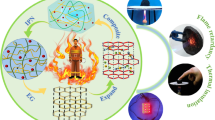
Abstract
Hydrogel-born fire resistance materials have attracted great attention due to their flame retardance and environmental friendliness. In this work, a facile strategy is presented to prepare a novel hydrogel–cotton fabric laminate with excellent thermal insulation and fire-retarding performance. The hydrogel–fabric laminates exhibited outstanding flame retardant behavior. The flame-retardant mechanism of this system was mainly due to the absorption of a large amount of energy as the water is heated and evaporated in the hydrogel layer. To increase the water retention capacity of the fire-resistant hydrogel, highly hydratable salt (CaCl2) was incorporated into the fire-resistant composite hydrogel composed of poly(N-isopropylacrylamide) (PNIPAAm)/sodium alginate (SA) to prolong water retention time. Here in this work, we aimed to investigate the effect of CaCl2 concentration on water retention capacity, fire-resistant and thermal insulating properties of hydrogel–cotton fabric laminates. Results indicated that the presence of hydratable salt successfully prolonged the water retention time and provided superior fire retardance property over traditional hydrogel. In additional, infrared imaging and vertical flammability test results confirmed that hydrogel–fabric laminates were capable of sustaining 1200 °C for 30 min without the cotton fabric layer burning, whereas natural cotton fabric was completely burned after 12 s. Finally, the hydrogel–cotton fabric laminates exhibited remarkable antibacterial activity against Staphylococcus aureus and Escherichia coli due to incorporated silver nanoparticles in hydrogels, and the bacteriostatic rates both exceeded 96%. The preparation of this hydrogel-born fire resistance materials is facile and can extended the period of protective time as fire resistant clothing for the firefighters.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fabrics from cotton, a natural cellulose fibre, have become an ideal candidate for thermal protective clothing with the advantages of its superb heat insulation, comfort, and not producing melting drops and toxic gas during fire exposure (Xu et al. 2020; Qin et al. 2020; Li et al. 2020a). However, the intrinsic flammability of natural cotton fabric limits its wide application in the field of fire-resistant apparel (Guo et al. 2020). According to the statistical data from National Fire Protection Association (NFPA), 20% of fire disasters were caused by flammable textiles including cotton fabric every year (Rosace et al. 2018). Thus, studies on the flame-retardant treatment of cotton fabrics have aroused great interest and extensive attention among researchers. In consideration of human health and environmental friendliness, the most widely used halogen-containing flame retardant was gradually replaced by phosphorus-containing flame retardant (Li et al. 2019; Liu et al. 2020b). Unfortunately, flame retardants only containing phosphorus exhibited poor flame-retardant efficiency, and failed to meet the practical fire prevention requirement. Consequently, there is growing demand in seeking the alternatives with highly efficient fire-retarding performance to traditional flame retardants (Xie et al. 2020).
At present, polymeric hydrogels as fire-resistant material has been attracted significant attention due to polymer chains of hydrogels filling with a large amount of water. (Wang et al. 2018). Water possesses high molar heat capacity [cM (H2O) = 4200 J/ (kg × °C)] and vaporisation enthalpy [ΔH°vap (H2O) = 2260 kJ/ (kg × °C)] (Mykhalichko et al. 2019). A large amount of water in hydrogels can absorb heat and cool temperature on combustible materials to suppress fire. Therefore, hydrogels combine cotton fabric can decrease the flammability of the composite materials when exposed to fire. Currently, some studies have reported that hydrogels were used in coal mine to prevent spontaneous combustion of coal. Li et al.(2020b) proposed a novel high-toughness, organic/inorganic double-network fire-retardant gel with acrylic acid (AA), acrylamide (AM) and sodium silicate (WG). The prepared hydrogel could uniformly cover the surface of the burning coal body forming a dense enwrapping to prevent fire burning. Jiang and Du (2020) grafted chitosan into poly(acrylic acid-co-methacrylamide) hydrogel to be applied in coal mining. Results indicated that the mixture of hydrogel exhibited excellent thermal stability even at high temperature. Ren et al. (2019) proposed a novel sodium silicate/polymer composite hydrogel for the prevention of spontaneous combustion of coal. Results also showed that the composite hydrogel was an ideal fire prevention and extinction material. Above researches revealed hydrogel has outstanding flame retardance in reducing the risk of spontaneous combustion of coal. Therefore, it may be an effective method that hydrogels are applied in textile to prevent and extinct fire.
Illeperuma et al. (2016) firstly proposed a concept that hydrogel laminated on fabric as fire-resistant protective gear to save lives during fire. The fire-resistant performance was attributed to a large amount of energy was absorbed as the inner water of hydrogel heating and evaporating at high temperature. The fabricated hydrogel–fabric laminates showed excellent heat resistance. Inspired by the Illeperuma’s concept of hydrogel–fabric laminates, our group previously prepared a novel fire-resistant material by laminating poly(N-isopropylacrylamide) (PNIPAAm)/sodium alginate (SA) hydrogel on cotton fabric (Yu et al. 2020). The flammability testing indicated that the composite hydrogel–fabric laminates displayed higher fire-resistant performance than pure cotton fabric. However, we also found the prepared hydrogels would fail to continue work when the inner water was evaporated. Thus, the water retention capacity is a key factor when hydrogel is used as a fire-resistant material. Generally, there are two methods to address the above issue: one way is to synthetize superhydrophilic hydrogel to increase the water absorb ability of composite material. Another way is to add additives into hydrogel prolonging water retention time. According to the literature (Bai et al. 2014), introducing highly hydratable salt can enhance water retention capacity of hydrogel. The hydratable salt will ionize into cations and anions when salt dissolves into water. Meanwhile, produced ions combine with water molecules to form hydrated ions, making the water difficult to lose (Cui et al. 2019). Therefore, a novel approach incorporating highly hydratable salts (CaCl2) into sodium alginate (SA)/poly (N-isopropylacrylamide) (PNIPAAm) fire-resistant hydrogel was proposed to improve water retention capacity in the present work.
Based on above the theory of electrolyte solutions, calcium chloride (CaCl2) ionized in an aqueous solution, the Ca2+ ions can form H2O–Ca2+ molecules to bond water (Zeng et al. 2016; Zhang et al. 2020). Moreover, Ca2+ incorporated SA polymers can influence the flame retardancy of cellulose fibres (Wang et al. 2020; Liu et al. 2020a). A few studies have found that calcium alginate (CA) is an inherently biobased flame-retardant polymer, whose limiting oxygen index (LOI) value is 34%. Therefore, the addition of CA also effectively increased the fire-resistant of composite hydrogels. Furthermore, PNIPAAm as an intelligent thermo-sensitive polymer contributed to water evaporate quickly at high temperature. PNIPAAm can change from hydrophilicity to hydrophobicity state in water when fire temperature was above low critical solution temperature (LCST) (32 °C) (Sun et al. 2019; Eklund et al. 2020). Therefore, PNIPAAm/CA hydrogel could rapidly release water to decrease the surface temperature of combustible when exposed to fire (Mou et al. 2014).
In the present work, CaCl2 was introduced into hydrogels to increase fire-resistant performance of the prepared PNIPAAm/CA hydrogel–cotton fabric laminates. We mainly investigated the effect of CaCl2 concentration on water retention capacity, fire-resistant and thermal insulating properties of hydrogel–cotton fabric laminates. Infrared imager and vertical flammability test were used to evaluated the flame retardant and thermal insulating properties. The prepared hydrogel–cotton fabric laminates as a fire-resistant material have potential applications for protective clothing.
Experimental
Materials
Pure cotton fabric with a density of 220 g/m2 was purchased from local market in Wuhan. N-isopropylacrylamide (NIPAAm, 98%) was supplied by Shanghai Macklin, Biochemical Co., Ltd. N,N-methylene-bis-acrylamide (MBAA, 98%, Mw = 154.17), ammonium persulfate (APS, 98%), N,N′,N′-tetra-methyl-ethylene-diamine (TEMED) and AgNO3 (99.8%) were purchased from China Pharmaceutical Group Chemical Reagent Co., Ltd. Sodium alginate (SA, 30–35%), calcium chloride (CaCl2, 95%) were supplied by Sino Pharm Chemical Reagent Co., Ltd.
Preparation of hydrogel–cotton fabric laminates
Synthesis of PNIPAAm/CA-Ag NP hydrogel
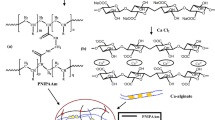
PNIPAAm/CA/Ag NP hydrogels were synthesised as shown in Fig. 1. Firstly, NIPAAm monomer (1 g) was dissolved in 10 mL of deionised water. Subsequently, crosslinker MBAA (0.2 g), initiator APS (0.02 g) and activator TEMED (6 μL) were added into the above solution to synthesis PNIPAAm at 4 °C for 24 h under nitrogen protection. Secondly, SA (0.6 g) was added with distilled water (10 mL) and stirred for 2 h until forming uniform SA solution. Then, 10 mmol/L of Ag NO3 solution was dissolved in the above SA solution and the mixture solution was adjusted to pH 11 by using NaOH. Next, the Ag NP suspension was successfully prepared under UV irradiation for 1 h. Thirdly, the obtained PNIPAAm polymer in the first step was transferred into Ag NP suspension and continuously stirred for 30 min to prepare PNIPAAm/SA/Ag NP composite hydrogel. Finally, obtained PNIPAAm/SA/Ag NP hydrogels was immersed in CaCl2 concentrations with different concentrations (1%, 3%, 5% and 7%) to increase the flame retardant and strength performance. The obtained fire-resistant hydrogels were described as P/CA(x)-Ag NP, where x refers to CaCl2 concentration.
Preparation of hydrogel–cotton fabric laminates
Firstly, a piece of natural cotton fabric (10 cm × 10 cm) was placed in a glass mold with a size of 10 × 10 × 1 cm3. Then the above resultant P/CA(x)-Ag NP hydrogel was transferred into the mold and then immersed in CaCl2 solution to obtain highly efficient hydrogel–cotton fabric laminates with the layer thickness of 2 mm.
Equilibrium swelling ratio of CA/PNIPAAm-Ag NP hydrogel
The water content ability of hydrogels with different CaCl2 concentrations (1%, 3%, 5%, and 7%) was determined by the equilibrium swelling ratio (ESR) in distilled water. Firstly, four hydrogel samples were immersed in distilled water at room temperature to achieve equilibrium state, then taken out and weighed as We. Secondly, the above swollen hydrogels were dried in an oven at 90 °C until the weight no longer changed. The hydrogels were then taken out, weighed and recorded as W0. Finally, the ESR of the four hydrogel samples was calculated by Eq. (1) as follows:
where the W0 is the weight of the dry completely hydrogel at 90 °C and We is the weight of the swollen hydrogel at equilibrium state.
Water retention capacity of CA/PNIPAAm-Ag NP hydrogel
The hydrogels were kept under ambient environment (relative humidity (RH) of 60%, 25 °C) to evaluate the water retention capacity. Firstly, the swollen hydrogels were weighed and recorded as We. Then, samples were placed in ambient environment to observe water loss. The hydrogel was weighted at same interval time and recorded as Wt. The water retention ratio was calculated by Eq. (2) as follows:
where Wt is the weight of hydrogel at time t and We is the weight of the swollen hydrogel at equilibrium state.
Characterisation of hydrogel–cotton fabric laminates
Vertical flammability test
Vertical flammability test was carried out using YG815B automatic vertical flammability cabinet (Ningbo Textile Instrument Factory, China) in accordance with the GB/T5455-2014 standard (Chen et al. 2020). Pure cotton fabric and hydrogel–cotton fabric laminate were cut into 300 mm × 89 mm before testing. The flame-retardant property was evaluated by the carbon length of the samples after burning time of 12 s.
Fire-resistance test
To investigate the fire-resistance performance of the hydrogel–cotton fabric laminates, a flame gun was used to simulate real fire scene environment. A butane flame with fire temperature of approximately 1200 °C was used to spray the surface of hydrogel layer (100 × 100 × 2 mm3). The temperature of fabric back was recorded by using the infrared imager. The schematic diagram of the infrared imager testing is shown in the Fig. 2.
Schematic diagram of infrared imager testing (Cai et al. 2020)
Thermal insulating test
Heat plate method was used to assess thermal insulating performance of hydrogel–cotton fabric laminates. The heat source was supported by the heat plate which was set at 120 °C (simulate radiant heat). The hydrogel layer of laminates (100 × 100 × 2 mm3) was placed on the surface of heat plat. Finally, the infrared imager was used to detect the surface temperature of hydrogel–cotton fabric laminates to investigate thermal insulation performance.
Antibacterial activity of hydrogel–cotton fabric laminates
In this work, the antibacterial activity of hydrogel–cotton fabric laminates against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) were evaluated. Shake flask method and agar diffusion plate method were carried out for quantitative and qualitative analyses according to the standard of GB/T 20944.3-2008 and GB/T 20944.1-2007, respectively (Ren et al. 2021).
Agar diffusion plate method
Firstly, hydrogel–cotton fabric laminates were cut into 6 mm in diameter. Afterwards, the samples were placed on cultured agar plates with S. aureus and E. coli, respectively. Finally, the cultured agar plates were incubated at 37 °C for 24 h in an incubator. The antibacterial activity of samples was evaluated by the inhibition zone. The average width of inhibition zone along the test specimen is calculated using the following Eq. (3):
where W is the width of inhibition zone (mm), T is the width of test specimen and inhibition zone (mm); and D is the width of test specimen (mm).
Shake flask method
Firstly, samples of hydrogel–cotton fabric laminates with and without Ag NP were cut into pieces (0.75 g) and sterilised by UV light. Then the test samples were placed in a jar with 70 mL of water and 5 mL of the bacterial suspension, followed by shaking at 37 °C for 24 h. After that, 0.1 mL of diluted bacterial suspension (1 × 104–2 × 104) was spread onto agar plates and then incubated at 37 °C for 24 h. Finally, antibacterial activity was determined using the percentage reduction of the viable cell counts, as shown in Eq. (4):
where R is the bacterial reduction (%), A and B are the viable cell counts of hydrogel–cotton fabric laminates with and without Ag, respectively.
Results and discussion
Preparation of CA/PNIPAAm-Ag NP hydrogel
The CA/PNIPAAm-Ag NP hydrogel with high water-retaining was successfully synthesised through simple physical interpenetrate polymer network (IPN) technology, as depicted in Fig. 3. In the inner of CA/PNIPAAm-Ag NP hydrogel, the SA chains were entangled within PNIPAAm chains by hydrogen bonding. Moreover, sodium ions (Na+) of SA were replaced by calcium ions (Ca2+) to enhance the mechanical strength of the IPN hydrogel. This is due to Ca2+ were surrounded by the carboxyl groups (–COO−) to form stable ‘eggshell’ structure. Furthermore, part of water in composite hydrogel will form hydrated salt with Ca2+ to improve water retention capacity.
Morphology of CA/PNIPAAm-Ag NP hydrogel
Hydrogels have micro porous structure like layer sponge, which are benefit for the absorb and retain water in networks. The morphologies of CA/PNIPAAm-Ag NP hydrogels were evaluated by SEM (Fig. 4). All the hydrogels exhibited porous and irregular network structures. Moreover, the pore sizes of CA/PNIPAAm-Ag NP hydrogels depended on exchanged ions concentration (Ca2+). The P/CA (1)-Ag NP hydrogel possessed the largest average pore sizes (100 µm), whereas the pore size of P/CA (7)-Ag NP hydrogel was the smallest (10 µm). This phenomenon is attributed to hydrogel would occur dehydration phenomenon in a highly concentrated CaCl2 aqueous solution. Higher crosslinking-network density of hydrogel means denser and uniformly porous structures (Wang et al. 2019). Such interconnected macroporous rough structures provided the hydrogels with good structural stability. Moreover, the pores with interconnectivity and irregular structures were also beneficial for maintaining moisture and absorbing water rapidly. Therefore, the inner network of the hydrogel had a significant effect on swelling-deswelling behaviour, and further influenced the flame-retarding performance as fire-resistant material.
Water content of CA/PNIPAAm-Ag NP hydrogel
It is well known that water is a determining factor for hydrogel as the fire-resistant material. The ESR was investigated the water content of different CA/PNIPAAm-Ag NP hydrogels, as shown in Fig. 5. The ESR values of all hydrogel samples were relatively high. This is because alginate is a hydrophilic polymer and a number of hydrophilic functional groups (such as –COO− and –OH) were presented in alginate chains. Besides, the PNIPAAm chains could absorb a large amount of water retaining inner network at room temperature (T < LCST). The ESR of P/CA (1)-Ag NP hydrogel was highest (1896%), followed by P/CA (3)-Ag NP hydrogel, P/CA (5)-Ag NP hydrogel and P/CA (7)-Ag NP (1098%). The CaCl2 concentration obviously had a remarkable effect on the ESR values. The influence of Ca2+ can be attributed to the dehydration of hydrogels in higher salt solutions. When hydrogel is immersed in CaCl2 solutions, the surrounding ion concentration is higher than the water environment. The hydrogel would gradually lose water until the internal and external ion concentrations were balanced due to the osmotic pressure. Therefore, increased Ca2+ content led to decreased water absorbency. High water-containing hydrogel could supply enough water when a fire-resistant material was exposed to fire. Therefore, hydrogel has potential applications in the flame-retardant field.
Water retention capacity of CA/PNIPAAm-Ag NP hydrogel
As fire resistant materials, high water retention capacity means the hydrogels are able to extend the protection time when exposed to fire. In this work, hygroscopic salt (CaCl2) was used to address the water loss in hydrogel. Figure 6a shows the evolutions of water loss with time in hydrogels. Results indicated that the water retention ratio of four hydrogels all decreased gradually with time going on until reaching a stable state. For the four samples, the water retention ratio would increase from 6.4 to 19.6% at 3000 min when the CaCl2 concentration increased from 1 to 7%. Moreover, the water inside the P/CA (1)-Ag NP hydrogel would be completely evaporated at 2000 min, which was the fastest among these four hydrogels. This result suggested that highly concentrated Ca2+could effectively reduce the dehydration behaviour of hydrogel. The different water loss ratios were due to the difference Ca2+ content in hydrogels. As shown in the Fig. 6b, the free water evaporated easily in the inner network of hydrogel under high temperatures. However, the bonded water in hydrogel was difficult to evaporate because of the strong bond between water molecules and salt ions. The stronger bond strength between Ca2+ and bonded water molecule resulted in the limitation of water molecules evaporation. In addition, when the Ca2+ content increased, the CA molecular chains showed denser clustering and the sizes of porous structures decreased. Therefore, the water in the hydrogel could not be easily lost and the water retaining capacity further improved. This work indicated that the presence of Ca2+ in hydrogel displayed an obvious effect on water retention capacity.
Thermal stability of CA/PNIPAAm-Ag NP hydrogels
Considering the hydrogel as fire resistant material practical application in fire, TG and DTG curves were used to determine the thermal behaviour of CA/PNIPAAm-Ag NP hydrogels, as shown in Fig. 7a, b. In the first stage, a slight weight loss at temperatures below 100 °C was shown as a result of evaporated moisture. The water loss in P/CA (1)-Ag NP hydrogel was faster than that of other hydrogels. This result verified that high Ca2+ content indeed improved the hydration ability to prolong the water retention time in hydrogel. The initial decomposition temperatures (T-5%) of hydrogels were at 130 °C, 180 °C, 210 °C and 235 °C, respectively. This stage of weight loss was mainly due to the cleavage of CA chains and the decomposition of PNIPAAm chains, which resulting in the destruction of the crosslinked structure in hydrogels. DTG thermograms displayed that the maximum decomposition temperature (T-max) increased with the increase in Ca2+ content during the decomposition process. This is owing to the Ca2+ possesses high thermal stability, which decreasing thermal degradation ratio of hydrogels at high temperatures. Thermal analysis indicated that prepared CA/PNIPAAm-Ag NP hydrogels displayed excellent thermal stability after exchanged Ca2+, which could be used in thermal protective clothing as fire resistant material.
Fire resistant performance of hydrogel–cotton fabric laminates
Vertical flammability test was used to examine the fire-resistant property of original cotton fabric and P/CA (7)-Ag NP hydrogel–cotton fabric laminates, as shown in Fig. 8a. The flame spread quickly and burned out completely after 12 s when the original cotton fabric was ignited. This phenomenon indicated that natural cotton fabrics have no fire-resistant property. In contrast, the hydrogel–cotton laminates did not burn when exposed to flame in 12 s. Therefore, there was no char length was observed. According to.
a Vertical flammability test images of original cotton fabric and hydrogel–cotton fabric laminates; b schematic of fire-resistant mechanism of hydrogel–cotton fabric laminates; c curves of maximum temperature on cotton fabric layer with time; d infrared images of P/CA (7)-Ag NP hydrogels; e photos of damaged P/CA (7)-Ag NP hydrogels
the above results, the hydrogel–cotton fabric displayed non-flammability. The fire-resistant mechanism of hydrogel–cotton fabric laminates can be explained as follows: firstly, water is a major composition of fire-resistant hydrogel. Water holds high specific heat capacity [cM (H2O) = 4200 J/ (kg × °C)] and large vaporisation enthalpy [ΔH°vap (H2O) = 2260 kJ/ (kg × °C)]. Therefore, when the hydrogel layer was exposed to fire, the water in hydrogel could absorb a large amount of heat and take away energy to reduce temperature on the cotton fabric surface, resulting in temperature dropping to below the ignition point temperature. Secondly, hydrogel would produce non-combustible gases when exposed to fire. These non-combustible gases such as H2O, CO2 and NO2 would dilute the oxygen concentration. Therefore, decreasing the combustibles of fire-resistant material due to the oxygen concentration under LOI value.
After the water was evaporated over, the hydrogel would form “dense skin layer” on the surface of cotton fabric and further displayed a positive role in inhibiting burning due to isolate the oxygen effect. Figure 8b illustrates the above flame-retardant mechanism.
To further investigate the fire resistance property of hydrogel–cotton fabric laminates, the high-temperature flame spray (1200 °C) was used to simulate real fire scene. The temperature–time curve directly exhibited the temperature variation of hydrogel–cotton fabric laminate back side when the front side was exposed to fire at 1200 °C. As shown in Fig. 8c, the temperature of the cotton fabric layer only increased to 400 °C within 1200 s. This is mainly due to a large amount of water retained in the hydrogel absorbed heat and reduced the temperature of the cotton fabric surface by water evaporation. After 1200 s, the temperature increased slowly from 400 to 449.8 °C until the hydrogel–cotton fabric laminate was burned completely. In addition, the infrared images (Fig. 8d) and burning photos (Fig. 8e) visually showed the fire resistance performance of P/CA (7)-Ag NP hydrogel–cotton fabric laminates. The laminate withstood the high temperature flame for a long period of time, until laminate was burned through within 35 min. All the results indicated that the hydrogel layer provided sufficient protection for cotton fabric when exposed to fire.
Thermal insulating performance of hydrogel–cotton fabric laminates
On the basis of the outstanding flame retardancy of hydrogel–cotton fabric laminates, the fire-resistant material will be applied in thermal protective clothing. To protect the skin from burn injuries by thermal radiation, the heat plate method was used to assess the thermal insulating performance of hydrogel–cotton fabric laminates. The temperature variation of the cotton fabric is depicted in Fig. 9. It was found that the temperature of pure cotton fabric increased rapidly, whereas the fire-resistant material increased relatively slowly. It was obviously seen that the surface of hydrogel–cotton fabric laminates would not over 90 °C when the heat plate was set at 120 °C. This was due to part of absorbed heat was taken away by water evaporation. Another reason was that microporous structure of hydrogel would provide excellent thermal insulation ability because the presence of static air in network after water evaporation over. The air possesses a low thermal conductivity (0.02227 W/m K) compared to dried fire-resistant laminates (0.05967 W/m K). Therefore, the good thermal insulation performance of hydrogel–cotton fabric laminates making them viable candidates in fire retarding field.
Antibacterial activity of hydrogel–cotton fabric laminates
The high water-retention capacity of CA/PNIPAAm hydrogel provided a wettability environment for bacteria. When the cotton fabric laminates are applied in thermal protective clothing, the progenitive bacteria in hydrogel may produce an unpleasant odor and harm to human heathy. Therefore, hydrogel–cotton fabric laminates must possess a certain antibacterial performance. The antibacterial activity of hydrogel–cotton fabric laminates against Gram-negative bacteria (E. coli) and Gram-positive bacteria (S. aureus) was evaluated in this study. As shown in Fig. 10a, the inhibition zone around the fire-resistant material was clearly observed. Result indicated that the present of Ag NP in hydrogel imparted excellent antibacterial performance against both E. coli and S. aureus. Moreover, it was also found that CaCl2 content in hydrogel did not influence the width of inhibition zone. For the shake flask method (Fig. 10b), results indicated that almost full bacterial appeared on the plates for the controlled sample without Ag NP, whereas sporadic bacterial colonies were observed for the sample with Ag NP. In this work, the bacterial reduction rates of E. coli and S. aureus both exceeded 96% for prepared P/CA (7)-Ag NP sample. This result revealed that Ag NP incorporated into the hydrogel greatly improved the antibacterial activity of laminates. The antibacterial mechanism of the hydrogel–cotton fabric laminates loaded with Ag NPs is illustrated in Fig. 10c. The release of Ag+ is the key factor in determining the antibacterial activity of Ag NPs. The Ag+ attached to the cell wall and penetrated the cytoplasm membrane to disrupt the membrane permeability (Lin et al. 2018; Homaeigohar and Boccaccini 2020). Then, Ag+ blocked the expression of proteins by introducing mutations into the bacterial genome (DNA) (Xie et al. 2019). Thus, the bacterial cellular structures were damaged and resulting in cell death.
Conclusion
In summary, a novel fire-resistant hydrogel with high water retention capacity has been prepared and then laminated to cotton fabric to increase the flame-retardant performance. Different concentration of CaCl2 was incorporated into hydrogel to increase the water retention capacity because of the strong hydration ability of Ca2+. The fire-retarding performance and thermal insulating property of the hydrogel–cotton fabric laminates were investigated by vertical flammability test and heat plate method. Results indicated that hydrogel–fabric laminates were capable of sustaining with 1200 °C for 30 min to prevent cotton fabric layer burning, whereas natural cotton fabric was completely burned after burning for 12 s. Moreover, Ag NPs were successfully incorporated into the composite hydrogels to prevent the hydrogel–cotton fabric laminates producing unpleasant odor and improve wearing comfort. Results indicated that the bacteriostatic rates against E. coli and S. aureus both exceeded 96%. Our understanding of interrelationship between CaCl2 content in hydrogel and fire-resistant ability can provide a guideline for designing and fabricating efficient flame-retardant thermal protective clothing used in fire-fighting.
References
Bai YY, Chen BH, Xiang F, Zhou JX, Wang H, Suo ZG (2014) Transparent hydrogel with enhanced water retention capacity by introducing highly hydratable salt. Appl Phys Lett 105(15):151903. https://doi.org/10.1063/1.4898189
Cai CY, Wei ZC, Huang YZ, Ding CF, Wang P, Song JY (2020) Ultralight programmable bioinspired aerogels with an integrated multifunctional surface for self-cleaning, oil absorption, and thermal insulation via coassembly. ACS Appl Mater Interfaces 12(9):11273–11286. https://doi.org/10.1021/acsami.0c00308
Chen JY, Xie HL, Lai XJ, Li HQ, Gao JF, Zeng XR (2020) An ultrasensitive fire-warning chitosan/montmorillonite/carbon nanotube composite aerogel with high fire-resistance. Chem Eng J 399:125729. https://doi.org/10.1016/j.cej.2020.125729
Cui XF, Zheng WJ, Zou W, Liu XY, Yang H, Yan J, Gao Y (2019) Water-retaining, tough and self-healing hydrogels and their uses as fire-resistant materials. Polym Chem-UK 10(37):5151–5158. https://doi.org/10.1039/c9py01015g
Eklund A, Zhang H, Zeng H, Priimagi A, Ikkala O (2020) Fast switching of bright whiteness in channeled hydrogel networks. Adv Funct Mater 30(28):2000754. https://doi.org/10.1002/adfm.202000754
Guo WW, Wang X, Huang JL, Zhou YF, Cai W, Wang JL, Song L, Hu Y (2020) Construction of durable flame-retardant and robust superhydrophobic coatings on cotton fabrics for water–oil separation application. Chem Eng J 398:125661. https://doi.org/10.1016/j.cej.2020.125661
Homaeigohar S, Boccaccini AR (2020) Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater 107:25–49. https://doi.org/10.1016/j.actbio.2020.02.022
Illeperuma WRK, Rothemund P, Suo ZG, Vlassak JJ (2016) Fire-resistant hydrogel–fabric laminates: a simple concept that may save lives. ACS Appl Mater Interfaces 8(3):2071–2077. https://doi.org/10.1021/acsami.5b10538
Jiang ZW, Du GL (2020) Preparation and Characterization of chitosan grafting hydrogel for mine-fire fighting. ACS Omega 5(5):2303–2309. https://doi.org/10.1021/acsomega.9b03551
Li SS, Lin XH, Li ZG, Ren XH (2019) Hybrid organic-inorganic hydrophobic and intumescent flame-retardant coating for cotton fabrics. Compos Commun 14:15–20. https://doi.org/10.1016/j.coco.2019.05.005
Li P, Wang B, Liu YY, Xu YJ, Jiang ZM, Dong CH, Zhang L, Liu Y, Zhu P (2020a) Fully bio-based coating from chitosan and phytate for fire-safety and antibacterial cotton fabrics. Carbohyd Polym 237:116173. https://doi.org/10.1016/j.carbpol.2020.116173
Li YS, Hu XM, Cheng WM, Shao Z, Xue D, Zhao YY, Lu W (2020b) A novel high-toughness, organic/inorganic double-network fire-retardant gel for coal-seam with high ground temperature. Fuel 263:116779. https://doi.org/10.1016/j.fuel.2019.116779
Lin J, Chen XY, Chen CY, Hu JT, Zhou CL, Cai XF, Wang W, Zheng C, Zhang PP, Cheng J, Guo ZH, Liu H (2018) Durably antibacterial and bacterially antiadhesive cotton fabrics coated by cationic fluorinated polymers. ACS Appl Mater Interfaces 10(7):6124–6136. https://doi.org/10.1021/acsami.7b16235
Liu Q, Yang S, Ren J, Ling SJ (2020a) Flame-retardant and sustainable silk ionotronic skin for fire alarm systems. ACS Mater Lett 2(7):712–720. https://doi.org/10.1021/acsmaterialslett.0c00062
Liu SD, Wan CY, Chen Y, Chen R, Zhang FX, Zhang GX (2020b) A novel high-molecular-weight flame retardant for cotton fabrics. Cellulose 27(6):3501–3515. https://doi.org/10.1007/s10570-020-03020-0
Mou CL, Ju XJ, Zhang L, Xie R, Wang W, Deng NN, Wei J, Chen QM, Chu LY (2014) Monodisperse and fast-responsive poly(N-isopropylacrylamide) microgels with open-celled porous structure. Langmuir 30(5):1455–1464. https://doi.org/10.1021/la4046379
Mykhalichko B, Lavrenyuk H, Mykhalichko O (2019) New water-based fire extinguishant: Elaboration, bench-scale tests, and flame extinguishment efficiency determination by cupric chloride aqueous solutions. Fire Saf J 105:188–195. https://doi.org/10.1016/j.firesaf.2019.03.005
Qin HB, Fang KJ, Ren YF, Zhang K, Zhang LY, Zhang XY (2020) Insights into influences of dye hydrophobicity on cleanliness and resolution of fabric ink jet printing. ACS Sustain Chem Eng 8:17291–17298. https://doi.org/10.1021/acssuschemeng.0c06447
Ren XF, Hu XM, Xue D, Li YS, Shao Z, Dong H, Cheng WM, Zhao YY, Xin L, Lu W (2019) Novel sodium silicate/polymer composite gels for the prevention of spontaneous combustion of coal. J Hazard Mater 371:643–654. https://doi.org/10.1016/j.jhazmat.2019.03.041
Ren YF, Fu RR, Fang KJ, Xie RY, Hao LY, Chen WC, Shi Z (2021) Clean dyeing of acrylic fabric by sustainable red bacterial pigment based on nano-suspension system. J Clean Prod 281:125295. https://doi.org/10.1016/j.jclepro.2020.125295
Rosace G, Castellano A, Trovato V, Iacono G, Malucell G (2018) Thermal and flame retardant behavior of cotton fabrics treated with a novel nitrogen-containing carboxyl-functionalized organophosphorus system. Carbohydr Polym 196:348–358. https://doi.org/10.1016/j.carbpol.2018.05.012
Sun XF, Zeng QH, Wang HH, Hao YW (2019) Preparation and swelling behavior of pH/temperature responsive semi-IPN hydrogel based on carboxymethyl xylan and poly(N-isopropyl acrylamide). Cellulose 26(3):1909–1922. https://doi.org/10.1007/s10570-018-2180-x
Wang ZF, Li HL, Tang ZJ, Liu ZX, Ruan ZH, Ma LT, Yang Q, Wang DH, Zhi CY (2018) Hydrogel electrolytes for flexible aqueous energy storage devices. Adv Funct Mater 28(48):1804560. https://doi.org/10.1002/adfm.201804560
Wang L, Zhang XH, Yang K, Fu YV, Xu TS, Li SL, Zhang DW, Wang LN, Lee CS (2019) A novel double-crosslinking-double-network design for injectable hydrogels with enhanced tissue adhesion and antibacterial capability for wound treatment. Adv Funct Mater 30(1):1904156. https://doi.org/10.1002/adfm.201904156
Wang B, Li P, Xu YJ, Jiang ZM, Dong CH, Liu Y, Zhu P (2020) Bio-based, nontoxic and flame-retardant cotton/alginate blended fibres as filling materials: thermal degradation properties, flammability and flame-retardant mechanism. Compos Part B Eng 194:108038. https://doi.org/10.1016/j.compositesb.2020.108038
Xie Y, Chen SQ, Zhang X, Shi ZQ, Wei ZW, Bao JX, Zhao WF, Zhao CS (2019) Engineering of tannic acid inspired antifouling and antibacterial membranes through co-deposition of zwitterionic polymers and Ag nanoparticles. Ind Eng Chem Res 58(27):11689–11697. https://doi.org/10.1021/acs.iecr.9b00224
Xie RY, Fang KJ, Liu Y, Chen WC, Fan JN, Wang XW, Ren YF, Song YW (2020) Z-scheme In2O3/WO3 heterogeneous photocatalysts with enhanced visible-light-driven photocatalytic activity toward degradation of organic dyes. J Mater Sci 55(26):11919–11937. https://doi.org/10.1007/s10853-020-04863-5
Xu QB, Shen LW, Duan PP, Zhang L, Fu FY, Liu XD (2020) Superhydrophobic cotton fabric with excellent healability fabricated by the “grafting to” method using a diblock copolymer mist. Chem Eng J 379:122401. https://doi.org/10.1016/j.cej.2019.122401
Yu ZC, Suryawanshi A, He HL, Liu JR, Li YQ, Lin XB, Sun ZH (2020) Preparation and characterization of fire-resistant PNIPAAm/SA/AgNP thermosensitive network hydrogels and laminated cotton fabric used in firefighter protective clothing. Cellulose 27(9):5391–5406. https://doi.org/10.1007/s10570-020-03146-1
Zeng HC, Yang JS, Han SY (2016) The synthesis and characteristics of sodium alginate/graphene oxide composite films crosslinked with multivalent cations. Appl Polym Sci 133(27):43616. https://doi.org/10.1002/app.43616
Zhang QF, Zhao LY, Wang J, Wang S, Liu YX, Liu XF (2020) High-strength and high-toughness sodium alginate/polyacrylamide double physically crosslinked network hydrogel with superior self-healing and self-recovery properties prepared by a one-pot method. Colloid Surf A 589:124402. https://doi.org/10.1016/j.colsurfa.2019.124402
Acknowledgments
The authors acknowledge financial support from Opening Project of Key Laboratory of High Performance fibers and products (Ministry of Education), Opening Project of Key Laboratory of Clean Dyeing and Finishing Technology of Zhejiang Province (Grant Number QJRZ1904), and Opening Project of Hubei Key Laboratory of Biomass Fibers and Eco-Dyeing & Finishing (Grant Number STRZ2020005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, Z., Liu, J., Suryawanshi, A. et al. Thermal insulating and fire-retarding behavior of treated cotton fabrics with a novel high water-retaining hydrogel used in thermal protective clothing. Cellulose 28, 2581–2597 (2021). https://doi.org/10.1007/s10570-021-03696-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-021-03696-y