Abstract
This article provides a perspective review on the use of modified cotton fabrics for oil–water separation. The principles of surface hydrophobicity of cotton fabrics are first described, from which the basis for producing superhydrophobic surfaces is presented. Then the preparation methods to convert hydrophilic cotton fabrics to hydrophobic fabrics are reviewed and discussed. Based on literature results the way to design novel preparation methods, the need to summarize testing protocols, and the comprehensive technoeconomic and sustainability analyses, are proposed. A demonstrative cotton fabrics test is used to reveal the significant role of conjugated fluid flows and surface interactions under different application scenarios for determining the separation efficiency of the oil–water mix.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oil spilling from life, industry and accident can adversely impact environment quality. The oil skimmer, centrifugation and air flotation processes were proposed to mitigate oil spilling pollution (Wang et al. 2020; Lu et al. 2020), which have drawbacks including low separation efficiency, high energy demand and time-consuming procedures (Shang et al. 2018; Dashairya et al. 2018). Oil-in-water wastewaters are generated in petrochemical, mineral and smelting, and food processing industries (Tao et al. 2014). Sufficient de-emulsification to convert stabilized micro droplets to large oil droplets can enhance oil–water separation efficiency in practice (Zhang et al. 2020a).
The porous sorbents, if applicable, can be effective to remediate oil pollution sites at low operational cost (Dashairya et al. 2019). A surface is considered hydrophobic with contact angle of water drop exceeding 90°. A surface is regarded superhydrophobic if the water contact angle is higher than 150° and the contact angle hysteresis is lower than 10° (Wang et al. 2016). A few superhydrophobic surfaces in nature can possess lotus leaf effect (Wei et al. 2010), rose petal effect (Shao et al. 2020) or butterfly wing effect (Bixler and Bhushan 2014). The porous sorbent with superhydrophobic surface should be able to adsorb oil from a water/oil mix (Hadji et al. 2020). For instance, a melamine sponge coated with hydrophobic TiO2 nanoparticles is shown to preferably separate oils and organic solvents from water (Cho et al. 2016). Cai et al. (2020) fabricated fluorine-free hydrophobic HDTMS-TiO2 coated mesh with high oil separation efficiency and cost effectiveness. Other modified matrixes including stainless mesh, polymer and melamine foams were applied for separating oil from water (Wang et al., 2020). However, these adsorbents are either produced in an environmentally unfriendly process or their final disposal can lead to secondary pollution (Lyon et al. 2020; Gong et al. 2019). The eco-friendly matrix with superhydrophobic surfaces is desired to tackle the oil spill pollution problems.
Cellulose is the most abundant biopolymer that is used as biodegradable and cost-effective oil adsorbent (He et al. 2020). Owing to the numerous hydroxyl groups presented on the pristine cellulose, hydrophobic modification of its surface should benefit the oil adsorption tendency from the spill (Mai et al. 2018). Cotton fabric is one of the cellulose-rich primary agricultural products, whose modification and use as oil spill adsorbent should have its promise. To apply hydrophilic cotton fabric for oil spill remediation acquires modifications to hydrophobilize the surfaces (Deng et al. 2020). This article summarized the commonly adopted modification methods including dip coating, in situ crosslinking, UV-triggered click reaction, self-affine modification and spray coating for fabricating superhydrophobic cotton fabric surface. A demonstration test with a modified cotton fabric is presented to reveal the needs to revise the testing method and to link to practical use of the fabricated cotton fabrics.
Water wetting and hydrophobicity
The hydrophobicity of a surface is attributed to the surface energy of substrate and determined by differences of the surface energies for the liquid and the solid surface (Krasowska et al. 2009). If a smooth surface, naturally or synthetically made, is subjected to a liquid drop in air; if the surface energy of the solid surface is higher than that of the liquid, the surface will be wet since the solid tends to pull the liquid molecules to it for minimizing their total energy level. Conversely if the surface energy of solid is lower than that of liquid, the solid would try to repel the liquid out to prevent increasing of its energy level.
The way to estimate the surface energy is to measure the contact angle between the solid and liquid. When a droplet contacts with a solid surface, three interfacial forces will act on the contact point: interfacial force between liquid and vapor (γL), interfacial force between solid and vapor (also mean the surface energy of solid) (γS) and interfacial force between solid and liquid (γSL). The contact angle (θ) is measured by the angle between solid surface and a liquid droplet on the surface (Kwok and Neumann, 1999). The interfacial force balance at the three-phase point for a smooth surface can be presented as the Young’s equation as follows:
Based on Eq. 1, high θ (> 90°) acquires low γS, low γL and/or high γSL. Low γS indicates a surface with both low polar and dispersive interactions with the surrounding molecules [> 250 mJ/m2 for metal and glass; 22–35 mJ/m2 for C/H polymer films (Fenouillot et al. 2009); 18 mJ/m2 for PFTE with only C and F atoms (Heitz and Dickinson 1999)]; low γL suggests a liquid with low inter-molecular interactions (18.4 mJ/m2 for n-hexane vs 72.8 mJ/m2 for water @ room temperature); high γSL corresponding to null work of adhesion, or there is no interaction between solid and liquid. The superhydrophobic surface can have high contact angle (θ) > 150°. At θ180o, γSL = γS + γL. with null work of adhesion; restated, the solid and liquid should extremely repel each other. The schematics of superhydrophobicity and superoleophobicity of a solid surface is shown in Fig. 1a.
a Schematics of superhydrophobicity and superoleophobicity of a surface. Typical superhydrophilic and superoleophilic surfaces are high energy metal surfaces; typical superhydrophobic and superoleophilic surfaces are lotus leaves (Tuteja et al. 2007), typical superhydrophilic and superoleophobic surfaces are ammonia treated heptadecafluorononanoic acid modified TiO2 coated SiO2 nanoparticles coated sponge (Xu et al. 2015); typical superhydrophobic and superoleophobic surfaces are 1H,1H,2H,2H-perfluorooctyltrichlorosilane coated porous silicon (Chu and Seeger 2014). b θ* versus θ* based on Wenzel model. r is the roughness ratio
The theory by Girifalco and Good (1957) estimated minimum γS = 6 mJ/m2, corresponding to maximum θ = 115.2°. By plotting the plots of literature data with γLV–γS versus cos θ, the limit contact angle is estimated to be 156° for water and 109° for hexadecane on a surface at γS = 0 limit (Shafrin and Zisman 1964). However, since no γS = 0 surface exists, the maximum apparent contact angle on a real-world smooth surface involving a sharp edge is estimated 120° (Oliver et al. 1977). Therefore, by a completely wet, smooth surface it is impractical to yield superhydrophobic surface using pure chemical modification pathways.
If a rough surface, naturally or synthetically made, is placed by a liquid drop in air, very large apparent contact angle (θ*) can be yielded by the contact angle hysteresis or by the air-trapped cavity to lift the drop from the solid surface. The Wenzel model predicts the link between θ* and θ as cos θ* = r cos θ, where r is the roughness ratio. Their correspondence is shown in Fig. 1b. For smooth surface (nearly unity r), θ* = θ; for very rough surface with r = 1.8, hydrophobic surfaces with θ of ca. 120° can yield superhydrophobic surface with θ* = 154°. Restated, the rough surfaces with hydrophobic properties can generate superhydrophobic effects in need.
Numerous surface modifications were adopted to affect the hydrophobicity of surface, including the doping/grafting nanoparticles (Dashairya et al. 2019b; Rostami et al. 2019), polymers (Qin et al. 2019; Jannatun et al. 2020), fluoroalkylsilanes (Cai et al. 2020) and alkyl silanes (Nanda et al. 2019; Guo et al. 2018a), were used for increasing hydrophobicity of the modified cotton fabrics. For instance, the nanoparticles, particularly zinc oxide nanoparticles with satisfactory biocompatibility and biodegradability, were doped onto targeted surface for increasing local roughness (Bai et al. 2019; Mendoza et al. 2019). The use of polymer, such as polyurethane monolith can be applied to produce interconnected pore structures on a solid surface via phase separation pathway so generating superhydrophobic effects on elastic substrate (Ye et al. 2020). Silane agents can react with the hydroxyl groups of substrates then anchor on the surface, so a hydrophilic surface can be converted into hydrophobicity with the hydrophobic poly silane groups with increased alkyl chain length (Qi et al. 2019). The surface hydrophobicity can be synergistically enhanced by the hierarchical structure and roughness which decreases the apparent surface energy (Wang et al. 2009). The surface grafted by long chain poly(fluoro-methacrylate), if the grafted layer thickness is thick enough to prevent water penetration, would yield > 140° contact angle (Guo et al. 2020). (Apparently the surface of the grafted layer should not be smooth otherwise the contact angle cannot exceed 120°). Wang et al. (2017a) utilized hexadecyltrimethoxysilane (HDTMS) constructing hierarchical structure to fabricate superhydrophobic steel mesh. Huang et al. (2020) fabricated fluoroalkylsilane-modified balsa wood sponge to clean up viscous crude oil.
A superamphiphobic or superomniphobic surface is a superhydrophobic and superoleophobic surface (Chu and Seeger 2014), which was first developed by Tuteja et al. (2007) by employment of re-entrant surface curvature and chemical composition modification. The superhydrophobic and superoleophobic surfaces are ready to foul in the oil–water separation; and since water is denser than oil, the hydrophobic adsorbent would hinder water to flow though (Wang et al. 2015). The superhydrophilic and superoleophobic surface is preferred for oil–water separation so water can flow over but oil can be prevented (Su et al. 2017). However, superoleophobic surface dislikes water, so it is hard to make it hydrophilic. The mesh with coating can make it a hydrophilic and oleophobic matrix for adsorbing oil from flowing through water stream.
As stated, the rough surface that can provide air trapped space beneath the drop is essential to generate superhydrophobic and/or superoleophobic effects (Tuteja et al. 2007). The nanoscale or microscale surface roughness can decrease the θ* if θ < 65° or increase the θ* if θ > 65° (Tian et al. 2013). Numerous methods were applied for generating the surface roughness, random or regular, including nanoparticle deposition, chemical etching or lithography, sol–gel reactions, and galvanic deposition (Chu and Seeger 2014). The natural fabrics such as cotton fabrics have surface roughness of all scales, providing sufficient air reentrant cavities all over the cotton fabric surfaces. The remaining part is to convert the hydrophilic nature of cotton fabrics to hydrophobic. The hydrophobic surface can be synthesized by grating with fluorinate polymers or fluorine monomers, polyhedral oligomeric silsesquioxane (POSS), deposited fluorinated nanoparticles, or other hydrophobic compounds. Xu et al. (2015) prepared a superhydrophobic and superoleophobic surface by dipping PS fabric or polyurethane sponge to SiO2 nanoparticles and heptadecafluorononanoic acid (CF3(CF2)7COOH) modified TiO2 sol. This coating can become superhydrophilic and superoleophobic after exposure to ammonia, which can adsorb water from bulk oil.
Preparation techniques to oil–water separation by modified cotton fabrics
Cotton fabrics were selected for oil spill separation based on its availability, degradability, and low cost (Singh and Singh 2019). Cotton fabrics are naturally grown fabrics with rough and hydrophilic surface, whose surface needs modification to make them fit the needs for oil–water separation (Zhou et al. 2013). As Fig. 1 lists, when using the modified cotton fabrics as oil adsorbent from oil–water mixture, a superhydrophobic and superoleophilic surfaces such as lotus leaves is desired. If the cotton fabric is used to remove trace water from an oil mix, superhydrophilic and superoleophobic surfaces such as the hot modified alkali treatment which introduce excess hydroxyl groups on the cotton fabric surface as proposed by Rana et al. (2016) is desired. This section categorizes the contemporary works that modified cotton fabrics surfaces for use in oil–water separation.
Dip coating
Nanoparticles ZnO (Boticas et al. 2019; He et al. 2019), SiO2 (Subramanian et al. 2020), TiO2 (Rostami et al. 2019) and Ag (Ahmed et al. 2020) were deposit to hydrophilic surfaces for reducing the surface energy of solids, so turning the surface to superhydrophobic (Fig. 2a). Zhang et al. (2013) adopted dip-coating method for fabricating superhydrophobic cotton fabric textiles using modified Zn nanoparticles and polystyrene as the water expeller (Zhang and Seeger 2011). Liu et al. (2013) fabricated superhydrophobic cotton fabrics with SiO2 nanoparticle deposition and octadecyltrichlorosilane modification. The key of dip coating is the affinity of nanoparticles and the surface of substrate to lead to strong particle attachment. Dip-coating can also be applied for fabricating functional membrane (Wenten et al. 2020) via sol–gel process (Matin et al. 2019) in large scale (Shafiee et al. 2019). This method is commonly simple to implement (Zhang et al. 2013), however, the disadvantages include easy detachment of particles as noted in some applications (Deng et al. 2020), which may yield health concerns and environmental concerns (Lee and Lee 2019). Prolong durability is essential for the success of the nanoparticle deposition method for fabricating superhydrophobic surfaces. To improve the durability, polymer compounds were used as binder between the substrate and nanoparticles (Anjum et al. 2020; Jeyasubramanian et al. 2016).
Schematics of modification methods for cotton with superhydrophobic surfaces. a Dip-deposition of nanoparticles onto a substrate surface; b in situ crosslinking method; c thiol-ene click reaction; d crosslink with amino-containing silane via Michael addition/Schiff base reactions; e spray coating method with modification of HNTs then spray coat HNTs to establish superhydrophobic surface
In situ crosslinking
Jannatun et al. (2020) fabricated SiO2/PVA/PDMS modified cotton fabric which used boric acid as a crosslinker. Guo et al. (2019) constructed hydrophobic cotton fabric by crosslinking of FAS15 and PDMS. Zhang et al. (2020b) synthesized superhydrophobic surface by immobilizing the ZnO nanoparticles and octyltriethoxysilane on the cotton fabric. In these cases, nanoparticles and polymer monomers can be mixed and crosslinking on the surfaces to form hydrophobic/superhydrophobic layer (Fig. 2b). The CuSA2-modified superhydrophobic cotton fabric that can separate n-heptane/water mixture up to 96% efficiency was fabricated (Pan et al. 2019). Cheng et al. (2019) established superhydrophobic cotton fabric via solvothermal polymerization. Song et al. (2020) synthesized ODTMS-HNTs modified cotton fabric which could maintain high separation efficiency after 40 cycles of oil/water separation. Table 1 lists the typical studies with in situ crosslinking modified cotton fabrics that can be applied for efficient oil–water separation. The modified substrate fabricated via in situ crosslinking possesses excellent durability against abrasion and chemical corrosion. Nevertheless, the fabrication procedure is energy-consuming by the high reaction temperature required.
The UV-triggered reactions were developed with high reactivity under mild and well-controlled reaction conditions (Jiang et al. 2019; Ning et al. 2019). Shen et al. (2019a) adopted ODA to crosslink with polydimethylsiloxane via UV stimulation to synthesize superhydrophobic coating. Shen et al. (2019b) synthesized superhydrophobic cotton fabric via click reaction under UV irradiation. Meng et al. (2020) constructed superhydrophobic surface via thiol-ene click reactions. The POSS (Deng et al. 2020; Yang et al. 2020), fluorine-containing molecules (Jiang et al. 2019; Yang et al. 2019), and long-chain molecules (Chen et al. 2019) were grafted to make superhydrophobic cotton fabrics via thiol-ene reactions under UV irradiation (Fig. 2c). Restated the hydroxyl groups on surface were first converted to thiol groups by thiol-containing silane, and then the so-produced thiol groups are reacted with POSS, fluorine-containing molecular, or long chain molecular to form double bonds under UV irradiation via thiol-ene click reactions. The final groups on surface can efficiently expel water molecules. Deng et al. (2020) fabricated POSS-grafted hydrophobic cotton fabrics via thiol-ene click reaction for oil/water separation. Hou et al. (2018) fabricated robust POSS-based superhydrophobic fabrics to absorb organic solvents from water. Table 2 lists the typical UV triggered reactions for superhydrophobilization of surfaces of cotton fabrics.
Self-affine modification
Nature-inspired methods received research interests owing to its sustainability, flexibility, and versatility to applications (Huang et al. 2016). Dopamine, 3,4-dihydroxyphenethylamine, is a hormone that plays essential regulating functions in human body. Inspired by the compositions of adhesive proteins in mussels, dopamine was applied in multifunctional polymer coating and in preparation of heparin immobilizing membrane (Jiang et al. 2010). Polydopamine (pDA) can be coated on a variety of inorganic and organic material surfaces, and then the coating can be further modified by numerous functional substances via grafting of macromolecules, deposition of long-chain molecules, and reduction of metal ions (Lee et al. 2007; Ahmed et al. 2020). Dopamine can be easily polymerized into pDA in mild conditions without stimulation (Wang et al. 2017b; Hu et al. 2006; Chen et al. 2019c), so leading to a method with potential for large-scale production (Xi et al. 2009). Xu et al. (2015) utilized silver nanoparticles which is reduced by dopamine to fabricate superhydrophobic melamine foam. Yan et al. (2020) synthesized dopamine-modified superhydrophobic cotton fabric via grafting octadecylamine (ODA). The major disadvantage of applying dopamine as modification agent is its high cost (Chen et al. 2018; Wang et al. 2019a) and excess time for reactions 12 h for TA/APTES, 9 h for TA/Ti (Hu et al. 2020) pDA/GO/PEI for 72 h (Li et al. 2019), and 80 h for pDA/Cu/eumelanin (Ball et al. 2013). In particular, the time to form pDA coating may take days (Zhang et al. 2016). Compared to pDA, tannic acid (TA), an affordable compound, also shows supreme adhesion tendency to wide ranges of substrate surfaces (Wang et al. 2018c). TA is a natural polyphenol which possesses abundant catechol functional groups, which can crosslink with amino-containing silane via Michael addition/Schiff base reactions (Fig. 2d) (Feng et al. 2019). However, the reaction time of tannic acid is still long compared to the UV-triggered reaction. Reducing the reaction time is essential for production efficiency. Other commonly adopted modification agents were also shown in the figure. POSS are organic–inorganic hybrid nanoparticles which have a cage with the general formula (RSiO1.5)n (Sun et al. 2016). Lui el at. (2019) utilized POSS to enhance the thermal stability and water resistance of polymeric film. Qiang el at. (2017) constructed POSS-graft superhydrophobic cotton fabric to improve the durability. The POSS-modified substrates possess UV resistance (Song et al. 2013; Deng et al. 2019), thermal stability (Liu et al. 2007; Huang et al. 2003), anti-corrosion ability (Yang et al. 2020), anti-abrasion (Zeng et al. 2019), low surface energy and good mechanical properties (McMullin et al. 2016).
Spray coating
Spray coating has considered as a promising method owing to its high efficiency (Zhang et al. 2019), simplicity (Lin et al. 2020), feasibility to be performed in large scale (Wang et al. 2019b). Guo et al. (2018a) fabricated superhydrophobic stainless steel mesh with spray coating of hexadecyltrimethoxysilane. For instance, the hydrophilic halloysite clay nanotubes (HNTs) were modified by long-chain silane to form hydrophobic nanotubes (Fig. 2e), and then the modified nanotubes were mix with curing agent and sprayed onto the targeted substrate surface. Superhydrophobic surface could be obtain by curing (Fig. 2e). Using this method, Yuan et al. (2019) synthesized FSiO2/HNTs for spray coating of aluminum plate to form superhydrophobic surface. Foorginezhad et al. (2019) established OV-POSS/PDMS superhydrophobic cotton fabrics that possessed chemical resistance and abrasion resistance. Guo et al. (2018b) constructed hierarchical nano-microstructure via free radical solution polymerization to create superhydrophobic surface. In Table 3, the polymeric materials such as resin (Zhou et al. 2019) or PDMS (Foorginezhad and Zerafat, 2019) were used as binder. Composites with polymeric materials and low surface energy nanoparticles (Gong et al. 2020; Esmeryan et al. 2019), wax (Celik et al. 2020; Torun et al. 2019) were applied to fabricate the superhydrophobic surface. However, the material including nondegradable polymer and detachment of nanoparticle are not friendly to environment (Lee and Lee 2019; Iwata 2015).
Other hydrophobicity modification method
Plasma treatment is efficient (Fu et al. 2018) and reducing waste generation (Kamlangkla et al. 2010). Li et al. (2015) synthesized hydrophobic cotton fabric via plasma-induced graft polymerization. Kamlangkla et al. (2010) utilized SF6 plasma treatment to fabricate hydrophobic cotton fabric. Kim et al. (2006) deposited hydrocarbon layer on cotton fabric to fabricate hydrophobic surface via plasma treatment. The equipment of plasma treatment is expensive and the plasma treatment lacks scale-up niche. Reducing the cost and making the process continuous instead of batchwise are essential for large-scale implementation of the plasma treatment production (Sarmadi 2013).
Discussions
Design of preparation method
Fabrication of superhydrophobic cotton fabric surfaces is readily achievable with simple method in mild conditions (Sect. 3). Individual method has its own advantage and disadvantage in preparation. Dip coating via sol–gel process to deposit nanoparticles on the substrates is simple and can product in large scale. But the interaction between nanoparticles and substrates is poor so the nanoparticles can be easily detached from the surface. In-situ crosslinking reaction endows the modified substrate with good mechanism resistance and good chemical resistance. However, this process is energy-consuming because of the high reaction temperature. Although the superhydrophobic matrix synthesized via UV-triggered click reaction are well-controlled and efficient, this reaction requires expensive UV irradiation equipment and is energy-consuming and costly, so can only be applied in small scale applications. Self-affine modification is sustainable to environment and is versatile to applications, but the reaction is time consuming compared to other methods. Spray coating is a method which can be applied in large scale with high efficiency; however, the material used in modification such as polymeric substance and fluorine-containing substances may cause adverse impacts on the receiving environment (Table 4).
Design of new preparation method for cotton fabrics can be based on the basic surface characteristics shown in Fig. 1a and the specific incentive to achieve. The pristine cotton fabric surface is superhydrophilic and superoleophobic, at the second quadrant of Fig. 1a. This cotton fabric is unfavorable to extract oil from the oil spilled sites. The modification methods reported in Sect. 3 is to fabricate superhydrophobic and superoleophilic surfaces, located at the forth quadrant of Fig. 1a. The so-generated surface would attract oil but repel water in the feed. If the modification method is to prepare a superhydrophobic and superoleophobic surface (the first quadrant) or a superhydrophilic and superolephilic surface (the third quadrant), separation is not achievable by surface affinity to oil and water phases.
Based on Fig. 1b, if a surface with contact angle exceeding 150° is needed, a rough surface is required. When a smooth surface is to be modified, deposition of nanoparticles to increase the surface roughness is often applied. When using nature cotton fabric surface as the substrate, the inherent surface roughness can be sufficient to serve as the air gap to support water drop with high contact angle.
New superhydrophobic cotton fabric preparation method can be proposed by combining those reported in Sect. 3. For instance, to combine self-affine modification and UV-triggered click reaction into one step can yield a simple cotton fabric preparation process using cost-effective and renewable feedstocks. For instance, the POSS-modified cotton fabrics (POSS-CT) can be fabricated under UV irradiation in one step instead of two steps adopted in literature. By mixing tannic acid, (3-mercaptopropyl)trimethoxysilane (MPTES), POSS and cotton fabrics together tannic acid can react with the thiol groups of MPTES via Michael addition reaction to form the TA hybrid coating that have hierarchical structures and high adhesion to cotton fabrics, and in the same medium POSS can react with the thiol-containing silane via thiol-ene click reaction to form the TA-MPTES-POSS modified cotton fabrics. Since the polymerization of TA by UV irradiation stimulation is a rapid reaction (Sadabad et al. 2017), the processing time of the newly prepared cotton fabric surfaces can be reduced. The price for TA is much cheaper than POSS, the replacement of TA to POSS can also sufficiently reduce the fabrication cost and fulfill the merit to apply renewable resources for applications. Shen et al. (2021) also synthesized TA-POSS composite membrane by crosslinking the tannic acid with POSS via Michael addition reactions. Additionally, the superior environmental compactibility of TA to that of POSS is another concern if the end disposal of the used cotton fabrics will be put into consideration.
Proper testing protocol is needed
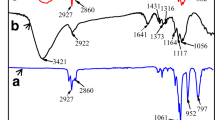
The oil–water separation efficiencies of the modified cotton fabrics adopted in literature were characterized using various protocols. We synthesized the hydrophobic cotton fabrics by a preparation method as follows: The 3-mercaptopropyltriethoxysilane (MPTS) and EtOH were mixed at weight ratio of 1:20 with pristine cotton fabrics being dipped at room temperature for 2 h. The dipped cotton fabrics was then washed with EtOH and was dried at 80 °C for 2 h to obtain samples SH-CT. The SH-CT samples were mixed in 1.6% w/w POSS solution with 0.16% w/w DMPA and 98.2%% w/w CH2Cl2 for 30 min. The dipped samples were then exposed to 365 nm UV light (@ 10 mW/cm2) to trigger thiol-ene click reaction for 60 min. Then the cotton fabrics were collected and washed with ethanol and further drying at 80 °C for 60 min. The obtained samples were the POSS-CT samples. The FTIR spectra of the cotton fabrics, SH-CT, and POSS-CT were shown in Fig. 3a, showing the attachment of the desired functional groups on the cotton fabric surfaces. The SEM–EDS images for the samples were revealed in Figs. 3b, clearly confirming the presence of C and O on cotton fabric surface, and that of Si on the POSS-CT surface. The produced POSS-CT surface has contact angles with water drops to be 145°., suggesting the successful fabrication of the hydrophobic cotton fabric surface (Fig. 3c). The yielded POSS-CT were used to packed into layer of thickness of 5 mm.
The testing methods for water–oil separation using modified cotton fabrics include the absorption test by applying the cotton fabrics to absorb oil from a clot of oil submerged in a water pool (Hou et al. 2018), and the pour test by pulling a bottle of segregated oil–water layers (a water layer with one or two immersible oil layer) to pass through the cotton fabric matrix for separation (Yang et al. 2020). The hypothesis is that the hydrophobic cotton fabrics would tend to absorb the oil from water (the absorption test) and to reject water with passage of the oil phase (the pour test). The POSS-CT was immersed to contact the DCM (red) at the bottom of the water pool and was noted to efficiently adsorb the DCM by the fabrics (Fig. 4a). This observation suggests that the POSS-CT has hydrophobic surfaces so can be used for oil–water separation. When the pristine cotton fabric was made contacting the DCM (red) under the water layer, the cotton fabric absorbed no DCM, correlating with the superhydrophilic nature of the fabric surface (Fig. 4b). However, when the hydrophilic cotton fabrics were made contacting the hexane (red) floating on the water pool, the cotton fabrics absorbed the hexane (Fig. 4c), contracting to the assumption that the hydrophilic cotton fabrics would not absorb oil compounds. This contradictory result was yielded by the bulk flow induced by capillarity of the macro pores of cotton fabrics with both oil and water phases inflow with minimal roles of surface hydrophobicity. As per the results in Fig. 4b, when the pristine cotton fabrics were immersed in water pool to absorb the DCM underneath the water layer, the macro pores would be filled with water first, so the bulk flow would be minimized, leading to negligible absorption of DCM into the pristine cotton fabrics.
Tests for oil–water separation for the tested samples. a Absorption test: POSS-CT for DCM (red) under water pool; b absorption test: pristine cotton for DCM (red) under water pool; c absorption test: pristine cotton for hexane (red) floating on water pool; d pour test: POSS-CT for DCM (red, bottom layer) and water (blue, top layer) separation; e pour test: POSS-CT for DCM (red) with SDS surfactant in the form of emulsion (looks purple) and water separation
The pour test using DCM (red, bottom layer) and water (blue, top layer) poured on the POSS-CT fabrics. The DCM passed through the fabrics and the water was kept on the POSS-CT, revealing separation (Fig. 4d). The separation efficiency of water from a oil/water mixture was characterized using the following equation:
where M1 and M2 are the quantities of initial sample and of collected sample after separation, respectively (Jiang et al. 2019). For instance, the separation efficiently for Fig. 4e was decreased about 30% after two cycles of separation. The durability and cyclability of the cotton fabrics were commonly described by observing the decline in separation efficiency over testing cycles. The mechanical properties of cotton fabrics such as flexural strength, flexural modulus, impact strength and fracture toughness were seldom described in related modification literature for cotton fabrics.
Conversely, when the DCM (red) was mixed with water and 20 mg/L surfactant sodium dodecyl sulfate (SDS) to generate an emulsion, the pour test with the generated emulsion onto the POSS-CT led to no separation, revealing complete passage of emulsion through the fabrics (Fig. 4e). Restated, although also with DCM and water (while the emulsion was with trace quantity of SDS) onto the POSS-CT, the pour tests showed distinct results from that in Fig. 4d owing to the different distribution of oil in the water pool. The pour tests with segregated water and oil layers, since the DCM is heavier than water and flows faster than water to contact the cotton fabrics, the macro pores were filled by DCM which rejected subsequent passage of water through the fabrics. When the oil was dispersed as oil-in-water emulsion, the collision of fine oil droplets with hydrophobic walls of macro pores dominate, leading to full rejection of DCM from the oil–water mix as noted in Fig. 4e. Illustrations in Fig. 4d and e showed that how the oil and water dispersed in the mix can lead to contradictory outcomes for separation.
The separation tests in Fig. 4 reveal the need to set up “standard” testing protocol for the modified cotton fabrics (and other matrix) for oil–water separation. Additionally, the “scenario” of the oil–water separation should also come into play. For instance, for recovery of spilled crude from a tanker on open sea, the as much as possible absorption of oil into the cotton fabrics is the target with the incorporated water and other impurities being non-essential factors and the operation should be considered batchwise or consider the used fabrics non-recyclable. To separate oil droplets in an oil-in-water wastewater from crude refinery, the efficient collisions of oil drops onto the hydrophobic pore surfaces for collection with water to pass though as fast as possible is the design target; however, the accumulation of oil inside macro pores with hydrophobic walls would lead to reduced filtration rate, while the continuous or batchwise deoiling steps from the cotton fabrics should be operated together with the oil–water separation processes. When handing water-in-oil emulsion, Lei et al. (2017) showed that their modified cotton fabrics can allow passage of oil and repel water droplets to accumulate on the top surface of the fabrics. It is clear that the conjugated fluid flow through macro pores and the involvement of surface interactions with the multi-phase fluid controls the separation efficiency. Further studies are needed.
Economy and sustainability analyses is needed
Comprehensive technoeconomic and sustainability analyses should be performed to confirm the use of modified cotton fabrics for oil–water separation. The use of cotton fabrics for oil–water separation should be justified on their economy and sustainability based on the fact that cotton fabrics are an economy plant used for textile since pre-historical era, and the modification chemicals used are not all environmentally friendly. If the cotton fabrics are to be used for contamination remediation, the quality of the recovered oil and the efficiency of the separation are not critical. However, if the separation is to be applied in a fine chemical production chain, the purity of the applied cotton fabrics and very high separation efficiency should be the determining factor for the process economy. When handling high-value products, whether the used cotton fabrics can be reused is of no concern. If the oil spill is to be confined, other waste besides cotton fabrics with excess hydroxyl groups may be also candidates for the feasible matrix for oil–water separation. The release of the modified chemicals into the environment after the modified cotton fabrics is disposed of after use.
Conclusions
This article presented a mini-review on the perspectives of applying modified cotton fabrics for oil–water separation. The principles of hydrophobicity of a surface is first stated, with the ways of producing superhydrophobic surfaces being described. The modification methods reported in literature are then summarized. The perspectives of using cotton fabrics for oil–water separation are highlighted. The needs to design new fabrication method, to develop standard testing protocols, and to perform technoeconomic and sustainability analyses for oil–water separation with the use of modified cotton fabrics are addressed.
References
Ahmed SB, Khalil MMA, Moataz S, Shaker E (2020) Novel superhydrophobic surface of cotton fabrics for removing oil or organic solvents from contaminated water. Cellulose 27:7703–7719
Anjum AS, Ali M, Sun KC, Riaz R, Jeong SH (2020) Self-assembled nanomanipulation of silica nanoparticles enable mechanochemically robust super hydrophobic and oleophilic textile. J Colloid Interf Sci 563:62–73
Bai X, Zhao Z, Yang H, Li J (2019) ZnO nanoparticles coated mesh with switchable wettability for on-demand ultrafast separation of emulsified oil/water mixtures. Sep Purif Technol 221:294–302
Ball V, Gracio J, Vila M, Singh MK, Boutigue MHM, Michel M, Bour J, Toniazzo V, Ruch D, Buehler MJ (2013) Comparison of synthetic dopamine-eumelanin formed in the presence of oxygen and Cu2+ cations as oxidants. Langmuir 29:12754–21276
Bixler GD, Bhushan B (2014) Rice- and butterfly-wing effect inspired self-cleaning and low drag micro/nanopatterned surfaces in water, oil, and air flow. Nanoscale 6:76–96
Boticas I, Dias D, Ferreira D, Magalhães P, Silva R, Fangueiro R (2019) Superhydrophobic cotton fabrics based on ZnO nanoparticles functionalization. SN App Sci 1:1376
Cai Y, Zhao Q, Quan X, Feng W, Wang Q (2020) Fluorine-free and hydrophobic hexadecyltrimethoxysilane-TiO2 coated mesh for gravity-driven oil/water separation. Colloid Surf A 586:124189
Celik N, Torun I, Ruzi M, Esidir A, Onses MS (2020) Fabrication of robust superhydrophobic surfaces by one-step spray coating: evaporation driven self-assembly of wax and nanoparticles into hierarchical structures. Chem Eng J 396:125230
Chen S, Xie Y, Xiao T, Zhao W, Li J, Zhao C (2018) Tannic acid-inspiration and post-crosslinking of zwitterionic polymer as a universal approach towards antifouling surface. Chem Eng J 337:122–132
Chen J, Zhou Y, Zhou C, Wen X, Xu S, Cheng J, Pi P (2019) Durable underwater superoleophobic and underoil superhydrophobic fabric for versatile oil/water separation. Chem Eng J 370:1218–1227
Cheng QY, Guan CS, Li YD, Zhu J, Zeng JB (2019) Robust and durable superhydrophobic cotton fabrics via a one-step solvothermal method for efficient oil/water separation. Cell 26:2861–2872
Cho EC, Jian CWC, Hsiao YS, Lee KC, Huang JH (2016) Interfacial engineering of melamine sponges using hydrophobic TiO2 nanoparticles for effective oil/water separation. J Taiwan Inst Chem Engrs 67:476–483
Chu Z, Seeger S (2014) Superamphiphobic surfaces. Chem Soc Rev 43:2784
Dashairya L, Rout M, Saha P (2018) Reduced graphene oxide-coated cotton as an efficient absorbent in oil-water separation. Adv Composit Hybrid Mater 1:135–148
Dashairya L, Barik DD, Saha P (2019) Methyltrichlorosilane functionalized silica nanoparticles-treated superhydrophobic cotton for oil–water separation. J Coat Technol Res 16:1021–1032
Deng Y, Han D, Zhou DL, Liu ZQ, Zhang Q, Li Y, Fu Q (2019) Monodispersed hybrid microparticles based on polyhedral oligomeric silsesquioxane with good UV resistance and high thermal stability: from organic to inorganic. Polymer 178:121609
Deng Y, Han D, Deng YY, Zhang Q, Chen F (2020) Facile one-step preparation of robust hydrophobic cotton fabrics by covalent bonding polyhedral oligomeric silsesquioxane for ultrafast oil/water separation. Chem Eng J. https://doi.org/10.1016/j.colsurfa.2019.123880
Esmeryan KD, Castano CE, Chaushev TA, Mohammadi R, Vladkova TG (2019) Silver-doped superhydrophobic carbon soot coatings with enhanced wear resistance and anti-microbial performance. Colloid Surf A 582:123880
Fang Y, Liu C, Li M, Miao X, Pei Y, Yan Y, Xiao W, Wu L (2020) Facile generation of durable superhydrophobic fabrics toward oil/water separation via thiol-ene click chemistry. Ind Eng Chem Res 59:6130–6140
Feng S, Li M, Zhang S, Zhang Y, Wang B, Wu L (2019) Superoleophobic micro-nanostructure surface formation of PVDF membranes by tannin and a condensed silane coupling agent. RSC Adv 9:32021–32026
Fenouillot F, Cassagnau P, Majesté JC (2009) Uneven distribution of nanoparticles in immiscible fluids: Morphology development in polymer blends. Polymer 50:1333–1350
Foorginezhad S, Zerafat MM (2019) Fabrication of superhydrophobic coatings with self-cleaning properties on cotton fabric based on octa vinyl polyhedral oligomeric silsesquioxane/polydimethylsiloxane (OV-POSS/PDMS) nanocomposite. J Colloid Interf Sci 540:78–87
Fu Y, Wang G, Ming X, Liu X, Hou B, Mei T, Li J, Wang J, Wang X (2018) Oxygen plasma treated graphene aerogel as a solar absorber for rapid and efficient solar steam generation. Carbon 130:250–256
Girifalco LA, Good RJ (1957) A theory for the estimation of surface and interfacial energies. I. Derivation and application to interfacial tension. J Phys Chem 61:904–909
Gong X, He S (2020) Highly durable superhydrophobic polydimethylsiloxane/silica nanocomposite surfaces with good self-cleaning ability. ACS Omega 5:4100–4108
Gong X, Wang Y, Zeng H, Betti M, Chen L (2019) Highly porous, hydrophobic, and compressible cellulose nanocrystals/poly(vinyl alcohol) aerogels as recyclable absorbents for oil-water separation. ACS Sustain Chem Eng 7:11118–11128
Guo D, Chen J, Hou K, Xu S, Cheng J, Wen X, Wang S, Huang C, Pi P (2018a) A facile preparation of superhydrophobic halloysite-based meshes for efficient oil–water separation. Appl Clay Sci 156:195–201
Guo D, Chen J, Wen L, Wang P, Xu S, Cheng J, Wen X, Wang S, Huang C, Pi P (2018b) A superhydrophobic polyacrylate film with good durability fabricated via spray coating. J Mater Sci 53:15390–15400
Guo H, Yang J, Xu T, Zhao W, Zhang J, Zhu Y, Wen C, Li Q, Sui X, Zhang L (2019) A robust cotton textile-based material for high-flux oil-water separation. ACS Appl Mater Interf 11:13704–13713
Guo Q, Zhang T, Xu Z, Li X, Zhao Y (2020) A single covalently grafted fluorolayer imparts intrinsically hydrophilic foams with simultaneous oleophobicity and hydrophilicity for removing water from oils. Colloids Surf A 605:125380
Hadji EM, Fu B, Abebe A, Bilal HM, Wang J (2020) Sponge-based materials for oil cleanups: a review. Front Chem Sci Eng 14:749–762
He Y, Wan M, Wang Z, Zhang X, Zhao Y, Sun L (2019) Fabrication and characterization of degradable and durable fluoride-free super-hydrophobic cotton fabrics for oil/water separation. Surf Coat Technol 378:125079
He T, Zhao H, Liu Y, Zhao C, Wang L, Wang H, Zhao Y, Wang H (2020) Facile fabrication of superhydrophobic titanium dioxide-composited cotton fabrics to realize oil-water separation with efficiently photocatalytic degradation for water-soluble pollutants. Colloid Surf A 585:124080
Heitz J, Dickinson JT (1999) Characterization of particulates accompanying laser ablation of pressed polytetrafluorethylene (PTFE) targets. Appl Phys A 68:515–523
Hou K, Zeng Y, Zhou C, Chen J, Wen X, Xu S, Cheng J, Pi P (2018) Facile generation of robust POSS-based superhydrophobic fabrics via thiol-ene click chemistry. Chem Eng J 33:150–159
Hu MX, Yang Q, Xu ZK (2006) Enhancing the hydrophilicity of polypropylene microporous membranes by the grafting of 2-hydroxyethyl methacrylate via a synergistic effect of photoinitiators. J Membr Sci 285:196–205
Huang JC, He CB, Xiao Y, Mya KY, Dai J, Siow YP (2003) Polyimide/POSS nanocomposites: interfacial interaction, thermal properties and mechanical properties. Polymer 44:4491–4499
Huang S, Zhang Y, Shi J, Huang W (2016) Superhydrophobic particles derived from nature-inspired polyphenol chemistry for liquid marble formation and oil spills treatment. ACS Sustain Chem Eng 4:676–681
Huang W, Zhang L, Lai X, Li H, Zeng X (2020) Highly hydrophobic F-rGO@wood sponge for efficient clean-up of viscous crude oil. Chem Eng J 386:123994
Iwata T (2015) Biodegradable and bio-based polymers: future prospects of eco-friendly plastics. Angew Chem Int Ed 54:3210–3321
Jannatun N, Taraqqi-A-Kamal A, Rehman R, Kumar Kuker J, Lahiri S (2020) A facile cross-linking approach to fabricate durable and self-healing superhydrophobic coatings of SiO2-PVA@PDMS on cotton textile. Eur Polym J. https://doi.org/10.1016/j.eurpolymj.2020.109836
Jeyasubramanian K, Hikku GS, Preethi AVM, Benitha VS, Selvakumar N (2016) Fabrication of water repellent cotton fabric by coating nano particle impregnated hydrophobic additives and its characterization. J Ind Eng Chem 37:180–189
Jiang JH, Zhu LP, Li XL, Xu YY, Zhu BK (2010) Surface modification of PE porous membranes based on the strong adhesion of polydopamine and covalent immobilization of heparin. J Membr Sci 364:194–202
Jiang C, Liu W, Yang M, Liu C, He S, Xie Y, Wang Z (2019) Robust multifunctional superhydrophobic fabric with UV induced reversible wettability, photocatalytic self-cleaning property, and oil-water separation via thiol-ene click chemistry. Appl Surf Sci 463:34–44
Kamlangkla K, Paosawatyanyong B, Pavarajarn V, Hodak JH, Hodak SK (2010) Mechanical strength and hydrophobicity of cotton fabric after SF6 plasma treatment. Appl Sur Sci 256:5888–5897
Kim JH, Liu G, Kim SH (2006) Deposition of stable hydrophobic coatings with in-line CH4 atmospheric rf plasma. J Mater Chem 16:977–981
Krasowska M, Zawala J, Malysa K (2009) Air at hydrophobic surfaces and kinetics of three phase contact formation. Adv Colloid Interf Sci 147–148:155–169
Kwok DY, Neumann AW (1999) Contact angle measurement and contactangle interpretation. Adv Colloid Interf Sci 81:167–249
Lee YJ, Lee DJ (2019) Impact of adding metal nanoparticles on anaerobic digestion performance—a review. Bioresour Technol 292:121926
Lee H, Dellatore SM, Miller WM, Messersmith PB (2007) Mussel-inspired surface chemistry for multifunctional coatings. Science 318:426–430
Li Y, Zhang Y, Zou C, Shao J (2015) Study of plasma-induced graft polymerization of stearyl methacrylate on cotton fabric substrates. Appl Sur Sci 357:2327–2332
Li S, Yang P, Liu X, Zhang J, Xie W, Wang C, Liu C, Guo Z (2019) Graphene oxide based dopamine mussel-like crosslinked polyethylene imine nanocomposite coating with enhanced hexavalent uranium adsorption. J Mater Chem A 7:16902
Lin J, Lin F, Liu R, Li P, Fang S, Ye W, Zhao S (2020) Scalable fabrication of robust superhydrophobic membranes by one-step spray-coating for gravitational water-in-oil emulsion separation. Sep Purif Technol 231:115898
Liu YR, Huang YD, Liu L (2007) Thermal stability of POSS/methylsilicone nanocomposites. Compost Sci Tech 67:2864–2876
Liu F, Ma M, Zang D, Gao Z, Wang C (2013) Fabrication of superhydrophobic/superoleophilic cotton for application in the field of water/oil separation. Carbohyd Polym 97:59–64
Liu D, Yuan L, Xu H, Tian H, Xiang A (2019) PVA grafted POSS hybrid for high performance polyvinyl alcohol films with enhanced thermal, hydrophobic and mechanical properties. Polym Comp 40:2768–2776
Lu T, Qi D, Zhang D, Fu K, Li Y, Zhao H (2020) Fabrication of recyclable multi-responsive magnetic nanoparticles for emulsified oil-water separation. J Clean Prod 255:120293
Lyon BP, Cowie WJ, Maes T, Quesne WJFL (2020) Marine plastic litter in the ROPME sea area: current knowledge and recommendations. Ecotoxicol Environ Saf 187:109839
Mai Z, Xiong Z, Shu X, Liu X, Zhang H, Yin X, Zhou Y, Liu M, Zhang M, Xu W, Chen D (2018) Multifunctionalization of cotton fabrics with polyvinylsilsesquioxane/ZnO composite coatings. Carbohyd Polym 199:516–525
Matin A, Baig U, Akhtar S, Merah N, Gondal MA, Bake AH, Ibrahim A (2019) UV-resistant and transparent hydrophobic surfaces with different wetting states by a facile dip-coating method Progr. Org Coat 136:105192
McMullin E, Rebar HT, Mather PT (2016) Biodegradable thermoplastic elastomers incorporating POSS: synthesis, microstructure, and mechanical Properties. Macromolecules 49:3769–3779
Mendoza AI, Moriana R, Hillborg H, Stromberg E (2019) Super-hydrophobic zinc oxide/silicon rubber nanocomposite surfaces. Surf Interf 14:146–157
Meng G, Yan J, Wu J, Zhang W, Wang Y, Wang Q, Liu Z, Guo X (2020) Thiol-ene click chemistry construct superhydrophobic cotton fabric for high-efficiency water-in-oil emulsion separation. Fibers Polym 21:245–251
Nanda D, Sahoo A, Kumar A, Bhushan B (2019) Facile approach to develop durable and reusable superhydrophobic/superoleophilic coatings for steel mesh surfaces. J Colloid Interf Sci 535:50–57
Ning N, Wang S, Zhang Z, Feng Z, Zheng Z, Yu B, Tian B, Zhang L (2019) Superhydrophobic coating with ultrahigh adhesive force and good anti-scratching on elastomeric substrate by thiol-ene click chemistry. Chem Eng J 373:318–324
Oliver JF, Huh C, Mason SG (1977) Resistance to spreading of liquids by sharp edges. J Colloid Interf Sci 59:568–581
Pan G, Xiao X, Ye Z (2019) Fabrication of stable superhydrophobic coating on fabric with mechanical durability, UV resistance and high oil-water separation efficiency. Surf Coat Technol 360:318–328
Qi Y, Chen S, Zhang J (2019) Fluorine modification on titanium dioxide particles: improving the anti-icing performance through a very hydrophobic surface. Appl Surf Sci 476:161–173
Qiang S, Chen K, Yin Y, Wang C (2017) Robust UV-cured superhydrophobic cotton fabric surfaces with self-healing ability. Mater Design 116:395–402
Qin X, Wang B, Zhang X, Shi Y, Ye S, Feng Y, Liu C, Shen C (2019) Superelastic and durable hierarchical porous thermoplastic polyurethane monolith with excellent hydrophobicity for highly efficient oil/water separation. Ind Eng Chem Res 58:20291–20299
Rana M, Chen JT, Yang S, Ma PC (2016) Biomimetic superoleophobicity of cotton fabrics for efficient oil-water separation. Adv Mater Interf 3:1600128
Rostami A, Pirsaheb M, Moradi G, Derakhshan AA (2019) Fabrication of durable superhydrophobic nanofibrous filters for oil-water separation using three novel modified nanoparticles (ZnO-NSPO, AlOO-NSPO, and TiO2-NSPO). Polym Adv Technol 31:1–16
Sadabad FB, Zhang H, Trouillet V, Welle A, Plumeré N, Levkin PA (2017) UV-triggered polymerization, deposition, and patterning of plant phenolic compounds. Adv Funct Mater 27:170012
Sarmadi M. 2013. Advantages and Disadvantages of Plasma Treatment of Textile Materials. 21st Int. Sym. on Plasma Chem
Shafiee BM, Torkaman R, Mahmoudi M, Emadi R, Karamian E (2019) An improvement in corrosion resistance of 316L AISI coated using PCL gelatin composite by dip-coating method. Progr Org Coat 130:200–205
Shafrin E.G., Zisman W.A. 1964. Upper limits to the contact angles of liquids on solids. In: Chapter 9. Contact Angle, Wettability, and Adhesion. Advances in Chemistry. American Chemistry Society, Washington DC
Shang Q, Liu C, Zhou Y (2018) One-pot fabrication of robust hydrophobia and superoleophilic cotton fabrics for effective oil-water separation. J Coat Technol Res 15:65–75
Shao Y, Zhao J, Fan Y, Wan Z, Lu L, Zhang Z, Ming W, Ren L (2020) Shape memory superhydrophobic surface with switchable transition between “lotus effect” to “rose petal effect.” Chem Eng J 382:122989
Shen L, Lai Y, Fu H (2019a) Fabrication of flower clusters-like superhydrophobic surface via UV curable coating of ODA and V-PDMS. J Appl Polym Sci 136:48210
Shen L, Pan Y, Fu H (2019b) Fabrication of UV curable coating for super hydrophobic cotton fabrics. Polym Eng Sci 59:452–459
Shen YJ, Kong QR, Fang LF, Qiu ZL, Zhu BK (2021) Construction of covalently-bonded tannic acid/polyhedral oligomeric silsesquioxanes nanochannel layer for antibiotics/salt separation. J Membr Sci 623:119044
Singh AK, Singh JK (2019) An efficient use of waste PE for hydrophobic surface coating and its application on cotton fibers for oil-water separator. Progr Org Coat 131:301–310
Song B, Meng L, Huang Y (2013) Preparation and characterization of (POSS/TiO2)n multi-coatings based on PBO fiber surface for improvement of UV resistance. Fibers Polym 14:375–381
Song Q, Wang H, Han S, Wang J, Zhang B, Zhang Y (2020) Halloysite nanotubes functionalized cotton fabric for oil/water separation. Progr Org Coat 148:105839
Su C, Yang H, Song S, Lu B, Chen R (2017) A magnetic superhydrophobic/oleophobic sponge for continuous oil-water separation. Chem Eng J 309:366–373
Subramanian BT, Alla JP, Essomba JS, Nishter NF (2020) Non-fluorinated superhydrophobic spray coatings for oil-water separation applications: an eco-friendly approach. J Clean Prod 256:120693
Sun D, Wang W, Yu D (2016) Preparation of fluorine-free water repellent finishing via thiol-ene click reaction on cotton fabrics. Mater Lett 185:514–518
Tao M, Xue L, Liu F, Jiang L (2014) An intelligent superwetting PVDF membrane showing switchable transport performance for oil/water separation. Adv Mater 26:1942–1948
Tian Y, Jiang L (2013) Intrinsically robust hydrophobicity. Nat Mater 12:291–292
Torun I, Ruzi M, Er F, Onses MS (2019) Superhydrophobic coatings made from biocompatible polydimethylsiloxane and natural wax. Progr Org Coat 136:105279
Tuteja A, Choi W, Ma ML, Mabry JM, Mazzella SA, Rutledge GC, McKinley GH, Cohen RE (2007) Designing superoleophobic surfaces. Science 318:1618–1622
Wang C, Yao T, Wu J, Ma C, Fan Z, Wang Z, Cheng Y, Lin Q, Yang B (2009) Facile approach in fabricating superhydrophobic and superoleophilic surface for water and oil mixture separation. ACS Appl Mater Interf 11:2613–2617
Wang CF, Huang HC, Chen LT (2015) Protonated melamine sponge for effective oil/water separation. Sci Rep 5:14294
Wang Z, Elimelech M, Lin S (2016) Environmental applications of interfacial materials with special wettability. Environ Sci Technol 50:2132–2150
Wang Q, Yu M, Chen G, Chen Q, Tian J (2017a) Robust fabrication of fluorine-free superhydrophobic steel mesh for efficient oil/water separation. J Mater Sci 52:2549–2559
Wang H, Zhou H, Liu S, Shao H, Fu S, Rutledge GC, Lin T (2017b) Durable, self-healing, superhydrophobic fabrics from fluorine-free, waterborne, polydopamine/alkyl silane coatings. RSC Adv 7:33986–33993
Wang Z, Ji S, Zhang J, Liu Q, He F, Peng S, Li Y (2018c) Tannic acid encountering ovalbumin: a green and mild strategy for superhydrophilic and underwater superoleophobic modification of various hydrophobic membranes for oil/water separation. J Mater Chem A 6:13959–13967
Wang Z, Han M, Zhang J, He F, Xu Z, Ji S, Peng S, Li Y (2019a) Designing preferable functional materials based on the secondary reactions of the hierarchical tannic acid (TA)-aminopropyltriethoxysilane (APTES) coating. Chem Eng J 360:299–312
Wang Y, Huang Z, Gurney RS, Liu S (2019b) Superhydrophobic and photocatalytic PDMS/TiO2 coatings with environmental stability and multifunctionality. Colloid Surf A 561:101–108
Wang H, Zhang C, Zhou B, Zhang Z, Shen J, Du A (2020) Hydrophobic silica nanorod arrays vertically grown on melamine foams for oil/water separation. Appl Nano Mater 3:1479–1488
Wenten IG, Khoiruddin K, Wardani AK, Aryantic PTP, Astuti DI, Komaladewi AAIAS (2020) Preparation of antifouling polypropylene/ZnO composite hollow fiber membrane by dip-coating method for peat water treatment. J Water Process Eng 34:101158
Xi ZY, Xu YY, Zhu LP, Wang Y, Zhu BK (2009) A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly(DOPA) and poly(dopamine). J Membr Sci 327:244–253
Xu ZG, Zhao Y, Wang HX, Wang XG, Lin T (2015) A superamphiphobic coating with an ammonia-triggered transition to superhydrophilic and superolephobic for oil-water separation. Angew Chem Int Ed 54:4527–4530
Yan X, Zhu X, Ruan Y, Xing T, Chen G, Zhou C (2020) Biomimetic, dopamine-modified superhydrophobic cotton fabric for oil–water separation. Cellulose 27:7873–7885
Yang M, Liu W, Jiang C, Xie Y, Shi H, Zhang F, Wang Z (2019) Facile construction of robust superhydrophobic cotton textiles for effective UV protection, self-cleaning and oil-water separation. Colloid Surf A 570:172–181
Yang M, Liu W, Liang L, Jiang C, Liu C, Xie Y, Shi H, Zhang F, Pi K (2020) A mild strategy to construct superhydrophobic cotton with dual self-cleaning and oil–water separation abilities based on TiO2 and POSS via thiol-ene click reaction. Cell 27:2847–2857
Ye S, Wang B, Shi Y, Wang B, Zhang Y, Feng Y, Han W, Liu C, Shen C (2020) Superhydrophobic and superelastic thermoplastic polyurethane/multiwalled carbon nanotubes porous monolith for durable oil/water separation. Compos Comm 21:100378
Yuan R, Liu H, Chen Y, Liu Z, Li Z, Wang J, Jing G, Zhu Y, Yu P, Wang H (2019) Design ambient-curable superhydrophobic/electroactive coating toward durable pitting corrosion resistance. Chem Eng J 374:840–851
Zeng T, Zhang P, Li X, Yin Y, Chen K, Wang C (2019) Facile fabrication of durable superhydrophobic and oleophobic surface on cellulose substrate via thiol-ene click modification. Appl Surf Sci 493:1004–1012
Zhang J, Seeger S (2011) Polyester materials with superwetting silicone nanofilaments for oil/water separation and selective oil absorption. Adv Funct Mater 21:4699–4704
Zhang M, Wang C, Wang S, Li J (2013) Fabrication of superhydrophobic cotton textiles for water–oil separation based on drop-coating route. Carbohyd Polym 97:59–64
Zhang C, Ou Y, Lei WX, Wan LS, Ji J, Xu ZK (2016) CuSO4/H2O2-induced rapid deposition of polydopamine coatings with high uniformity and enhanced stability. Angew Chem Int Ed 55:3054–3057
Zhang Z, Li W, Wang W, Wang S, Qin C (2019) Reactive superhydrophobic paper from one-step spray-coating of cellulose based derivative. Appl Surf Sci 497:143816
Zhang J, Huang D, Wu G, Chen SC, Wang YZ (2020a) Highly-efficient, rapid and continuous separation of surfactant-stabilized oil/water emulsions by selective under-liquid adhering emulsified droplets. J Hazard Mater 400:123132
Zhang J, Saleem R, Wang P, Wen H, Zhu Z, Huang W, Ibrahim MAM, Liu C (2020b) Polymer brush-grafted ZnO-modified cotton for efficient oil/water separation with abrasion/acid/alkali resistance and temperature ‘‘switch” property. J Colloid Interf Sci 580:822–833
Zhou X, Zhang Z, Xu X, Guo F, Zhu X, Men X, Ge B (2013) Robust and durable superhydrophobic cotton fabrics for oil/water separation. ACS Appl Mater Interf 5:7208–7214
Zhou H, Chen R, Liu Q, Liu J, Yu J, Wang C, Zhang M, Liu P, Wang J (2019) Fabrication of ZnO/epoxy resin superhydrophobic coating on AZ31 magnesium alloy. Chem Eng J 368:261–272
Funding
This research received no financial support.
Author information
Authors and Affiliations
Contributions
TCL: Investigation; formal analysis; DJL Conceptualization; Writing—original draft.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interests.
Availability of data and material
Can be obtained from corresponding author by reasonable requests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, TC., Lee, DJ. Cotton fabrics modified for use in oil/water separation: a perspective review. Cellulose 28, 4575–4594 (2021). https://doi.org/10.1007/s10570-021-03850-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-021-03850-6