Abstract
The protective clothing of firefighters requires specialised fire-resistant materials to ensure their safety. In this work, a novel fire-resistant material was prepared by laminating an interpenetrating polymer network (IPN) hydrogel on cotton fabric. The hydrogel-fabric laminates can be used as a flame-retardant material to produce firefighter protective clothing. The IPN hydrogel layer comprised poly (N-isopropylacrylamide) (PNIPAAm), sodium alginate (SA) and silver nanoparticles (Ag NPs). The chemical structures, swelling ratio, thermal properties, microstructures and tensile properties of the synthesised IPN hydrogel were investigated using Fourier transform-infrared spectroscopy (FTIR), X-ray diffraction, thermogravimetric analysis, differential scanning calorimetry, scanning electron microscopy and tensile testing, respectively. Results revealed that the water content of IPN hydrogel was approximately 2186% at 20 °C, indicating excellent ability to absorb energy as water heats up and evaporate. FTIR results showed that the PNIPAAm and SA were only physical interpenetrated within the IPN hydrogel. Moreover, compared with pure PNIPAAm, IPN hydrogel displayed better elastic and breaking strength. Vertical burning findings indicated that the hydrogel-fabric laminates did not burn when exposed to flame for 12 s, whereas natural cotton fabric was burned out. Finally, the fire-resistant hydrogel displayed excellent antibacterial activity against Staphylococcus aureus and Escherichia coli through the introduction of Ag NPs, and the antibacterial activity for both microorganisms exceeded 96%. Overall, this study provided an easy approach to producing a fire-resistant material by laminating a hydrogel and a fabric that may save lives.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
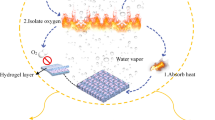
Firefighters struggle in a working environment with flash fire during operations and rescue missions. Thus, fire-resistance fabrics are in large demand to protect firefighters from burn injuries and ensure their safety (Illeperuma et al. 2016; Xue et al. 2016; Li et al. 2017). Water is an excellent extinguisher because of its high heat capacity and latent heat of vaporisation. However, water cannot be directly used as fire protection materials because water is in the liquid state and has no fixed shape at room temperature. At present, hydrogel is attracting research attention to produce firefighter protective clothing because of its high water content (Cui et al. 2019).
Hydrogel is an aggregate of water and a polymer network, having water as the primary component (Wang et al. 2019a, b, c). Hydrogel can absorb a large amount of heat and then released through moisture evaporates. Therefore, a fire-resistant material can be produced by laminating a hydrogel and a fabric that may save lives. The hydrogel-fabric laminates display an inverse thermal protective mechanism compared with traditional thermal insulation materials. Most of the fire-retardant insulation materials protect firefighters from burn injuries by resisting heat passing through the fabrics, whereas the hydrogel-fabric laminates resist fires by absorbing energy and releasing through water evaporates. Therefore, the hydrogel–fabric laminates can be potentially applied in firefighter-protective clothing to stop flames or reduce the temperature to an acceptable level for the human body when exposed to fire.
At present, the poly (N-isopropylacrylamide) (PNIPAAm) is well known and extensively studied because of its thermosensitive behaviour and water retention properties. The temperature-responding feature of PNIPAAm hydrogel is based on its lower critical solution temperature (LCST) at 30 °C–32 °C with water. Below the LCST, the PNIPAAm hydrogel contains high amount of water and is in a swelling and expansion state. When heating above LCST, the phase separation occurs, and the hydrogel shrinks releasing water (Krakovsky et al. 2019; Wang et al. 2016). Thus, using PNIPAAm hydrogel as a flame-retardant material to protect people from burn injuries by shrinking and releasing water is appropriate because the LCST of PNIPAAm is near body temperature.
However, the major factor limiting the application of PNIPAAm hydrogel as a fire-resistant material is its poor mechanical strength. In general, hydrogel cannot be directly used as a fire-resistant material because of its poor strength property. Laminating hydrogel with fabric is an effective method to obtain satisfactory flame-retardant and wearable fire-resistant materials. In recent years, cotton fabrics are frequently used worldwide because of their excellent properties and advantages with regard to thermal insulation, biocompatibility and great moisture absorption and breathability performances (Liu et al. 2019; Annalisa et al. 2016). These advantages indicate potential applications of cotton fabrics in protective clothing and human health (Wang et al. 2019a, b, c). However, natural cotton fabric is highly flammable and will rapidly burn out. This fatal drawback reveals a potential danger and limits the use of cotton fabrics. Therefore, treating cotton fabrics to obtain fire-resistant cotton fabrics is important. Compared to chemical fibers, natural cotton fabric will not produce melting drip and poisonous gas during fire exposure (Liu et al. 2017). These advantages make cotton fabrics viable candidates in life saving application such as fire-retardant protective clothing. To endow natural cotton fabrics with a favourable fire-resistant property, a novel method is developed to prepare a high-performance fire-resistant cotton fabric by laminating interpenetrating polymer network (IPN) hydrogel and a fabric that may save lives. The flame-retardant performance of hydrogel-fabric laminates is primarily due to the internal water in the IPN hydrogel. However, the cotton fabric in the hydrogel–fabric laminates will be more susceptible to bacterial attack due to the high water content in hydrogel. Therefore, silver nanoparticles (Ag NPs) are embedded in the synthesised hydrogel using Ag NO3 as a prime metal and sodium alginate (SA) as a green reducing agent, which is a polysaccharide polymer with excellent inherent fire-resistant properties. In the present work, fire-resistant IPN hydrogel, including PNIPAAm, SA and Ag NPs, was firstly produced. The role of SA in the IPN hydrogel was to form a physical interpenetration with PNIPAAm and to eliminate the poor flame retardancy and low strength of PNIPAAm. The chemical structures, swelling ratio (SR), thermal properties, microstructures and mechanism properties of synthesised IPN hydrogel were investigated using different methods, such as Fourier transform-infrared spectroscopy (FTIR), X-ray diffraction (XRD), thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), scanning electron microscopy (SEM), tensile tester and antibacterial activity. Moreover, the vertical flame test was also used to investigate the flame-retardant property of hydrogel–fabric laminates. This work provided a new, simple strategy to produce a high-performance flame-retardant material used as firefighter protective clothing.
Experimental
Materials
N-Isopropylacrylamide (NIPAAm), N,N-methylene-bis-acrylamide (MBAA, Mw = 154.17), N,N,N',N'-tetra-methyl-ethylene-diamine (TEMED), N,N-methylene-bis-acrylamide (MBAA), AgNO3 and ammonium persulphate (APS) were purchased from Sinopharm Chemical Reagent Co., Ltd. Staphylococcus aureus (S. aureus) (ATCC 6538) and Escherichia coli (E. coli) (ATCC 8099) were purchased from Shanghai Luwei Microbial Technology Co., Ltd. Cotton fabric (ends per inch/picks per inch = 75/68, areal density = 75 g/m2) was purchased from Wuhan local market. All reagents used in the present study were analytically pure.
Characterization
The absorption characteristic peak of Ag NP suspension was measured using a UV–Vis spectrophotometer (UV-8000S, China). The particle size of Ag NPs in suspension was determined by a Zetasizer Nano-ZS particle size analyser (ZEN3690, Malvern, UK). The chemical structures of the fire-resistant IPN hydrogel were investigated using FTIR (Tensor 27, Germany) in the range from 4000 to 500 cm−1 at room temperature. The surface morphology of synthesised hydrogel was recorded using SEM (MVE016, Phenom, Netherlands). The sample was sputter coated with a gold layer before observation. TGA of hydrogel was conducted using a thermal analyser (Q5000, USA) ranging from 30 to 500 °C. Determination of the phase-separation temperatures of the dried IPN hydrogels was achieved using a DSC apparatus (Q2000, USA).
Synthesis of fire-resistant IPN hydrogel
The fire-resistant IPN hydrogel was synthesised (Fig. 1). Firstly, 0.6 g SA and 10 mL of AgNO3 solution (50 mmol/L) were dissolved in deionised water (10 mL). Then, the Ag NP suspension was successfully prepared at different pH values (5, 7, 9) under ultraviolet (UV) radiations (wavelength 365 nm). Secondly, NIPAAm monomer (1.0 g), MBAA (0.02 g), BTCA (0.2 g), co-initiator APS (0.005 g) and SHP (0.1 g) were added into the above-mentioned Ag NP suspension with continuous stirring. The initiator TEMED (6 µL) was then injected in the mixed solution by a syringe. Finally, the mixture was stirred vigorously for 60 s, and polymerisation was conducted under nitrogen protection in a refrigerator at 5 °C for 24 h. After the crosslinking reaction, the obtained IPN hydrogel was soaked in CaCl2 solution (4.0 wt%) for 12 h to obtain Ca-alginate/PNIPAAm IPN hydrogel. Afterwards, the hydrogel was vacuum dried for 24 h at 25 °C and then further vacuum dried for 3 min at 150 °C. Finally, the dried hydrogel was immersed in distilled water for 2 days to reach equilibrium swelling ratio (SR) at 20 °C.
Preparation of hydrogel–fabric laminates
The schematic illustration of producing hydrogel–fabric laminates is presented in Fig. 2. Firstly, a piece of natural cotton fabric with a dimension 15 × 10 × 0.1 cm3 was placed into a mould. Secondly, all IPN hydrogel synthesis reagents were poured into the mould with fabric. The synthetic reaction condition was the same as that of the previously synthesised IPN hydrogel. The above-mentioned mould was kept in the fridge for 24 h under nitrogen protection condition. Afterwards, hydrogel–fabric laminates were removed and immersed into the CaCl2 solution (4.0 wt%) for 24 h to exchange the sodium ions and to remove the remaining impurities. Then, the hydrogel–fabric laminates were vacuum dried for 24 h at 25 °C and then further vacuum dried for 3 min at 150 °C. Finally, the dried hydrogel-fabric laminates were immersed in distilled water to reach the largest water content in hydrogel and the layer thickness of hydrogel on the surface of the cotton fabric was 1.5 mm and contains around 2000% water in hydrogel.
Dynamic thermo-responsive volume change of IPN hydrogel
The dynamic thermo-responsive volume change of synthesised fire-resistant hydrogel could be characterised by the equilibrium SR at different temperatures. Firstly, the IPN hydrogel was cut into pieces with the dimension of 5.0 × 5.0 × 3.0 cm3. Secondly, the hydrogel samples were immersed into distilled water to reach a full swelling state under 20 °C and then calculated the equilibrium SR. Thirdly, the equilibrium SR of fire-resistant hydrogel at 20 °C–50 °C was investigated. The equilibrium SR of synthesised fire-resistant IPN hydrogel at different temperatures was calculated as follows:
where Ws and Wd are the sample masses in the equilibrium swelling and dry state, respectively. The mass of Md of hydrogel was obtained by drying in an oven at 100 °C for 6 h.
Antibacterial activity of fire-resistant hydrogels
The antibacterial activity of the fire-resistant hydrogel was tested against S. aureus (E. coli) and E. coli (S. aureus) in accordance with the agar diffusion plate (AATCC90-2011) and shake flask (AATCC100-2004) methods. For the inhibition zone method, a piece of hydrogel with a diameter of 2.5 cm was gently pressed onto the surface of the agar medium with bacteria and then incubated at 37 °C for 24 h (Bu et al. 2019). The antibacterial activity was evaluated through the observation inhibition zone around the sample. The schematic diagram of the inhibition zone method is shown in Fig. 3.
For the shake flask method, 1.0 mL of the bacterial suspension (1–2 × 105 CFU) was loaded onto one test sample (diameter 4.8 cm) in a jar. Afterwards, 100 mL of bacteria-free water was added into the jar and then shake incubated at 37 ± 2 °C for 24 h before being assayed for bacterial population density. Finally, the number of colonies was counted by the pour plate method, and the percentage reduction of bacteria was calculated as follows:
where R is the bacterial reduction percentage %; A and B are the number of bacteria recovered from the inoculated synthesised hydrogel after 24 h and zero contact time, respectively (Fig. 4).
Fire resistance test
The vertical burning test was performed on a YG815B automatic vertical flammability cabinet (Ningbo Textile Instrument Factory, China) according to GB/T5455-2014. The test sample had sheet dimensions of 300 mm × 89 mm. The contact time between the flame and sample was set as 12 s. Finally, the flame retarding effect was evaluated by recording the afterflame time, afterglow time and damaged length.
Results and discussion
Synthesis of Ag NPs
SA is a hydrophilic and biodegradable linear polysaccharide copolymer, which is extensively used as a flame-retardant material through carbonisation (Xu et al. 2019). In the current work, SA was used as the physically interpenetrated composition to increashge the strength and fire-resistance property of the synthesised IPN hydrogel. As the carboxyl and hydroxyl groups of alginates possessed a certain reduction property, SA can react with Ag+ and reduce to Ag0 (Thangaraj et al. 2018). In this work, Ag NP suspension was firstly prepared before synthesising the hydrogel by reducing AgNO3 using SA as reducer and stabiliser under UV irradiation.
Figure 5 shows the UV–Vis absorption spectra of AgNO3 and SA solution under different pH values. At first, no characteristic absorption peak was found in the wavelength range from 300 to 800 nm for the silver nitrate and SA solution. Moreover, no new peak appeared after stirring the solution for 30 min without UV irradiation. However, a typical absorption region can be observed between 400 and 450 nm when the solution was exposed to UV irradiation for half an hour, particularly under alkaline condition. In previous studies, the absorption peak at approximately 415 nm could reveal successful synthesis of Ag NPs in the mixed solution (Aladpoosh et al. 2014; Rac-Rumijowska et al. 2017). These results indicated that pH and UV irradiation displayed a key factor in the synthesis of Ag NPs. Furthermore, it was also observed that the colour of the solution gradually changed from white to yellow under alkaline synthesis bath with UV irradiation (Fig. 6). Further, it turned out that the Ag NPs were successfully synthesised in the synthesis bath through reduction of silver ions by SA.
Based on the result of UV–Vis absorption spectra, the optimal reduction condition was in the alkaline medium. In this work, the zeta particle size analyser was futher used to analyse the intensity and size distribution of Ag NPs in the synthesis bath with pH 9. Figure 7 shows that the mean size of Ag NPs was in the range of 20–60 nm, which implies having even particle size.
Structural characterisation of fire-resistant IPN hydrogel
The chemical structure of the samples is investigated using FTIR in the optical range from 4000 to 500 cm−1 (Fig. 8). Results show that the OH-stretching vibration is responsible for the characteristic absorption peak at approximately 3200–3400 cm−1. In the PNIPAAm spectrum, the strong peaks at 1649 and 1540 cm−1 corresponded to the C = O stretching peak and N–H bending peak, respectively. The absorption peak of PNIPAAm at 1649 cm−1 and N–H bending peak were observed in any spectra, indicating the presence of PNIPAAm and SA in the synthesis of fire-resistant INP hydrogel. The intensity of the N–H bending peak at 1649 cm−1 will decrease in the IPN hydrogel, which indicated the decreasing amount of PNIPAAm chain present in the IPN (Wang et al. 2019a, b, c; Pallmann et al. 2019). For the three chemical structures in FTIR, no new characteristic peak was added or disappeared, suggesting that the introduced SA and Ag NPs did not influence the structure of PNIPAAM.
Figure 9 shows the XRD spectra of the fire-resistant IPN hydrogel with and without Ag NPs. The XRD pattern of the synthesised hydrogel with Ag NPs shows three strong peaks at 34.25°, 44.30° and 77.52°, corresponding to the silver crystal planes 111, 200 and 311, respectively. The previous results demonstrated that Ag+ was successfully reduced by SA and deposited inside of the fire-resistant IPN hydrogel (Xu et al. 2017; Cui et al. 2018). The presence of Ag NPs in fire-resistant IPN hydrogel can avoid wound infection of firefighters suffering from burn injuries.
Thermal behaviour of fire-resistant hydrogel
The thermal behaviour of the synthesised fire-resistant IPN hydrogel in a solid state was investigated by the DSC and TGA devices. The DSC curves of PNIPAAm and synthesised fire-resistant IPN hydrogel are shown in Fig. 10. As can be seen in Fig. 10, a typical exothermic peak for the IPN hydrogel was observed in the DSC curve. The intersection point between the two tangent lines in the DSC curve was the LCST of the synthesised IPN thermosensitive hydrogel. The result indicated that the LCST value of fire-resistant hydrogel was approximately 31 °C, which was similar to the pure PNIPAAm. The result also revealed that the introduction of SA in PNIPAAm almost had no influence on the LCST because of physically interpenetration crosslink between the SA and the PNIPAAm molecules.
The TG–DTG evaluation was carried out to characterise the thermal stability of composite hydrogels. Figure 11 shows the TG and DTG curves of the PNIPAAm and fire-resistant IPN hydrogel. TG curves indicated that the mass loss of hydrogel would increase with the increase of temperature. Figure 11a indicates that the weight loss of IPN hydrogel was lower than that of PNIPAAm in the whole heating process. In addition, the thermal degradation stage of the samples primarily included three steps. The first process was mostly ascribed to the moisture loss of the hydrogel between 25 and 200 °C. The second process was the primary thermal decomposition step from 200 to 550 °C due to the breaking of the PNIPAAm backbone. The IPN hydrogel showed evidently a decomposition peak at 260 °C and 405 °C (peak 3 in Fig. 11b). In this step, the mass residue of the samples was dropped sharply because of material carbonisation and decomposition of the SA and PNIPAAm. The third step was the temperature over 550 °C, and the weight loss of the sample displayed almost no change. As shown in Fig. 11a, the semi-decomposition temperature of the IPN and PNIPAAm hydrogel 504 °C and 408 °C, respectively. The result showed that the embedded SA in the PNIPAAm hydrogel would remarkably improve the thermal stability of the IPN hydrogel. Moreover, the weight loss of fire-resistant IPN hydrogel was evidently lower than the PNIPAAm hydrogel when the sample state was in the thermal degradation equilibrium stage within the range 550 °C–600 °C. This result can be due to the presence of calcium chloride in the IPN hydrogel, which was difficult to decompose under heat exposure condition. Furthermore, these results indicated that the synthesised fire-resistant IPN hydrogel worked well as fire-retardant materials.
Surface morphology
SEM was used to observe the morphology of the fire-resistant IPN hydrogel. The uniform porous structure of freeze-dried IPN hydrogel was observed by SEM (Fig. 12). The average pore size of IPN hydrogels was approximately from 30 to 60 μm. The microstructure indicated that the porous structures of the IPN hydrogel were important to transport the moisture from the human body to the atmospheric environment, improving the wear comfort of the hydrogel-fabric laminates. Moreover, a good compatibility between the PNIPPAm and SA in the polymer matrix can be seen from the SEM images. Overall, the pore characteristics indicated that hydrogel can accommodate a large amount of water and cause strong fire-resistant ability, but also possessed great moisture permeability property.
Swelling properties
Water is vital for fire-resistant polymer hydrogel when exposed to fires. The trapped water in hydrogel could absorb a large amount of energy by water heats up and evaporates. To determine the water content of hydrogel, dry IPN hydrogel was soaked into water until constant weight was attained at a given temperature to obtain the equilibrium swelling rate. The equilibrium swelling rate of synthesised IPN hydrogel at different temperatures is shown in Fig. 13. The experimental results showed a decrease in SR with increased temperature of the synthesised IPN hydrogel. The fire-resistant hydrogel showed the maximum SR of 2186% at 20 °C, whereas the lowest SR value of 1500% was observed at 55 °C. The change in SR was primarily caused by the thermoresponsive behaviour of PNIPAAm in fire-resistant IPN hydrogel. It was also worth noting that the SR would have a sharp decrease around 31 °C, which corresponding to the LCST of the synthesised IPN hydrogel. Above the LCST, phase-separation of the IPN hydrogel would occur, resulting in released water from the hydrogel (Fig. 14). The LCST from the SR observation coincided with the DSC curves. Moreover, the SR will not decline further between 40 and 55 °C. This result indicated that the deswelling process of IPN hydrogel would stop when the hydrogel temperature was higher than the LCST value.
Tensile properties
The PNIPAAm hydrogel was often fragile, such as gelatin and tofu (Zhang et al. 2018). Thus, the poor mechanical property limited the application of PNIPAAm hydrogel. In the current work, the SA was diffused into the IPN hydrogel as a physical interpenetration crosslinking component to improve the strength of synthesised IPN hydrogel. The tensile stress–strain behaviour of swelling IPN hydrogel at 20 °C is shown in Fig. 15. The result showed that with an increase in tensile strength, the elongation of IPN hydrogel would gradually increase. For the IPN hydrogel, the tensile strength would reach up to 180 kPa, and elongation at breaking point was over 17%. The dependence between the strain and the tensile stress was almost linear. In the present study, the fire-resistant IPN hydrogel did not show a fracture phenomenon when the elongation was lower than 17%. In addition, we were not able to perform the tensile stress–strain test of the PNIPAAm hydrogel because of its high brittleness.
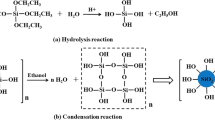
Based on the the above-mentioned analysis, the IPN hydrogel displayed better mechanical property compared with the PNIPAAm hydrogel, which primarily due to two reasons. Firstly, the SA was inserted into the PNIPAAm hydrogel and formed a new type of double network (DN) hydrogel composed of thermoresponsive PNIPAAm and high hydrophilic SA polymers. SA polymer had a longer chain network structure and breaking strength properties compared with PNIPAAm. When the synthesised fire-resistant hydrogel was stretched, the PNIPAAm will be the first to reach the breaking point because of its short-chain network structure. By contrast, the long-chain network structure of SA polymer retained its elasticity and continued to dissipate energy.Therefore, the IPN hydrogel performed better breaking strength and breaking elongation than PNIPAAm. Figure 16 shows the stretching process of the DN hydrogel which consisted of two polymer networks.
Secondly, the good tensile strength of IPN hydrogel was probably due to the introduction of the BTCA crosslinking agent in the preparation of fire-resistant hydrogel. The –OH group in the SA could be crosslinked by the –COOH group in BTCA (Fig. 17). The chemical crosslink between the –OH in the SA and the –COOH in the BTCA could limit the motion of SA in the interpenetrating network. This result led to a strong adhesion between PNIPAAm and SA polymer in the water matrix composite. Therefore, the above-mentioned chemical reactions would further enhance the strength of the synthesised fire-resistant IPN hydrogel.
Fire-resistant performance
To further understand the fire-resistant performance of hydrogel-fabric laminates, the vertical burning test was carried according to standard GB/T5455-2014. Figure 18 shows the visual vertical burning results of the natural cotton fabric and hydrogel–fabric laminates. As shown in Fig. 18, the natural cotton fabric combusted quickly and did not self-extinguish until the fabric was burned off. The damaged length of the natural cotton fabric could reach up to 30 cm, indicating its poor fire-resistant property. However, the hydrogel–fabric laminates did not burn at all when exposed to flame for 12 s in the vertical burning process. Only a char length of 0.8 cm was found in the hydrogel–fabric laminates, indicating great fire-resistant performance because of internal water content in the IPN hydrogel. When the hydrogel layer on the surface of the cotton fabric was exposed to flame, a large amount of energy would be absorbed by the IPN hydrogel as water heats up and water evaporates. Based on this method, prepared hydrogel–fabric laminates could successfully provide superior protection ability compared with natural cotton fabrics. Considering that the water content was limited in hydrogel, the flame retarding capability of hydrogel–fabric laminates may not be effective over long periods of time. To shield the skin from the flame for a long time as the fire-resistant material, the laminated thickness of water matrix composite hydrogel on the surface of the cotton fabric should be further increased under flame exposure condition.
Antibacterial activity evaluation
Considering that the fire-resistant hydrogel–fabric laminate can be used as firefighter-protective clothing, the synthesised hydrogel should be loaded with antimicrobial components to avoid wound infection when suffering from burn injuries. In the current work, Ag NPs embedded in IPN hydrogel were successfully synthesised and laminated on the surface of cotton fabrics. Ag NPs had gained considerable attention because of their broad inhibitory activity towards various bacterial species. As the loading amount of Ag NPs in the IPN hydrogel will influence the antibacterial activity, the Ag NPs content was investigated using the energy dispersive X-ray spectroscopy (EDX). Figure 19 depicts the EDX spectra of the synthesised fire-resistant hydrogel. The EDX spectrum of hydrogel displayed a strong peak at 22 and 25 keV, corresponding to the Ag element. This spectrum confirmed the presence of Ag NPs, and the loading amount of Ag NPs in the IPN hydrogel was approximately 14.6%.
In the present study, the inhibition zone and shaking flask methods were used to determine the antibacterial activity of the fire-resistant composite hydrogel using E. coli and S. aureus bacteria. For comparison, the IPN hydrogel without Ag NPs was produced under identical conditions. The zone of the inhibition test result for fire-resistant IPN hydrogels is given in Fig. 20. As shown in Fig. 20, no inhibition zone was observed around the hydrogel without Ag NPs, whereas the hydrogel containing Ag NPs displayed an evident inhibition zone. The results revealed that the synthesised fire-resistant hydrogel showed a great antibacterial performance against E. coli and S. aureus bacteria. This result also indicated that AgNO3 was successfully reduced to Ag NPs by the SA in the synthesised hydrogel. The antibacterial mechanism was primarily attributed to the released Ag+ ions from silver penetrated into the cell and reacted with cellular proteins and DNA, leading to bacterial cell death (Sandri et al. 2019; Pant et al. 2019).
The zone of inhibition test was only a qualitative method to determine antibacterial activity. This method cannot quantitatively analyse the antibacterial activity and showed bacterial reduction in percentage. In the current work, the shaking flask method was further used to investigate the bacterial reduction percentage of the fire-resistant hydrogel. Figure 21 shows the images of colony counts incubated in agar plates with dilutions of 104. In the present study, the antibacterial efficiency of the hydrogel containing Ag NPs was 96% against E. coli and 98% against S. aureus.
Conclusions
A novel fire-resistant material was produced by laminating a hydrogel and a natural cotton fabric. The achievement of ideal fire-resistant performances was primarily attributed to the water retained in the synthesised IPN hydrogel. The hydrogel that contained water would prevent the natural cotton fabric from burning when exposed to flame. Therefore, hydrogel–fabric laminates can protect the human skin from burn injuries as firefighter-protective clothing.
In addition, a new type of fire-resistant IPN hydrogel was prepared by combining PNIPAAm with SA. The two IPN had good compatibility and strong adhesion with each other. The LCST of the fire-resistant IPN hydrogel was detected at approximately 31 °C. To impart the antibacterial ability and avoid burn infection, a new green route was explored to incorporate Ag NPs into the hydrogel using SA as the reducing agent. The results indicated that the incorporation of Ag NPs into the synthesised hydrogel showed good antibacterial behaviour against S. aureus and E. coli bacteria. Furthermore, the FTIR, swelling ratio, XRD, TG, fire-resistant and the mechanism properties of IPN hydrogel were investigated. The results indicated that the hydrogel laminate displayed higher thermal degradation temperature and mechanical strength compared with PNIPAAm. The vertical burning results suggested that the hydrogel–fabric laminates did not burn at all when exposed to flame for 12 s, whereas the untreated cotton fabric was burn out. Thus, the synthesised IPN hydrogels can be potentially utilised as fire-resistant coating for fireproof clothing. A further work will be conducted to better understand the heat resistance and stored energy performance of the fire-resistant hydrogel–fabric laminates.
References
Aladpoosh R, Montazer M, Samadi N (2014) In situ green synthesis of silver nanoparticles on cotton fabric using Seidlitzia rosmarinus ashes. Cellulose 21:3755–3766. https://doi.org/10.1007/s10570-014-0369-1
Annalisa C, Francesca B, Giulio M, Chiara M, Monica P (2016) DNA-chitosan crosslinking and photografting to cotton fabrics to improve washing fastness of the fire-resistant finishing. Cellulose 23(6):3963–3984. https://doi.org/10.1007/s10570-016-1067-y
Bu YM, Zhang SY, Cai YJ, Yang YY, Ma ST, Huang JJ, Yang HJ, Ye DZ, Zhou YS, Xu WL, Gu SJ (2019) Fabrication of durable antibacterial and superhydrophobic textiles via in situ synthesis of silver nanoparticle on tannic acid-coated viscose textiles. Cellulose 26(3):2109–2122. https://doi.org/10.1007/s10570-018-2183-7
Cui YF, Xing ZJ, Yan J, Lu YH, Xiong XQ, Zheng LJ (2018) Thermosensitive behavior and super-antibacterial properties of cotton fabrics modified with a sercin-NIPAAm-AgNPs interpenetrating polymer network hydrogel. Polymers 10(8):1–14. https://doi.org/10.3390/polym10080818
Cui XF, Zheng WJ, Zou W, Liu XY, Yang H, Yan J, Gao Y (2019) Water-retaining, tough and self-healing hydrogels and their uses as fire-resistant materials. Polym Chem 10(37):5151–5158. https://doi.org/10.1039/c9py01015g
French AD (2017) Glucose, not cellobiose, is the repeating unit of cellulose and why that is important. Cellulose 24:4605–4609. https://doi.org/10.1007/s10570-017-1450-3
Gai HJ, Wu J, Wu CY, Sun XJ, Jia FJ, Yu YQ (2015) Synthesis and characterization of thermosensitive hydrogel with improved mechanical properties. J Mater Res 30(16):2400–2407. https://doi.org/10.1557/jmr.2015.233
Illeperuma WRK, Rothemund P, Suo ZG, Vlassak JJ (2016) Fire-resistant hydrogel-fabric laminates: a simple concept that may save lives. ACS Appl Mater Interfaces 8(3):2071–2077. https://doi.org/10.1021/acsami.5b10538
Krakovsky I, Kourilova H, Hrubovsky M, Labuta J, Hanykova L (2019) Thermoresponsive double network hydrogels composed of poly (N-isopropylacrylamide) and polyacrylamide. Eur Polym J 116:415–425. https://doi.org/10.1016/j.eurpolymj.2019.04.032
Li XM, Zhang KK, Shi R, Ma XM, Tan LW, Ji Q, Xia YZ (2017) Enhanced flame-retardant properties of cellulose fibers by incorporation of acid-resistant magnesium-oxide micro-capsules. Carbohydr Polym 176:246–256. https://doi.org/10.1016/j.carbpol.2017.08.096
Liu MS, Huang S, Zhang GX, Zhang FX (2019) Synthesis of P-N–Si synergistic flame retardant based on a cyclodiphosphazane derivative for use on cotton fabric. Cellulose 26(12):7553–7567. https://doi.org/10.1007/s10570-019-02608-5
Liu Y, Zhao JC, Zhang CJ, Cui L, Guo Y, Zhu P, Zhang H, Zheng ZW, Wang DY (2017) Flame retardancy and thermal degradation properties of cotton/alginate fabric. J Therm Anal Calorim 127(2):1543–1551
Pant B, Park M, Park SJ (2019) One-step synthesis of silver nanoparticles embedded polyurethane nano-fiber/net structured membrane as an effective antibacterial medium. Polymers 11(7):1–11. https://doi.org/10.3390/polym11071185
Pallmann J, Ren YL, Mahltig B, Huo TG (2019) Phosphorylated sodium alginate/APP/DPER intumescent flame retardant used for polypropylene. J Appl Polym Sci 136(29):1–10. https://doi.org/10.1002/app.47794
Rac-Rumijowska O, Fiedot M, Karbownik I, Suchorska-WozniakP TH (2017) Synthesis of silver nanoparticles in NMMO and their in situ doping into cellulose fibers. Cellulose 24:1355–1370. https://doi.org/10.1007/s10570-016-1168-7
Sandri G, Miele D, Faccendini A, Bonferoni MC, Rossi S, Grisoli P, Taglietti A, Ruggeri M, Bruni G, Vigani B, Ferrari F (2019) Chitosan/glycosaminoglycan scaffolds: the role of silver nanoparticles to control microbial infections in wound healing. Polymers 11(7):1–1. https://doi.org/10.3390/polym11071207
Thangaraj V, Mahmud S, Li W, Yang F, Liu HH (2018) Greenly synthesised silver-alginate nanocomposites for degrading dyes and bacteria. IET Nanobiotechnol 12(1):47–51. https://doi.org/10.1049/iet-nbt.2017.0074
Wang BX, Wu XL, Li J, Hao X, Lin J, Cheng DH, Lu YH (2016) Thermosensitive behavior and antibacterial activity of cotton fabric modified with a chitosan-poly (N-isopropylacrylamide) interpenetrating polymer network hydrogel. Polymers 8(4):1–14. https://doi.org/10.3390/polym8040110
Wang BX, Zhang S, Wang YF, Si B, Cheng DH, Liu L, Lu YH (2019a) Regenerated antheraea pernyi silk fibroin/poly(N-isopropylacrylamide) thermosensitive composite hydrogel with improved mechanical strength. Polymers 11(2):1–14. https://doi.org/10.3390/polym11020302
Wang S, Du XS, Deng S, Fu XH, Du ZL, Cheng X, Wang HB (2019b) A polydopamine-bridged hierarchical design for fabricating flame-retarded, superhydrophobic, and durable cotton fabric. Cellulose 26(11):7009–7023. https://doi.org/10.1007/s10570-019-02586-8
Wang Y, Joshee N, Cao WX, Wu QL, Tahir N (2019c) Continuous hydrogel production by dark and photo co-fermentation using a tubular multi-cycle bio-reactor with paulownia biomass. Cellulose 26(15):8429–8438. https://doi.org/10.1007/s10570-019-02468-z
Xue CH, Zhang L, Wei PB, Jia ST (2016) Fabrication of superhydrophobic cotton textiles with flame retardancy. Cellulose 23(2):1471–1480. https://doi.org/10.1007/s10570-016-0885-2
Xu P, Shao PY, Zhang Q, Cheng W, Li ZC, Li Q (2019) A Novel inherently flame-retardant composite based on zinc alginate/nano-Cu2O. Polymers 11(10):1–12. https://doi.org/10.3390/polym11101575
Xu QB, Xie LJ, Diao HLN, Li F, Zhang YY, Fu FY, Liu XD (2017) Antibacterial cotton fabric with enhanced durability prepared using silver nanoparticles and carboxymethyl chitosan. Carbohydr Polym 177:187–193. https://doi.org/10.1016/j.carbpol.2017.08.129
Zhang WL, Liu X, Wang J, Tang JD, Hu J, Lu TQ, Suo ZG (2018) Fatigue of double-network hydrogels. Eng Fract Mech 187:74–93. https://doi.org/10.1016/j.engfracmech.2017.10.018
Funding
This research was supported from the Opening Project of Key Laboratory of High Performance fibers and products (Ministry of Education), and undergraduate innovation and entrepreneurship training program from Hubei Province (S201910495027; S201910495070).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, Z., Suryawanshi, A., He, H. et al. Preparation and characterisation of fire-resistant PNIPAAm/SA/AgNP thermosensitive network hydrogels and laminated cotton fabric used in firefighter protective clothing. Cellulose 27, 5391–5406 (2020). https://doi.org/10.1007/s10570-020-03146-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03146-1