Abstract
Cotton fabrics were subjected to modification with fluorine-free polysiloxanes. In the first stage a series of polysiloxanes substituted with alkoxysilyl groups and/or alkyl chains were synthesized. Two methods of functionalization of vinyl group-containing polysiloxanes were used, i.e. hydrosilylation and hydrothiolation. Cotton fabrics were modified via dip-coating technique in solutions of the prepared compounds or by thiol–ene click reaction directly on the surface of cotton. In the first stage of the latter method cotton fabrics were modified by sol–gel technique with 3-mercaptopropyltrimethoxysilane in order to obtain mercapto-functionalized samples. After grafting SH groups on the surface, the fabrics were easily functionalized using the photoclick thiol–ene reaction with vinyl groups containing polysiloxane or vinyl- and alkyl-groups containing polysiloxane. The hydrophobicity was determined by measuring the water contact angle. Changes in the surface morphology were examined by FTIR spectroscopy and scanning electron microscopy (SEM). The coatings of the modified fabrics were subjected to elemental analysis using SEM–EDS techniques. All modified cotton fabrics showed highly hydrophobic properties. All obtained hydrophobic fabrics were fully resistant to the washing process, which proves the durability of the developed modifications.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton fabric, due to its great properties, is very interesting for scientific research as well as for industrial applications (Li et al. 2007). Its most important desirable features include softness, warmness, breathability, comfort and low cost (Bea et al. 2009; Gao et al. 2016). Moreover cotton belongs to natural fabrics, made of renewable raw materials and environmentally friendly, which is a crucial issue nowadays (Ismail 2016). Pristine cotton fabrics due to the presence of hydroxyl groups on their surface are also characterized by high polarity and hydrophilic character, therefore their usefulness in many areas may be limited by the poor water and dirt repellency (Shateri-Khalilabad and Yazdanshenas 2013). Water absorption by natural fabrics also promotes the growth of microorganisms on their surface and weakens them. On the other hand, the presence of OH groups enables the functionalization of cotton by chemical modifications towards obtaining their water resistance (Przybylak et al. 2016). Fabrics with the ability to repel liquid particles from the surface are used in the production of everyday and special-purpose clothing, including protective clothing in the medical and military sector (Grozea et al. 2016).
A large number of compounds of different binding chemistry, properties and structures have been used to modify natural fabrics. A very interesting group of modifiers are organosilicon compounds such as silanes, polysiloxanes or silsesquioxanes. Polysiloxanes show a number of unique properties such as flexibility, transparency, air permeability, environment-friendliness and low surface energy. Polysiloxanes can also be easily functionalized (Drozdov et al. 2018) by introducing functional groups to their structure. Moreover synthesis of siloxane derivatives is inexpensive (Liu et al. 2019). Polysiloxane has also been used to functionalize cotton fabrics, however classical polysiloxanes without reactive functional groups have poor adhesion to cotton fibers and durability of bonding with cellulose surface. Therefore, it is necessary to introduce functional groups into the polysiloxane structure that would have the ability to form bonds with cellulose hydroxyl groups. One of the best known methods of finishing cotton textiles is the sol-gel process using alkoxysilane, which allows the formation of a siloxane layer on the surface of the fibers (Hayn et al. 2011; Vasiljević et al. 2014; Hoefnagels et al. 2007; Xu et al. 2011; Vasiljević et al. 2017; Periolatto et al. 2013; Xu et al. 2010). Our previous research has shown that difunctional polysiloxanes have excellent modifying properties. Due to the presence of alkoxysilyl groups, they form permanent covalent bonds with the cellulose OH groups. In addition, the introduction of appropriate functional groups into the polysiloxane structure may endow the modified fabrics with new properties. Modification with polyslioxane containing alkoxysilyl groups and fluorinated chains gave fabrics superhydrophobic properties (Przybylak et al. 2016). On the other hand, impregnation of fabrics in solution of polysiloxane substituted with quaternary ammonium salts endowed the fibers with both hydrophobic and biocidal properties (Przybylak et al. 2018). In addition, thanks to their structure, polysiloxanes can produce durable and flexible coatings on the surface of fibers.
So far, our research group has synthesized difunctional polysiloxanes by hydrosilylation reaction, which is one of the most commonly used processes for obtaining and modification of organosilicon compounds. However, Pt-based catalysts commonly used in hydrosilylation reaction are expensive and not resistant to many types of functional groups (Xue et al. 2013). Furthermore the synthesis usually takes place at elevated temperature (Marciniec 2008), which requires a higher energy input and thus is less favorable in terms of cost. Taking into account these limitations, new strategies of functionalization should be developed. One of them is hydrothiolation reaction. Compared to hydrosilylation, this reaction does not require expensive heavy-metal catalysts and an elevated temperature, and the synthesis reactions can be carried out in the thiol–ene radical addition of thiols containing various types of functional groups (Xue et al. 2013; Crowe-Willoughby and Genzer 2009; Ścibiorek et al. 2000; Herczynska et al. 1999; Zuo et al. 2014). Moreover this type of reaction has many advantages such as versatility, high efficiency, simplicity or mild reaction conditions (Jiang et al. 2019a, b). Thiol–ene click reaction has become increasingly popular for functionalization of many surfaces of materials like glass (Li et al. 2015; Biggs et al. 2016; Oberleitner et al. 2013; Lundberg et al. 2010), wood (Kostić et al. 2017), cotton (Bai et al. 2019; He et al. 2014) paper (Wang et al. 2019; Guo et al. 2018). aluminum (Sparks et al. 2013), marble (Sparks et al. 2013) or sandstone (Sparks et al. 2013). Thiol–ene click reactions were used to obtain new organosilicon derivatives in bulk synthesis (in solution) as well as for functionalization directly on the cotton surface. In the first stage of the latter method cotton fabric can be modify by sol–gel technique with 3-mercaptopropyltrialkoxysilane in order to obtain mercapto-functionalized samples (Xu et al. 2017a, b; Sun et al. 2016; Zeng et al. 2019, Xue et al. 2019). Then, on the thus prepared surface click reactions can be carried out using various types of compounds containing unsaturated bonds. This type of reaction was used to obtain fireproof, biocidal or hydrophobic surfaces (Xu et al. 2017a,b Sun et al. 2016; Zeng et al. 2019; Qiang et al. 2017; Fei et al. 2018; Xue et al. 2019; Jiang et al. 2019a, b). However, the developed methods of synthesis and textiles modification are complex and multi-stage, in addition, in some cases they need the use of fluorine. Nevertheless, there are also another possibilities for the hydrophobic treatment of cotton fabrics with the use of fluorine-free compounds (Zhong and Netravali 2016; Patil and Netravali 2019).
The aim of this study was to find new, simple and inexpensive methods for hydrophobization of cotton fabric. In the present work the hydrophobic effect of polysiloxanes obtained by hydrosilylation and hydrothiolation reactions was compared. The polysiloxane containing vinyl groups was functionalized with silanes or thiols in order to introduce alkoxysilyl groups capable of forming bonds with cellulose. One of the main goals of this study was comparison of thiol–ene click reaction at bulk synthesis (in solution) with direct reaction on cotton surface. Moreover, the expected practical outcome of our study was to obtain hydrophobic textiles resistant for multiple washing which are able to create durable bonds with cellulose.
Experimental
Materials
A bleached cotton fabric with an areal density of 145 g/m2 was supplied by the Textile Factory in Łódź (Poland). Karstedt catalyst, 1-octanethiol, 2,2-dimethoxy-2-phenylacetophenone (DMPA), chloroform-d and 1-octene were purchased from Aldrich. 3-mercaptopropyltrimethoxysilane, poly(vinylmethyl-co-dimethyl)siloxane and lithium aluminum hydride were purchased from ABCR. Triethoxysilane was purchased from Unisil Company Ltd. Poland. Dimethylchlorosilane was purchased from Alfa Aesar. Solvents were purchased from POCH. Ammonia, sodium hydroxide and acidic acid were purchased from Merck.
The compound n-octyldimethylsilane used in this study was synthesized in two stages. In the first stage n-octyldimethylchlorosilane was obtained by hydrosilylation with the use 1-octene, dimethylchlorosilane and Karstedt catalyst and in the second stage it was converted to n-octyldimethylsilane by lithium aluminum hydride in THF according to a literature method (Januszewski et al. 2019).
Preparation of functionalized polysiloxanes
Functionalized polysiloxanes were synthesized by two types of reactions, i.e. hydrosilylation and hydrothiolation.
Preparation of functionalized polysiloxanes via hydrosilylation reactions
Hydrosilylation was performed in the presence of Karstedt catalyst, with the use of triethoxysilane and n-octyldimethylsilane at elevated temperature according to Scheme 1.
Synthesis and spectroscopic characterization of polysiloxane P1
A three-necked round-bottomed flask equipped with a magnetic stirrer, reflux condenser and thermometer, was loaded with poly(vinylmethyl-co-dimethyl)siloxane 5.50 g (1.63 mmol), triethoxysilane 1.34 g (8.16 mmol) and Karstedt’s catalyst (5 × 10− 5 mol Pt/mol SiH). The reaction mixture was heated for 2 h at 115 °C. The product was purified from volatile impurities under reduced pressure at 60 °C. The reaction gave the product in 99% yield (6.78 g), mainly β isomer.
1H NMR (CDCl3, δ, ppm) = 5.96 (=CH2); 5.79 (=CH); 3.82; 3.71 (OCH2CH3); 1.22 (OCH2CH3); 1.08; 0.94, 0.57 (CH2CH2, CHCH3); 0.15, 0.08 (SiCH3). 13C NMR (CDCl3, δ, ppm) = 137.26–136.96 (=CH2); 133.17-132.87 (=CH–); 59.30, 58.47 (OCH2), 18.46 (OCH2CH3); 8.56, 7.72, 5.79, 1.96–(− 1.16) (CH2CH2, CHCH3, CH3). 29Si NMR (CDCl3, δ, ppm) = 7.21 (Si(CH3)3); − 21.42–(− 21.97) (Si(CH3)2, CH3SiCH2); − 35.09-(− 35.48) (SiCH=CH2); − 44.68, − 46.07 (Si(OCH2CH3)3).
Synthesis and spectroscopic characterization of polysiloxane P2
A three-necked round-bottomed flask equipped with a magnetic stirrer, reflux condenser and thermometer, was loaded with poly(vinylmethyl-co-dimethyl)siloxane 3.00 g (0.891 mmol), n-octyldimethylsilane 1.537 g (8.92 mmol), Karstedt’s catalyst (1.25 × 10− 4 mol Pt/mol SiH) and toluene (4 ml). The reaction mixture was heated for 3 h at 115 °C and then triethoxysilane 0.732 g (4.456 mmol) and Karstedt’s catalyst (1.10− 4 mol Pt/mol SiH) were added and the mixture was heated for additional 1 h at 115 °C. The reaction gave the product in 99% yield (5.22 g), mainly β isomer.
1H NMR(CDCl3, δ, ppm) = 5.96 (=CH2); 5.80 (= CH); 3.81, 3.72 (OCH2CH3); 1.28 (CH2)6; 1.22 (OCH2CH3); 1.08 (CHCH3Si(OCH2CH3)3); 1.02 (CHCH3Si(CH3)2Oct); 0.94 (CH); 0.88 (CH2CH3); 0.55 (CH2CH2Si(OEt)3); 0.42 (O2SiCH2CH2Si(Me)2CH2); 0.15, 0.08, − 0.06 (SiCH3). 13C NMR (CDCl3, δ, ppm) = 137.25, 132.86 (CH =CH2); 58.47 (OCH2); 33.94, 32.14, 29.52, 24.11, 22.86 (CH2); 18.48 (OCH2CH3); 14.94, 14.27 (CH2); 9.63–6.37, 1.94–(− 1.12) (CH2CH2, CHCH3, CH3); − 3.79 (Si(CH3)2Oct. 29Si NMR (CDCl3, δ, ppm) = 7.23 (Si(CH3)3); 4.09, 3.49 (Si(CH3)2Oct); − 21.95 (Si(CH3)2, CH3SiCH2); − 35.81 (SiCH=CH2); − 44.54, − 44.70 (Si(OCH2CH3)3).
Preparation of functionalized polysiloxanes via hydrothiolation reactions
The poly(vinylmethyl-co-dimethyl)siloxane copolymer was functionalized by hydrothiolation reaction with two different thiols, i.e. 3-mercaptopropyltrimethoxysilane and 1-octanethiol in the presence of 2,2-dimethoxy-2-phenylacetophenone as photoinitiator under UV irradiation according to Scheme 2.
Synthesis and spectroscopic characterization of PS1
A round-bottomed flask equipped with a magnetic stirrer was loaded with poly(vinylmethyl-co-dimethyl)siloxane 5.50 g (1.63 mmol), 3-mercaptopropyltrimethoxysilane 1.604 g (8.17 mmol) and 2,2-dimethoxy-2-phenylacetophenone 21.0 mg (0.0819 mmol). The reaction mixture was irradiated for 1 h. The reaction gave product in quantitative yield, which was used without further purification.
1H NMR (CDCl3, δ, ppm) = 5.95 (=CH2); 5.78 (=CH); 3.55, 3.45 (OCH3); 2.53 (CH2SCH2); 1.68 (CH2CH2CH2); 0.85 (SiCH2); 0.74 (CH2Si(OCH3)3); 0.18, 0.07 (SiCH3). 13C NMR (CDCl3, δ, ppm) = 136.95, 133.12 (CH =CH2); 50.77, 50.58 (OCH3); 34.96 (CH2CH2Si); 26.46 (CH2S); 23.02 (SCH2); 18.54 (SiCH2); 8.78 (CH2Si(OCH3)3); 1.92, 1.14, -0.51 (SiCH3). 29Si NMR (CDCl3, δ, ppm) = 7.19 (Si(CH3)3); − 21.43–(− 21.98) (Si(CH3)2); − 24.43–(− 24.70) (SiCH2); − 35.08–(− 35.47) (SiCH=CH2); − 42.37 (Si(OCH3)3).
Synthesis and spectroscopic characterization of PS2
A round-bottomed flask equipped with a magnetic stirrer was loaded with poly(vinylmethyl-co-dimethyl)siloxane 5.50 g (1.63 mmol), 3-mercaptopropyltrimethoxysilane 1.604 g (8.17 mmol), 1-octanethiol 2.39 g (16.34 mmol) and 2,2-dimethoxy-2-phenylacetophenone 62.8 mg (0.245 mmol). The reaction mixture was irradiated for 2 h. The reaction gave product in quantitative yield, which was used without further purification.
1H NMR (CDCl3, δ, ppm) = 5.97 (=CH2); 5.80 (=CH); 3.55, 3.47 (OCH3); 2.52 (CH2SCH2); 1.68 (SCH2CH2CH2Si(OCH3)3); 1.56 (SCH2CH2); 1.36, 1.27 (CH2)5; 0.87 (SiCH2, CH2CH3); 0.07 (SiCH3). 13C NMR (CDCl3, δ, ppm) = 136.73, 133.01 (CH=CH2); 50.93, 50.62 (OCH3); 35.08 (CH2CH2Si); 32.11, 31.97, 29.74, 29.38, 29.16 (S(CH2)6); 26.58 (CH2S); 23.00 (SCH2); 22.79 (CH2CH3); 18.50 (SiCH2); 14.22 (CH2CH3); 8.80 (CH2Si(OCH3)3); 1.91, 1.16, -0.14, -0.51 (SiCH3). 29Si NMR (CDCl3, δ, ppm) = 7.33 (Si(CH3)3); − 21.50–(− 21.94) (Si(CH3)2); -24.31 (SiCH2); -35.16 (SiCH=CH2); − 42.35 (Si(OCH3)3).
Synthesis and spectroscopic characterization of PS3
A round-bottomed flask equipped with a magnetic stirrer was loaded with poly(vinylmethyl-co-dimethyl)siloxane 8.00 g (2.38 mmol), 1-octanethiol 3.48 g (23.79 mmol) and 2,2-dimethoxy-2-phenylacetophenone 60.9 mg (0.238 mmol). The reaction mixture was irradiated for 1 h. The reaction gave product in quantitative yield, which was used without further purification.
1H NMR (CDCl3, δ, ppm) = 5.99 (=CH2); 5.81 (=CH); 2.54 (CH2SCH2); 1.57 (SCH2CH2); 1.37, 1.28 (CH2)5; 0.87 (SiCH2, CH2CH3); 0.08 (SiCH3). 13C NMR (CDCl3, δ, ppm) = 136.93, 133.35 (CH=CH2); 31.99, 29.77, 29.40, 29.18 (S(CH2)6); 26.61 (CH2S); 22.80 (CH2CH3); 18.52 (SiCH2); 14.24(CH2CH3); 1.93, 1.18; -0.18; -0.48 (SiCH3). 29Si NMR (CDCl3, δ, ppm) = 7.21 (Si(CH3)3; − 21.43–(− 21.97) (Si(CH3)2; − 24.19–(− 24.68) (SiCH2); -35.09–(− 35.43) (SiCH=CH2).
Modification of cotton fabrics
Mercerization of cotton
The fabric surface has been activated in the mercerization process. Activation of cotton textile was carried out at 2 vol% solution of sodium hydroxide in water for 20 min at 70 °C.
Grafting of –SH groups onto cotton surface
Solution of 5 vol% of 3-mercaptopropyltrimethoxysilane in ethanol and water (2:8 v/v) was hydrolyzed for 2 h at room temperature and then the mercerized and non-mercerized fabrics were immersed in the prepared coating solution for 30 min. The excess of the modifier solution was removed by squeezing followed by fabrics fixing for 5 min at 120 °C to get SH-functionalized cotton fabrics.
Cotton textile modification with polysiloxane solution
A mixture of 5 vol% of unmodified polysiloxane (P0) and 5 vol% of acetic acid or ammonia in toluene was hydrolyzed for 30 min at room temperature and then the mercerized and non-mercerized fabrics were immersed in the prepared coating solution for 30 min. Then the excess of the modifier solution was removed by squeezing and the samples were dried for 1 h at 80 °C and fixed for 3 min at 120 °C.
Thiol–ene click reaction on the cotton fabrics surface
Two methods were used, according to the first one SH-functionalized cotton fabric was immersed in the solution of unmodified polysiloxane P0 or polysiloxane PS3 (5 wt%) and the initiator DMPA (0.01 wt%) in toluene and subjected to UV irradiation for 15 min (7.5 min each site) at room temperature. According to the second one, cotton fabric was immersed in the solution for 15 min, removed from the solution and then irradiated for 15 min (7.5 min each site). The fabric was exposed to a 280–600 nm light source (LQ-400 mercury lamp, Gröbel UV-Elektronik GmbH, 400 W). The lamp was placed ∼ 5 cm away from the samples. After the above treatments, the samples were rinsed with distilled water, the excess of the modifier solution was wiped into paper tower and the fabrics were dried for 1 h at 60 °C to get the final products.
Washing process
The durability of the modifications was determined by measuring the water contact angle directly after the modification as well as after the washing process. Washing process of modified fabric samples was carried out according to the standard PN-EN ISO 105-C06:2010 Textiles—Tests for color fastness—Color fastness to domestic and commercial laundering. The process was performed at 40 °C for 60 min. using a detergent and this was followed by rinsing. The washing was repeated five times.
Characterization
Determination of the amount of modifiers applied on the fabrics (add-on)
The total dry solid add-on in cotton fabric samples (A) was determined by weighing a fabric sample before (Wi) and after (Wf) its modification with a composition used and thermal fixing. The analytical balance Ohaus was used for the measurements. The uptake (Table 1) was calculated according to the following equation:
FTIR analysis
FTIR spectra of the modified fabrics were taken on a Bruker spectrometer, model Tensor 27, with a Specac Golden Gate single-reflection diamond ATR accessory.
NMR
NMR spectra were recorded using 400 and 500 MHz spectrometer in commercially available CDCl3.
Analysis of the elemental composition of the applied coatings
The analysis was carried out by employing the SEM–EDS technique to determine the ultimate elements (Si, O, C and S) present in the modifying mixtures. A Hitachi S-3500 N scanning electron microscope (SEM) equipped with an EDS (energy-dispersive X-ray) detector (Ultra Dry Silicon Drift X-ray Detector made by Thermo Scientific) was used for the measurements.
Determination of hydrophobic properties
The water contact angles were measured using an automatic video contact-angle testing apparatus, Krüss, model DSA 100 Expert. A 5 µl volume of water was applied to the treated cotton fabrics, and the contact angle was determined from the video camera images of the drop in the course of its formation. Each measurement is an average of five drops.
Studies of surface topography
Microscopic evaluation of surface changes of modified and unmodified samples of the cotton fabric was carried out using a Hitachi S-3400 N scanning electron microscope; the samples were coated with a thin layer of gold before observations. Moreover, SEM images were taken using an FEI Quanta 250 FEG instrument equipped with a large field detector (LFD) that records the secondary electrons (SEs). For the LFD SE measurements the samples were not covered by any layers. The microscope was operated at low vacuum mode (70 Pa), and the accelerating voltage was 10 kV.
Results and discussion
Vinyl group-containing polysiloxanes were used as a starting reactant in the study taking into account the possibility of their functionalization by two well-known and facile methods i.e. hydrosilylation and hydrothiolation. Application of both methods enables introduction of two types of functional groups i.e. alkoxysilyl and alkyl chains in the reactions of two different silanes or thiols to obtain functionalized polysiloxanes with high efficiency.
Poly(vinylmethyl-co-dimethyl)siloxane P0 copolymer was functionalized by hydrosilylation reaction with two different silanes, triethoxysilane and n-octyldimethylsilane to give copolymers P1 and P2 of the general formula 1 (Fig. 1). Functionalization by hydrothiolation reaction with two different thiols, 3-mercaptopropyltrimethoxysilane and 1-octanethiol was performed to obtain copolymers PS1, PS2 and PS3 of the general formula 2 (Fig. 1).
Both types of reactions used (hydrosilylation and hydrothiolation) enable obtaining functionalized polysiloxanes with high yields (≥ 99%). According to the NMR spectra, the hydrosilylation reactions gave mixtures of α and β isomers. In the hydrothiolation α isomer is either not formed or formed in a too small amount to be observed and detected by NMR spectroscopy.
Polysiloxanes with trialkoxysilyl groups (P1, P2, PS1, PS2) were introduced on cotton surface by dip-coating process using a 5 vol% solution of organosilicon compound. Unmodified polysiloxane P0 and derivative PS3 were used as substrates in the photoclick reactions performed directly on the surfaces of the fabrics, previously modified with mercaptosilane. Schemes 3 and 4 illustrate the overall chemistry of the polysiloxanes grafting on the cotton fabric surface. In the first step the cotton fabric was immersed in a solution of 3-mercaptopropyltrimethoxysilane in order to graft thiol groups onto its surface (Scheme 3). Then, the click reactions took place between the SH groups and the unsaturated bonds of the polysiloxanes, in the presence of UV irradiation and a photoinitiator (Scheme 4).
The hydrophobic effect of all modified samples was evaluated by measuring the water contact angle. The samples were analyzed directly after modification and after five cycles of washing process. Figure 2 shows the WCA values of the samples modified with polysiloxanes P1 and PS1 in the dip-coating process. Modifications were performed in acidic (A) or basic (B) conditions.
Figure 2 shows that all compositions endowed the modified surfaces with high hydrophobicity. Comparing both polysiloxanes used, it was noted that the A comparison of the results obtained for the two polysiloxanes used revealed that the organosilicon compound obtained in the hydrothiolation reaction (PS1) gives a little higher WCA. This observation may be due to the difference in the lengths of the chains connecting the polysiloxanes to the fabric. Compound PS1 contains five carbon atoms and a sulfur atom in the spacer between the polysiloxane chain and Si(OR)3 group. Meanwhile, the derivative obtained as a result of hydrosilylation (P1) contains only two carbon atoms in the spacer. A longer spacer permits a better orientation of polysiloxane on the surface of the fabric. In addition, it makes the coating more flexible, which has a positive result on the hydrophobic effect. The issue of the spacer length has been noticed before (Maciejewski et al. 2014). Figure 2 shows that the base-catalyzed compositions resulted in slightly lower WCA values than those catalyzed by an acid. An alkaline environment promotes condensation. It can therefore be assumed that the condensation of polysiloxanes begins before applying the modifier onto the fabric. Hydrolysis and condensation of Si(OR)3 groups before application to the fabric reduced the number of reactive groups that can attach to the fabric. This resulted in less effective bonding of the modifier to the fabric. It should be emphasized that the samples after the washing process retained hydrophobic character at the same level as before washing. Maintaining the WCA values at the same level indicates the durability of the bond between the modifier and the fabric. The fabrics were also modified with polysiloxanes containing additional alkyl side chains. The first of the polysiloxanes was obtained by hydrosilylation (P2) and the second one by hydrothiolation (PS2) (Fig. 3).
Comparing Figs. 2 and 3, it can be seen that the fabrics modified with the alkyl chains-containing polysiloxanes resulted in lower WCA than those modified with analogous compounds that have no alkyl groups. These results were quite surprising because it was expected that the presence of additional alkyl chains would further increase the hydrophobic character of the fabrics. It is well known that long alkyl chains are capable of endowing a modified surface with good hydrophobic properties. Several factors could have led to the results obtained. In our research, the short-chain polysiloxane composed of 40 segments (50% of vinylmethylsiloxane segments) was used. During the synthesis of P2 and PS2 polysiloxanes, each of them was substituted with 15 functional groups in total. The introduction of so many substituents into the short-chain polysiloxane could have increased its rigidity. Probably the presence of long alkyl chains interfered with the formation of bonds between the fabric and the much shorter chain containing Si(OR)3 groups. The presence of alkyl chains could have made the polysiloxanes partly just settle on the fabric instead of forming bonds with it. In the above chart, the samples subjected to the washing process are characterized by higher WCA values than those not washed, so washing off the occluded modifier from the cotton surface increases the hydrophobic character. In addition, the washing process may permit the alkyl chains to assume a better orientation. The fabrics modified with polysiloxane PS2 are characterized by higher WCA and thus hydrophobic character. Moreover, the WCA values of these samples practically did not change after washing. Higher hydrophobicity and similar WCA before and after washing of the fabric modified with polysiloxane PS2 can be a result of a longer spacer between the polysiloxane chain and the alkoxysilyl groups. It can be concluded that the presence of alkyl groups in the polysiloxane obtained in hydrothiolation reaction does not have such a great impact on hydrophobicity like their presence in the polysiloxane obtained by hydrosilylation. It can be seen that the washed samples presented in Fig. 3 have very similar WCA values as the analogous samples shown in Fig. 2. This confirms that after washing away the occluded particles, both types of polysiloxanes endow the textile surface with similar hydrophobic properties.. The hydrophobic effect of the alkyl chains may have been eliminate bystiffening of the polysiloxane. Stiffer polysiloxane is less able to accurately protect the fibers and their morphology becomes less favorable. The effect of polysiloxane chain length has already been observed in our previous studies. The longer and more flexible polysiloxane gave higher fabric hydrophobicity than the shorter stiffer compound.
Thiol–ene click reactions were also carried out directly on the fabrics surfaces. Two procedures of modification were used. In the first procedure, the fabric soaked by the modifying composition was UV irradiated (M1). In the second procedure, the fabric was UV irradiated while being immersed in the solution of the modifier (M2). Before grafting SH groups onto the fabrics, they were subjected to mercerization process. Figure 4 presents the values of WCA of the fabrics modified by thiol–ene click reactions directly on the cotton surfaces.
WCA values of fabrics modified by click reactions directly on their surfaces. (M1)—UV irradiation of fabric soaked by the modifying composition, (M2)—UV irradiation of samples immersed in the solution of the modifier, PS3—polysiloxane containing alkyl chains, P0—polysiloxane without alkyl and alkoxysilyl groups
The results presented in Fig. 4 show that all modification gave very good results. All obtained samples were highly hydrophobic. The fabrics were modified by two methods differing in the way of UV irradiation. It can be seen in the Figure above that direct UV irradiation of the fabric soaked with the modifier solution (M1) gave better results but the differences were not significant. Higher hydrophobicity of the fabrics directly exposed to UV irradiation may be caused by better access of UV light to thiol groups present on the surface of the fibers.
During irradiation of the fabric immersed in a solution of the modifier, UV light must pass through the layer of the solution, which could adversely affect the effectiveness of the click reactions. Comparing the results in all three above bar charts (Figs. 2, 3 , 4), it can be seen that the click reaction on the surface of the fabrics gave a better hydrophobic effect than impregnation of the fabric with ready-made polysiloxanes via dip-coating technique. Higher hydrophobicity can be caused by a larger number of reactive groups in polysiloxanes that can bond to the SH groups present on the surface of the fabrics. At the same time the unmodified polysiloxane (P0) contains 20 vinyl groups capable of forming bonds with SH groups present on the cotton as well as polysiloxane PS3 contains 10 vinyl groups that can undergo a click reaction. The polysiloxanes obtained via hydrothiolation during bulk synthesis (in solution) contain only 5 alkoxysilyl groups able to bind to the surface of the fabric. Hydrophobization of the fabrics by thiol–ene click reactions on their surface is more effective thanks to the greater number of reactive groups and possibility to create more bonds with the reactive groups present on the cotton fibers. The possibility of creating more bonds with the fibers resulted in a larger amount of polysiloxane attached to the fabric. Greater amount of polysiloxane on the fabric surface resulted in a higher hydrophobicity. The best results were observed for the sample with grafted mercapto-silane, soaked in a solution of unmodified polysiloxane and then exposed to UV irradiation to perform the click reaction (M1P0). The shape of a water drop on the cotton surface modified using the aforementioned method is shown in Fig. 5.
The fabrics modified by the click reactions on their surface also showed very high resistance to repeated washing. This confirms that the bonds formed between the thiol groups present on the fiber surfaces and the vinyl groups of polysiloxanes are stable. Figure 6 shows the drops applied to the M1P0 modified fabric before (a) and after (b) the washing process.
The fabrics were subjected to mercerization prior to the modification with mercaptosilane. This process was carried out for further activation of the fibers surfaces. Mercerization activates fiber surfaces by increasing active centers as a result of breaking hydrogen bonds inside cellulose molecules, which facilitates further fabric refinement. This kind of pre-treatment of fabrics prior to mercaptosilane modification has been reported in several publications (Yu et al. 2017; Xu et al. 2017a). It has been shown that mercerized samples become stiffened as a result of modification, which is very undesirable because it limits their performance. Therefore, in the next stage of research, the modification was not preceded by a mercerization process. Figure 7 shows the WCA values of non-mercerized samples modified by the method analogous to that of the fabrics characterized in Fig. 4.
WCA values of fabrics modified during click reactions directly on cotton surfaces without pre-treatment by mercerization process (nM). (M1)—UV irradiation of fabric soaked by the modifying composition, (M2)—UV irradiation of samples immersed in the solution of the modifier, PS3—polysiloxane containing alkyl chains, P0—polysiloxane without alkyl and alkoxysilyl groups
Non-mercerized fabrics were not stiffened as a result of modification with organosilicon compounds. The samples obtained by this method were characterized by hydrophobic character and maintain flexibility at the same time. As can be seen in Fig. 7, all modified samples were highly hydrophobic both before and after washing. The values of the WCA of non-mercerized fabrics modified with P0 polysiloxane are very similar to those of the fabrics mercerized before impregnation. This means that the mercerization of cotton before application of silane did not affect the final hydrophobic effect. However, omission of mercerization process prior to modification with the polysiloxane containing alkyl side groups (PS3) resulted in a decrease in fabric hydrophobicity. The reason for this phenomenon as well as changes in fabric stiffness will be discussed later.
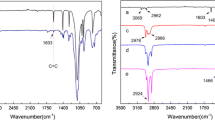
The effectiveness and durability of the modifications were verified by several analytical methods. The modified fabrics after the washing process were subjected to FTIR analysis (Figs. 8 , 9).
FTIR spectra of pristine cotton fabric (a), water repellent cotton fabrics after washing obtained by click reaction directly on the surface M1P0 W (b), M1PS3 W (c), M1P0nM W (d), M1PS3nM W (e), (M1)—UV irradiation of fabric soaked by the modifying composition, (nM)—non mercerized, (W)—washing process
The FTIR analysis confirmed the presence of the modifier on the fabric surface as well as the formation of covalent bonds between the polysiloxane and cotton fibers. The spectra of all obtained samples showed the presence of characteristic bands before and after washing process. In the spectra of samples (P1A W) and (PS1A W) the bands at about 3056 cm− 1 and 1598 cm− 1, originating from the vibrations of C=C unreacted vinyl groups in polysiloxane, were observed (Fig. 8). The same bands were observed for samples (M1P0) and (M1P0nM) (Fig. 9). All four polysiloxanes contained a large number of vinyl groups in their structure whose presence was manifested by specific bands in the spectra. However these bands were not present in the spectra of samples (P2A W), (PS2A W), (M2P0) and (M1P0nM), which can be accounted for by a smaller number of vinyl groups in bifunctional polysiloxanes containing additional alkyl chains. Intensive bands at about 1259 cm− 1 and 796 cm− 1 confirm that all cotton fabrics with water repellent properties have been coated with polysiloxane. In addition, they confirm the formation of permanent covalent bonds between the fibers and polysiloxane. These two peaks originate from Si–CH3 (1259 cm− 1) and Si–O–C (796 cm− 1). The cotton fabric treated with polysiloxanes containing alkoxysilyl and alkyl groups (P2A W), (PS2A W), (M2P0) and (M1P0nM) shows intensive bands at 2959 cm− 1, 2924 cm− 1 and 2856 cm− 1 corresponding to the stretching vibration of C–H (CH3) and C–H (CH2). The bands in the range 980–1100 cm− 1 are attributed to the characteristic peaks of cellulose and overlap the Si–O–Si bands. The FTIR spectra of all water repellent fabrics contain more intensive bands in the range 1100–1020 cm− 1 than pristine cotton fabric, which confirms the presence of polysiloxane on the fabric surface.
Before and after the modification processes the samples were weighed to determine the add-on value. The content of elements of hydrophobic fabrics was also examined. Both unwashed and washed samples were analyzed. Acid-catalyzed samples were selected for analysis from the fabrics modified by the both methods. Textiles modified with mercaptosilane, i.e. mercerized (M-SH) and non-mercerized (nM-SH) samples were also analyzed. The results of the analysis are presented in Table 1; Fig. 10.
The data collected in Table 1 clearly show that all modifications were effective and the samples obtained are durable and resistant to the washing process. All hydrophobic fabrics contained in their composition both silicon and sulfur, which confirms successful modification. The exception were the samples impregnated with polysiloxanes obtained by hydrosilylation reactions that did not contain sulfur atoms in their structure (P1A, P2A). The contents of Si and S in the fabrics modified by the dip-coating process were lower than those in the samples obtained as a result of click reaction on the cotton surface, which is a consequence of application of smaller amount of compounds that is confirmed by the add-on values. It is worth noting that the fabrics modified by polysiloxane PS1, despite the low add-on, had high WCA (around 140°) both before and after washing. According to the results, the alkyl-substituted polysiloxanes were applied in a smaller amount than the polysiloxanes that contained only alkoxysilyl groups (PS2A, P2A). These results were confirmed by the values of both add-on and SEM–EDS analysis and were reflected in lower WCA values of modified textiles. In addition, the content of S and Si in the aforementioned samples decreased after washing, which confirmed the washing off of the unbound compounds. The result of this process is an increase in WCA values after washing. Elemental analysis of mercerized and non-mercerized SH-functionalized fabrics shows big differences between them. The mercerized samples are characterized by higher add-on values and higher content of Si and S elements than the non-mercerized ones. Mercerization process activates OH groups present on fiber surfaces, which promotes binding with mercaptoalkoxysilane. In general all mercerized samples are characterized by greater add-on values and greater content of silicon and sulfur elements than non-mercerized ones.
The results presented in Table 1 show that greater amount of polysiloxane covers the fibers during direct UV irradiation of the fabrics surfaces, i.e. obtained by M1rather than M2 method. Elemental analysis confirmed that the UV irradiation of the fabric immersed in the modifier’s solution is less effective due to the layer of solution between the lamp and the fibers. Elimination of the mercerization process reduced the add-on values. In addition, the samples modified with the polysiloxanes without additional alkyl side chains have a high content of Si and S elements (M1P0 nM, M2P0 nM). It can therefore be assumed that despite the smaller number of SH groups, still much polysiloxane has been attached to the surface of the fibers. This thesis is confirmed by comparably high values of contact angles of modified fabrics. It should be emphasized that the fabrics modified in this way retained their elasticity and soft grip. Comparing all samples UV irradiated on the fibers surface, the lowest add-on value and content of sulfur and silicon elements have the non-mercerized fabrics impregnated with alkyl chain substituted polysiloxanes (M1PS3 nM, M2PS3 nM). This may be due to a smaller number of SH groups on the surface of the fibers and the low content of vinyl bonds in the modifier structure, which proved to be insufficient for effective attachment of the compound. These samples also had lower contact angles relative to the others.
To analyze the surfaces of the modified fibers, SEM pictures were taken (Fig. 11).
The SEM analysis showed that the modifications carried out were effective. Polysiloxanes tightly covered the entire fibers surface to form a hydrophobic layer. The sample modified with polysiloxane in the dip-coating process (b) is characterized by a thin, even layer of the modifier on the surface of the fibers. Mercerized fabric subjected to UV irradiation is covered with a thick siloxane layer which partially glued the fibers (c). In contrast, fibers modified in the same way, without mercerization, have an even thin layer of the modifier (d). The SEM pictures taken explain the reason for the stiffening of the mercerized fabrics and the preservation of the elasticity of non-mercerized fabrics.
Conclusions
As a result of the presented research, an easy, cheap and simple method of durable hyrdophobization of textile was developed. Comparison of polysiloxane synthesis by hydrosilylation and hydrothiolation showed that the hydrothiolated compounds give slightly higher hydrophobicity of fabrics. In addition, hydrothiolation is a much simpler method of synthesis, does not require transition metal complex catalysts, and can be carried out at room temperature without the use of solvents. The best results were obtained in a two-step synthesis using two commercially available compounds, 3-mercaptopropylotrimethoxysilane and polysiloxane containing vinyl groups. No need to synthesize new compounds is an important benefit for the length of the process and from the commercial point of view. From the two methods of modification by thiol–ene click reaction on the surface of the fibers, the one involving direct UV irradiation of the solution-soaked fabric modifier was more effective. It was also method from the technological point of view. In addition, our research has shown no need of mercerization treatment prior to mercaptosilane modification, which further facilitates and reduces the cost of the process. The developed hydrophobizing compounds are fluorine-free, which makes them environmentally friendly and is important for producers of functional textiles.
References
Bae GY, Min BG, Jeong YG, Lee SC, Jang JH, Koo GH (2009) Superhydrophobicity of cotton fabrics treated with silica nanoparticles and water-repellent agent. J Colloid Interface Sci 337:170–175. https://doi.org/10.1016/j.jcis.2009.04.066
Bai S, Xu R, Wang W, Yu D (2019) Dual–response of temperature and humidity asymmetrical cotton fabric prepared based on thiol–ene click chemistry. Colloids Surfaces A 567:104–111. https://doi.org/10.1016/j.colsurfa.2019.01.050
Biggs CI, Walker M, Gibson MI (2016) “Grafting to” of RAFTed responsive polymers to glass substrates by thiol–ene and critical comparison to thiol–gold coupling. Biomacromol 17:2626–2633. https://doi.org/10.1021/acs.biomac.6b00662
Crowe-Willoughby JA, Genzer J (2009) Formation and properties of responsive siloxane‐based polymeric surfaces with tunable surface reconstruction kinetics. Adv Funct Mater 19:460–469. https://doi.org/10.1002/adfm.200800622
Drozdov FV, Cherkaev GF, Demachenko NF, Muzarafov AM (2018) Synthesis of new functional hexamethyltrisiloxanes and telechelic polydimethylsiloxanes based on them. J Appl Polym Sci 135:46848. https://doi.org/10.1002/app.46848
Fei Z, Liu B, Zhu M, Wang W, Yu D (2018) Antibacterial finishing of cotton fabrics based on thiol-maleimide click chemistry. Cellulose 25:3179–3188. https://doi.org/10.1007/s10570-018-1771-x
Gao Q, Hu J, Li R, Pang L, Xing Z, Xu L, Wu G (2016) Preparation and characterization of superhydrophobic organic–inorganic hybrid cotton fabrics via γ-radiation-induced graft polymerization. Carbohydr Polym 149:308–316. https://doi.org/10.1016/j.carbpol.2016.04.124
Grozea CM, Huang S, Liu G (2016) Water-based, heat-assisted preparation of water-repellent cotton fabrics using graft copolymers. RSC Adv 6:20135–20144. https://doi.org/10.1039/C5RA27056A
Guo J, Filpponen I, Johansson LS, Heiβler S, Li L, Levkin P, Rojas OJ (2018) Micro-patterns on nanocellulose films and paper by photo-induced thiol–ene click coupling: a facile method toward wetting with spatial resolution. Cellulose 25:367–375. https://doi.org/10.1007/s10570-017-1593-2
Hayn RA, Owens JR, Boyer SA, McDonald RS, Lee HJ (2011) Preparation of highly hydrophobic and oleophobic textile surfaces using microwave-promoted silane coupling. J Mater Sci 46:2503–2509. https://doi.org/10.1007/s10853-010-5100-5
He XM, Zhu GT, Zhu YY, Chen X, Zhang Z, Wang ST, Feng YQ (2014) Facile preparation of biocompatible sulfhydryl cotton fiber-based sorbents by “thiol–ene” click chemistry for biological analysis. ACS Appl Mater Interfaces 6:17857–17864. https://doi.org/10.1021/am505876b
Herczynska L, Lestel L, Boileau S, Chojnowski J, Polowinski S (1999) Modification of polysiloxanes by free-radical addition of pyridylthiols to the vinyl groups of the polymer. Eur Polym J 35:1115–1122. https://doi.org/10.1016/S0014-3057(98)00084-6
Hoefnagels HF, Wu D, De With G, Ming W (2007) Biomimetic superhydrophobic and highly oleophobic cotton textiles. Langmuir 23:13158–13163. https://doi.org/10.1021/la702174x
Ismail WNW (2016) Sol–gel technology for innovative fabric finishing—a review. J Sol Gel Sci Tech 78:698–707. https://doi.org/10.1007/s10971-016-4027-y
Januszewski R, Kownacki I, Maciejewski H, Marciniec B (2019) Transition metal-catalyzed hydrosilylation of polybutadiene—the effect of substituents at silicon on efficiency of silylfunctionalization process. J Catal 371:27–34. https://doi.org/10.1016/j.jcat.2019.01.026
Jiang C, Liu W, Yang M, Liu C, He S, Xie Y, Wang Z (2019) Robust multifunctional superhydrophobic fabric with UV induced reversible wettability, photocatalytic self-cleaning property, and oil–water separation via thiol–ene click chemistry. Appl Surf Sci 463:34–44. https://doi.org/10.1016/j.apsusc.2018.08.197
Jiang C, Liu W, Yang M, Zhang F, Shi H, Xie Y, Wang Z (2019) Robust fabrication of superhydrophobic and photocatalytic self-cleaning cotton textiles for oil–water separation via thiol–ene click reaction. J Mater Sci 54:7369–7382. https://doi.org/10.1007/s10853-019-03373-3
Kostić S, Berg JK, Casdorff K, Merk V, Burger I, Cabane E (2017) A straightforward thiol–ene click reaction to modify lignocellulosic scaffolds in water. Green Chem 19:4017–4022. https://doi.org/10.1039/C7GC01601H
Li S, Xie H, Zhang S, Wang X (2007) Facile transformation of hydrophilic cellulose into superhydrophobic cellulose. Chem Commun 46:4857–4859. https://doi.org/10.1039/B712056G
Li J, Li L, Du X, Feng W, Welle A, Trapp O, Levkin PA (2015) Reactive superhydrophobic surface and its photoinduced disulfide-ene and thiol–ene (bio) functionalization. Nano Lett 15:675–681. https://doi.org/10.1021/nl5041836
Liu J, Dong C, Wei D, Zhang Z, Xie W, Li Q, Lu Z (2019) Multifunctional antibacterial and hydrophobic cotton fabrics treated with cyclic polysiloxane quaternary ammonium salt. Fibers Polym 20:1368–1374. https://doi.org/10.1007/s12221-019-1091-2
Lundberg P, Bruin A, Klijnstra JW, Nyström AM, Johansson M, Malkoch M, Hult A (2010) Poly (ethylene glycol)-based thiol–ene hydrogel coatings—curing chemistry, aqueous stability, and potential marine antifouling applications. ACS Appl Mater Interfaces 2:903–912. https://doi.org/10.1021/am900875g
Maciejewski H, Karasiewicz J, Dutkiewicz A, Dutkiewicz M, Dopierała K, Prochaska K (2014) Synthesis and properties of polysiloxanes containing mixed functional groups. React Funct Polym 83:144–154. https://doi.org/10.1016/j.reactfunctpolym.2014.07.019
Marciniec B (ed) (2008) Hydrosilylation: a comprehensive review on recent advances, vol 1. Springer, New York
Oberleitner B, Dellinger A, Déforet M, Galtayries A, Castanet AS, Semetey V (2013) A facile and versatile approach to design self-assembled monolayers on glass using thiol–ene chemistry. Chem Commun 49:1615–1617. https://doi.org/10.1039/C2CC38425F
Patil NV, Netravali AN (2019) Direct assembly of silica nanospheres on halloysite nanotubes for “green” ultrahydrophobic cotton fabrics. Adv Sustain Syst 3(8):1900009
Periolatto M, Ferrero F, Montarsolo A, Mossotti R (2013) Hydrorepellent finishing of cotton fabrics by chemically modified TEOS based nanosol. Cellulose 20:355–364. https://doi.org/10.1007/s10570-012-9821-2
Przybylak M, Maciejewski H, Dutkiewicz A, Dąbek I, Nowicki M (2016) Fabrication of superhydrophobic cotton fabrics by a simple chemical modification. Cellulose 23:2185–2197. https://doi.org/10.1007/s10570-016-0940-z
Przybylak M, Maciejewski H, Dudkiewicz A, Walentowska J, Foksowicz-Flaczyk J (2018) Development of multifunctional cotton fabrics using difunctional polysiloxanes. Cellulose 25:1483–1497. https://doi.org/10.1007/s10570-017-1621-2
Qiang S, Chen K, Yin Y, Wang C (2017) Robust UV-cured superhydrophobic cotton fabric surfaces with self-healing ability. Mater Des 116:395–402. https://doi.org/10.1016/j.matdes.2016.11.099
Ścibiorek M, Gladkova NK, Chojnowski J (2000) Controlled synthesis of amphiphilic siloxane–siloxane block copolymers with carboxyl functions. Polym Bull 44:377–384. https://doi.org/10.1007/s002890070087
Shateri-Khalilabad M, Yazdanshenas ME (2013) One-pot sonochemical synthesis of superhydrophobic organic–inorganic hybrid coatings on cotton cellulose. Cellulose 20:3039–3051. https://doi.org/10.1007/s10570-013-0040-2
Sparks BJ, Hoff EF, Xiong L, Goetz JT, Patton DL (2013) Superhydrophobic hybrid inorganic–organic thiol–ene surfaces fabricated via spray-deposition and photopolymerization. ACS Appl Mater Interfaces 5:1811–1817. https://doi.org/10.1021/am303165e
Sun D, Wang W, Yu D (2016) Preparation of fluorine-free water repellent finishing via thiol-ene click reaction on cotton fabrics. Mater Lett 185:514–518
Vasiljević J, Tomšič B, Jerman I, Orel B, Jakša G, Simončič B (2014) Novel multifunctional water-and oil-repellent, antibacterial, and flame-retardant cellulose fibres created by the sol–gel process. Cellulose 21:2611–2623. https://doi.org/10.1007/s10570-014-0293-4
Vasiljević J, Zorko M, Štular D, Tomšič B, Jerman I, Orel B, Simončič B (2017) Structural optimisation of a multifunctional water-and oil-repellent, antibacterial, and flame-retardant sol–gel coating on cellulose fibres. Cellulose 24:1511–1528. https://doi.org/10.1007/s10570-016-1187-4
Wang M, Wang Y, Gao B, Bian Y, Liu X, He Z, Gu Z (2019) Fast strategy to functional paper surfaces. ACS Appl Mater Interfaces 11:14445–14456. https://doi.org/10.1021/acsami.9b00512
Xu QF, Wang JN, Sanderson KD (2010) A general approach for superhydrophobic coating with strong adhesion strength. J Mater Chem 20:5961–5966. https://doi.org/10.1039/C0JM00001A
Xu L, Zhuang W, Xu B, Cai Z (2011) Fabrication of superhydrophobic cotton fabrics by silica hydrosol and hydrophobization. Appl Surf Sci 257:5491–5498. https://doi.org/10.1016/j.apsusc.2010.12.116
Xu L, Wang W, Yu D (2017a) Durable flame retardant finishing of cotton fabrics with halogen-free organophosphonate by UV photoinitiated thiol-ene click chemistry. Carbohyd Polym 172:275–283. https://doi.org/10.1016/j.carbpol.2017.05.054
Xu L, Wang W, Yu D (2017b) Preparation of a reactive flame retardant and its finishing on cotton fabrics based on click chemistry. RSC Adv 7(4):2044–2050
Xue L, Wang D, Yang Z, Liang Y, Zhang J, Feng S (2013) Facile, versatile and efficient synthesis of functional polysiloxanes via thiol–ene chemistry. Eur Polym J 49:1050–1056. https://doi.org/10.1016/j.eurpolymj.2013.01.017
Xue CH, Fan QQ, Guo XJ, An QF, Jia ST (2019) Fabrication of superhydrophobic cotton fabrics by grafting of POSS-based polymers on fibers. Appl Surf Sci 465:241–248
Yu D, Xu L, Hu Y, Li Y, Wang W (2017) Durable antibacterial finishing of cotton fabric based on thiol–epoxy click chemistry. RSC Adv 7:18838–18843. https://doi.org/10.1039/C6RA28803K
Zeng T, Zhang P, Li X, Yin Y, Chen K, Wang C (2019) Facile fabrication of durable superhydrophobic and oleophobic surface on cellulose substrate via thiol-ene click modification. Appl Surf Sci 493:1004–1012
Zhong Y, Netravali AN (2016) ‘Green’surface treatment for water-repellent cotton fabrics. Surf Innov 4(1):3–13
Zuo Y, Lu H, Xue L, Wang X, Wu L, Feng S (2014) Polysiloxane-based luminescent elastomers prepared by thiol–ene “click” chemistry. Chem Eur J 20:12924–12932. https://doi.org/10.1002/chem.201402746
Acknowledgments
The authors acknowledge financial support from the National Science Centre (Poland), Project OPUS 2018/29/B/ST8/00913, entitled “Synthesis and characterization of materials with defined surface properties”.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Przybylak, M., Szymańska, A., Maciejewski, H. et al. Durable, highly hydrophobic modification of cotton fabric with fluorine-free polysiloxanes obtained via hydrosilylation and hydrothiolation reactions. Cellulose 27, 8351–8367 (2020). https://doi.org/10.1007/s10570-020-03341-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03341-0