00339J
Maciejewski H, Karasiewicz J, Dutkiewicz M, Marciniec B (2015) Hydrophobic materials based on fluorocarbofunctional spherosilicates. SILICON 7:201–209. https://doi.org/10.1007/s12633-014-9264-5
Maciejewski H, Karasiewicz J, Marciniec B (2012) Effective synthesis of fluorofunctional (poly)siloxanes. Polimery 57:449–455
Marra A, Staderini S, Berthet N, Dumy P, Renaudet O, Dondoni A (2013) Thiyl glycosylation of propargylated octasilsesquioxane: synthesis and lectin-binding properties of densely glycosylated clusters on a cubic platform. Eur J Org Chem 2013:1144–1149. https://doi.org/10.1002/ejoc.201201453
Ni B, Dong XH, Chen Z, Lin Z, Li Y, Huang M, Fu Q, Cheng SZD, Zhang WB (2014) “Clicking” fluorinated polyhedral oligomeric silsesquioxane onto polymers: a modular approach toward shape amphiphiles with fluorous molecular clusters. Polym Chem 5:3588–3597. https://doi.org/10.1039/C3PY01670F
Przybylak M, Maciejewski H, Dutkiewicz A (2016) Preparation of highly hydrophobic cotton fabric by modification with bifunctional silsesquioxanes in the sol-gel process. Appl Surf Scie 387:163–174. https://doi.org/10.1016/J.APSUSC.2016.06.094
Przybylak M, Szymańska A, Maciejewski H, Makowska K (2020) Durable, highly hydrophobic modification of cotton fabric with fluorine-free polysiloxanes obtained via hydrosilylation and hydrothiolation reactions. Cellulose 27:8351–8367. https://doi.org/10.1007/s10570-020-03341-0
Resetco C, Hendriks B, Badi N, Du Prez F (2017) Thiol–ene chemistry for polymer coatings and surface modification–building in sustainability and performance. Mater Horiz 4:1041–1053. https://doi.org/10.1039/C7MH00488E
Rissing C, Son DY (2009) Application of thiol− ene chemistry to the preparation of carbosilane− thioether dendrimers. Organometallics 28:3167–3172. https://doi.org/10.1021/om9001395
Rozga-Wijas K, Chojnowski J (2012) Synthesis of new polyfunctional cage oligosilsesquioxanes and cyclic siloxanes by thiol-ene addition. J Inorg Organomet Polym Mater 22:588–594. https://doi.org/10.1007/s10904-012-9652-5
Shang Q, Liu C, Chen J, Yang X, Hu Y, Hu L, Zhou Y, Ren X (2020) Sustainable and robust superhydrophobic cotton fabrics coated with castor oil-based nanocomposites for effective oil–water separation. ACS Sustain Chem Eng 8:7423–7435. https://doi.org/10.1021/acssuschemeng.0c01469
Sinha AK, Equbal D (2019) Thiol− ene reaction: synthetic aspects and mechanistic studies of an anti-Markovnikov-selective hydrothiolation of olefins. Asian J Org Chem 8:32–47. https://doi.org/10.1002/ajoc.201800639
Sun D, Wang W, Yu D (2017) Highly hydrophobic cotton fabrics prepared with fluorine-free functionalized silsesquioxanes. Cellulose 24:4519–4531. https://doi.org/10.1007/s10570-017-1388-5
Walczak M, Franczyk A, Dutkiewicz M, Marciniec B (2019) Synthesis of bifunctional silsesquioxanes (RSiMe2O)∼4(R′SiMe2O)∼4Si8O12 via hydrosilylation of alkenes. Organometallics 38:3018–3024. https://doi.org/10.1021/acs.organomet.9b00350
Wang Y, Ma S, Chen Y, Zhang L, Ou J, Shen Y, Ye M (2018) Thiol-radical-mediated polymerization for preparation of POSS-containing polyacrylate monoliths in capillary liquid chromatography. Talanta 190:62–69. https://doi.org/10.1016/j.talanta.2018.07.061
Wendeln C, Rinnen S, Schulz C, Arlinghaus HF, Ravoo BJ (2010) Photochemical microcontact printing by thiol− ene and thiol− yne click chemistry. Langmuir 26:15966–15971. https://doi.org/10.1021/la102966j
Xue CH, Fan QQ, Guo HJ, An QF, Jia ST (2019) Fabrication of superhydrophobic cotton fabrics by grafting of POSS-based polymers on fibers. Appl Surf Scie 465:241–248. https://doi.org/10.1016/j.apsusc.2018.09.156
Yang M, Liu W, Jiang C, Xie Y, Shi H, Zhang F, Wang Z (2019) Facile construction of robust superhydrophobic cotton textiles for effective UV protection, self-cleaning and oil-water separation. Colloids Surf A Physicochem Eng Asp 570:172–181. https://doi.org/10.1016/j.colsurfa.2019.03.024
Yang M, Liu W, Liang L, Jiang C, Liu C, Xie Y, Shi H, Zhang F, Pi K (2020a) A mild strategy to construct superhydrophobic cotton with dual self-cleaning and oil–water separation abilities based on TiO2 and POSS via thiol-ene click reaction. Cellulose 27:2847–2857. https://doi.org/10.1007/s10570-019-02963-3
Yang M, Liu W, Liang L, Jiang C, Liu C, Xie Y, Shi H, Zhang F, Pi K (2020b) A mild strategy to construct superhydrophobic cotton with dual self-cleaning and oil–water separation abilities based on TiO 2 and POSS via thiol-ene click reaction. Cellulose 27:2847–2857. https://doi.org/10.1007/s10570-019-02963-3
Yu B, Chan JW, Hoyle CE, Lowe AB (2009) Sequential thiol-ene/thiol-ene and thiol-ene/thiol-yne reactions as a route to well-defined mono and bis end-functionalized poly (N-isopropylacrylamide). J Polym Sci A Polym Chem 47:3544–3557. https://doi.org/10.1002/pola.23436
Zeng T, Zhang P, Li X, Yin Y, Chen K, Wang C (2019) Facile fabrication of durable superhydrophobic and oleophobic surface on cellulose substrate via thiol-ene click modification. Appl Surf Sci 493:1004–1012. https://doi.org/10.1016/j.apsusc.2019.07.040
Acknowledgments
The authors acknowledge financial support from the National Science Centre (Poland), project OPUS UMO-2018/29/B/ST8/00913, entitled “Synthesis and characterization of materials with defined surface properties”.
Funding
This work was supported by funds from the National Science Centre (Poland), project OPUS 2018/29/B/ST8/00913, entitled “Synthesis and characterization of materials with defined surface properties”.
Author information
Authors and Affiliations
Contributions
Performed cotton fabrics modifications and wrote the manuscript—MP; synthesized and characterized the obtained compounds –thiol-ene click reaction, participated in writing the manuscript—AS; participated in the conceptualization, provided guidance during the planning of experiments and reviewed the manuscript—HM; Washing process, thiol-ene click reaction –AP
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, nor in the decision to publish the results.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
All authors gave explicit consent to participate.
Consent for publication
All authors gave explicit consent to submit.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Szymańska, A., Przybylak, M., Maciejewski, H. et al. Thiol-ene chemistry as an effective tool for hydrophobization of cotton fabrics. Cellulose 29, 1231–1247 (2022). https://doi.org/10.1007/s10570-021-04357-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-021-04357-w