Abstract
A novel reactive flame retardant with two phosphoric acid ester groups and one ammonium phosphorus acid group, the ammonium salt of 2,7-bis(dimethoxyphosphoryl)-3,7-dimethyl-3-phosphonic acid (FR–LO), was synthesized. The treated cotton fabrics displayed a high flame retardancy and excellent durability because the FR–LO molecule contains two phosphoric acid ester groups without an ammonium ion that can be eliminated and decrease the flame retardancy. The cotton fabrics treated with 30% FR–LO had limiting oxygen indexes of 43.4, and 29.7 after 50 laundry cycles according to AATCC 61–2013 2A standard. Scanning electron microscope images and energy-dispersive X-ray spectroscopy showed that the treated cotton fibers retained their complete structure after combustion, and nitrogen and phosphorus were present in the treated cotton fibers. Thermogravimetric and thermogravimetric-infrared analyses showed that the treated cotton produced more carbon residue and released fewer combustible gases during thermal decomposition. Microscale combustion calorimetry showed that the heat release rate and total heat release of the treated cotton during combustion were much lower than those of pre-treated cotton. The stiffness of cotton treated with FR–LO increased, and its tensile strength decreased compared with pre-treated cotton.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton fabrics have good hygroscopicity, anti-static properties, and comfort for personal wear (Sun et al. 2020; Zhu et al. 2020); however, cotton fabric burns easily and vigorously when ignited and can cause casualties and property damage (Atakan et al. 2019; Li et al. 2020). Therefore, cotton fabrics are often treated with flame retardants that typically contain halogens, phosphorus, nitrogen, boron, or silicon. Other specialty flame-retardant materials include graphite and alkaline earth metallic compounds (Molaba et al. 2016; Salmeia et al. 2016a). Halogen-containing flame retardants are highly effective at capturing high-energy free radicals during thermal decomposition process, but they have been banned in many countries and regions because they release carcinogenic and toxic compounds (van der Veen and de Boer 2012). Boron-containing flame retardants often lack durability and are inefficient (Di Blasi et al. 2007), and while alkaline earth metal compounds have satisfactory flame resistance, they require high weight loadings, which affects the substrate properties(Costes et al. 2017; Li et al. 2010). Nitrogenous additives are often combined with other flame-retardant elements to improve their flame resistance (Xie et al. 2013; Zhang et al. 2017)
Recently, many researchers have investigated nanomaterials (e.g., clay, carbon nanotubes, and polyhedral oligomeric silsesquioxanes) as novel flame retardants (Decher and Hong 1991; Fabia et al. 2020; Fu et al. 2017; Jiang et al. 2018; Liang et al. 2013), because they can form continuous protective network layers that act as physical barriers to reduce flame spread (Kashiwagi et al. 2005). Although the heat release rate of treated samples is lower, other important fire parameters (e.g., time to ignition and total heat release) are not improved. Lignin is a kind of sustainable polymer with abundant reserves. Recently, it has been reported that lignin can be used as a synergistic agent to improve the flame retardancy of polymers (Lukawski et al. 2020; Song et al. 2011). But there is no evaluation of its durability. Phosphorous-containing flame retardants do not produce dust and have eco-friendly advantages, making them the most studied type of flame retardant (Nazir and Gaan 2020; Salmeia et al. 2016b). Proban and Pyrovatex CP are two typical organophosphorus flame retardants used in cotton fabrics (Horrocks 2013). However, cotton fabrics treated with these two flame retardants will release formaldehyde during usage. In recent years, many researchers have focused on the use of green flame retardants, and a wide range of source materials, including renewable biomass materials, have been investigated. Some bio-based substances such as deoxyribonucleic acid, casein, hydrophobin, and amino acids have potential applications as environment-friendly flame retardants (Alongi et al. 2013, 2014; Xu et al. 2019). However, cotton fabrics treated with these kind of flame retardants have poor durability.
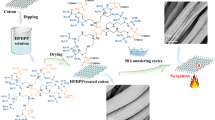
In our previous research, we developed several ammonium organic phosphorus acid flame retardants (Huang et al. 2019; Jia et al. 2017; Tian et al. 2019; Wan et al. 2019a; Xu et al. 2019). The cotton fabrics treated with these flame retardants passed 50 laundering cycle tests under AATCC 61–2013 1A standard (40 °C, 10 steel balls), but they did not pass the AATCC 61–2013 2A standard (49 °C, 50 steel balls), because while the flame retardants contain many ammonium phosphorus acid groups, not enough hydroxyl groups of cellulose were exposed because of the high crystallinity of the cotton fibers. Therefore, during laundering, the ammonium ions were easily replaced by metal ions such as Na+, Ca2+, and Mg2+, and the flame retardancy of the treated cotton fabrics decreased greatly. So, if a flame retardant molecule contains several phosphoric acid ester groups (which do not combine with metal ions) and a few ammonium phosphorus acid groups that will react with cellulose, the cotton fabrics treated with this kind of flame retardants will have a high durability and may meet the AATCC 61–2013 2A standard.
Linalool is a colorless, oily liquid from biomass with two alkenes that can be functionalized with two phosphoric acid ester groups via an addition reaction, while the hydroxyl group of linalool can be used to introduce an ammonium phosphorus acid group. In this research, a novel flame retardant for cotton fabrics was synthesized from linalool, phosphoric acid, and dimethyl phosphite. It is expected that the cotton fabrics treated with this flame retardant will have high flame retardancy and excellent durability without producing formaldehyde.
Experimental section
Materials
Cotton fabrics (159.91 g/m2) were obtained from Jinzhou Rongshun Textile Co., Ltd. (Hebei, China). Linalool and dimethyl phosphite were procured from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). Phosphoric acid, sodium hydroxide, xylene and ethanol were purchased from Chongqing Chuandong Chemical Co., Ltd. (Chongqing, China). Urea and dicyandiamide were supplied by Chengdu Kelong Chemical Co., Ltd. (Sichuan, China). All reagents were used as received.
Pretreatment of cotton fabrics
Cotton fabrics were dipped into a 25 wt% sodium hydroxide solution at 25 °C for 5 min with a bath ratio 1:20. Then, the wet cotton fabrics were washed with a large amount of water to remove excess sodium hydroxide. The pre-treated cotton fabrics were dried in an oven at 110 °C for later use. (The pretreatment of cotton fabric with sodium hydroxide solution can transform part of the crystalline cellulose into amorphous cellulose (mercerization), thereby increasing the number of reaction sites and improving the graft rate.)
Flame retardant synthesis
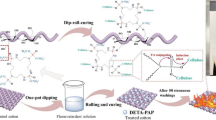
The reactions used to synthesize the ammonium salt of 2, 7-bis(dimethoxyphosphoryl)-3,7-dimethyl-3-phosphonic acid (FR–LO) are illustrated in Scheme 1. Linalool (1, 23.1 g, 0.15 mol), phosphoric acid (2, 16.7 g, 0.17 mol) and xylene (100 ml) were added to a 250 mL three-neck flask equipped with a magnetic stirrer, a thermometer, a Dean-Stark trap, and a reflux condenser. The mixture was heated to reflux at 125 °C for 4 h. At the end of the reaction, an obvious stratification occurred between the orange oily product and the light yellow solvent. The product was separated from the solvent by liquid separation. Then the intermediate, dimethyl phosphite (3, 33.0 g, 0.30 mol) and 50 ml distilled water were poured into a three-neck flask with a magnetic stirrer, a thermometer, and a reflux condenser. The reaction lasted for 3 h at 65 °C. Then, the product was poured into a large beaker. Urea (4, 18.0 g, 0.3 mol) was also added to the beaker, and the mixture was stirred at 140 °C until it reached a neutral pH. The crude product was purified by ethanol precipitation and dried in an oven at 60 °C.
The structure of FR–LO was characterized by proton nuclear magnetic resonance (1H NMR), carbon-13 nuclear magnetic resonance (13C NMR), and phosphorus-31 nuclear magnetic resonance (31P NMR) spectroscopy, as follows:
1H NMR results (D2O, 600 MHz) δ (ppm): 0.55, 0.67 (s, CH, H2), 2.72 (s, 3CH2, H4, H5, H6); 3.03 (s, 4CH3, H1, H8, H9, H10); 3.41(s, 4O–CH3, H11, H12, H13, H14); 4.93(D2O); 13C NMR results (D2O, 600 MHz) δ (ppm): 15.29 (s, C1, C8, C9, C10, C11, C12, C13, C14); 16.52 (s, C4, C5, C6), 57.24 (s, C2), 61.66 (s, C3, C7); 31P NMR results (D2O, 600 MHz) δ (ppm): −0.63 (s, P1); 4.70 (s, P2); 7.06 (s, P3). (Supplementary Material, Fig. S1, S2, S3).
Flame retardant finishing of cotton fabrics
FR–LO solutions with different concentrations (15 wt%, 20 wt%, and 30 wt%) were prepared, and each solution contained 5 wt% dicyandiamide as a catalyst. Pretreated cotton fabrics were immersed in these solutions at 70 °C for 40 min with a bath ratio 1:20. The cotton fabrics were then padded to reach a wet pickup of about 100% and baked in an automatic continuous-shaping dryer at 180 °C for 5 min. Finally, the treated cotton fabrics were washed with distilled water, and dried at 110 °C in an oven. The reaction between cellulose and FR–LO is illustrated by Scheme 2.
The weight gain (WG) of the cotton fabrics after treatment can be calculated by Eq. (1):
where W1 and W2 represent the weight of the cotton fabric before and after treatment, respectively.
Measurements
Nuclear magnetic resonance (NMR)
The structural analyses of the FR–LO (1H, 13C, and 31P) were conducted on a Bruker AVIII 600 MHz liquid nuclear magnetic resonance spectrometer (USA) with deuterium oxide (D2O) as a solvent. The 31P chemical shifts are reported in δ relative to external 85% aqueous phosphoric acid (H3PO4).
Scanning electron microscope (SEM)
The surface morphologies of the control cotton fibers, the pre-treated cotton fibers and the cotton fibers treated with 30% FR–LO before and after combustion were observed using a Phenom Prox Holland SEM with magnifications of 1000 × , 3000 × , and 5000 × , and a voltage of 10 kV. All samples weresputtered with gold.
Energy-dispersive X-ray spectroscopy (EDS)
The elemental compositions and atomic contents of the pre-treated cotton fibers and the cotton fibers treated with 30% FR–LO before and after combustion and after 50 LCs were tested using an energy-dispersive X-ray spectroscopy (JEOL-6300F) with a voltage of 15 kV.
Fourier transform infrared spectroscopy (FT-IR)
The functional groups and chemical bonds of the pre-treated cotton fibers, the cotton fibers treated with 30% FR–LO and the FR–LO powders were analyzed with an FT-IR (PE Co., USA) at wavenumbers from 4000 cm−1 to 400 cm−1 and a resolution of 4.0 cm −1. The potassium bromide tablet method was used in the infrared spectrum analysis of this experiment.
Durability tests
The durability of the cotton fabrics treated with different concentrations of FR–LO were tested according to the AATCC 61–2013 2A standard method (The washing temperature: 49 °C; the amount of detergent: 150 mL, 0.15%; the number of steel balls: 50; the washing time: 45 min, the effect of five typical hand or home washes on the fabric can be simulated in a 45-min test.)
Limiting oxygen index (LOI) and vertical flammability tests (VFT)
The flame retardancy of the pre-treated cotton fabric and the cotton fabric treated with different concentrations of FR–LO were tested using an M606B digital display oxygen index apparatus (Qingdao Shanfang Instrument Co. Ltd., China) according to the ASTMD2863-2000 standard, and a YG815B vertical fabric flame-retardancy tester (Nantong Sansi Electromechanical Science and Technology Co. Ltd., China) in accordance with the ASTM D6413-99 standard.
Thermogravimetric analysis (TGA)
TGA was conducted on a Pyris 1 TGA (PerkinElmer, USA). The mass losses of the pre-treated cotton and cotton treated with 30% FR–LO were measured using 3–5 mg samples heated from 40 to 700 °C at a heating rate of 20 K/min under a nitrogen atmosphere.
TGA-FTIR
TGA–FTIR analyses were conducted on a Netzsch STA 409 PC thermal analyzer coupled with a Bruker Tensor 27 FTIR spectrometer via a Teflon tube. The analyses were conducted using 3–5 mg samples heated from 40 to 700 °C at a heating rate of 20 K/min under a nitrogen atmosphere. The wavenumber range was 4000–600 cm−1 with a 4.0 cm−1 resolution and 4 scans.
Microscale combustion calorimetry (MCC)
The thermal properties of the cotton fabrics treated with different concentrations of FR–LO were measured on a FTT001 micro fire testing equipment (Fire Testing Technology Ltd, HL, UK) according to ASTM E1354 standard. Samples were heated in an atmosphere in which the ratio of nitrogen-to-oxygen was 4–1 from 100 to 700 °C at a heating rate of 1 K/s.
Bending lengths and tensile strengths
The stiffness and tensile strengths of the cotton fabrics treated with different concentrations of FR–LO were evaluated using a YG (B) 022D fabric stiffness tester (Wenzhou Darong Textile Machinery Co. Ltd, Zhejiang, China) according to the ASTM D 1388–96(2002) standard and an electronic fabric tension tester (HD026N, Nantong Hongda Experiment Instruments Co. Ltd., China) according to the ASTM 5035-2006 standard, respectively.
X-ray diffraction (XRD)
The XRD patterns of the pre-treated cotton and the cotton treated with 30% FR–LO were evaluated using a Rigaku XD-3 wideangle diffractometer (Beijing Purkinje General Instrument Co. Ltd., Beijing, China) at 36 kV and 20 mA. The diffractogram scattering angle ranged from 5 to 50° with a step size of 0.02° (λ = 0.154 nm).
X-ray photoelectron spectroscopy (XPS)
The elemental compositions of the pre-treated cotton and the cotton treated with 30% FR–LO were measured by X-ray photoelectron spectrometer (Thermo Fisher Scientific K-Alpha, USA) with a Al Ka excitation radiation (hv = 1253.6 eV). The working voltage is 15 kV. The test passing-energy is 50 eV, step length is 0.05 eV.
Results and discussion
SEM–EDS
The surface morphologies of samples were observed using SEM, and the corresponding micrographs are presented in Fig. 1. The control cotton fibers (Fig. 1 a, b, c) showed a spiral structure with many distinct folds (Jia et al. 2017), while the pre-treated cotton fibers (Fig. 1 d, e, f) had columnar structures, because the cotton fabrics were modified through mercerization. (Tarbuk et al. 2014). The surface morphologies of cotton fibers treated with 30% FR–LO (Fig. 1 g, h, i) were similar to those of pre-treated cotton fibers, and no obvious coating was observed on the surfaces. This implied that FR–LO entered the inner spaces of the cotton fibers and grafted to cellulose (Huang et al. 2019). After combustion, the treated cotton fibers (Fig. 1 j, k, l) retained their complete structures and lots of bubbles remained on the cotton fiber surface. This was because FR–LO released phosphoric acid, which promoted formation of cellulose char during combustion (Zhao et al. 2016). Ammonia released by FR–LO during combustion diffused from the fiber interior to the exterior to form dense bubbles on the fiber surfaces (Feng et al. 2017).
SEM images of the control cotton fibers a, b, c; pre-treated cotton fibers d, e, f; cotton fibers treated with 30% FR–LO g, h, i; and treated cotton fibers after combustion j, k, l at magnifications of 1000 × a, d, g, j, 3000 × b, e, h, k, and 5000 × c, f, i, l. Images with the same magnification share the same scale bars
The elemental compositions and atomic contents of samples were measured using EDS, and the corresponding data are recorded in Fig. 2 and Table 1. Only carbon and oxygen elements were detected in the pre-treated cotton fibers, and their atomic ratio matched that of cellulose (Klemm et al. 2005). In addition to carbon and oxygen, nitrogen and phosphorus were detected in fibers treated with FR–LO, treated fibers after 50 LCs, and treated fibers after combustion. The introduction of nitrogen and phosphorus in the cotton fibers showed that FR–LO was successfully grafted to the cotton fabrics, and the phosphorus content of the treated cotton fibers did not significantly decrease after 50 LCs. This demonstrated the excellent durability of the treated cotton fibers.
FT-IR spectra
The FT-IR spectra of samples are shown in Fig. 3, and the data corresponding to the characteristic absorption peaks of each sample are listed in Table 2. The FT-IR spectra agreed with the structure of FR–LO. P = O (Thach-Mien et al. 2013), P–O–C (Hu et al. 2019), and P–N (Zhang et al. 2019) bonds were found in the spectra of FR–LO and of cotton treated with 30% FR–LO, but not in pre-treated cotton. These results were consistent with the EDS results and indicate that FR–LO was successfully grafted onto cotton fabrics. And the spectra of cotton treated with 30% FR–LO after 50LCs showed the esters could hydrolyze. The P–O–C bond at about 1060 is weaker than it was before washing.
Vertical flammability, limiting oxygen index(LOI), and durability
The flame retardancy of cotton fabrics treated with different concentrations of FR–LO before and after laundering was analyzed by vertical flammability and LOI tests. The vertical flammability test images are shown in Fig. 4, and the parameters of the vertical flammability test and LOI tests are summarized in Table 3, 4, respectively. In the vertical flammability tests, the pre-treated cotton fabric burned down after the ignition source was removed, and smoldering lasted for 45 s without producing ash. However, the cotton fabrics treated with FR–LO did not burn, and there was no after-flame or after-glow. The length of the char frames decreased upon increasing the FR–LO concentration. The char lengths of the cotton fabrics treated with 20% and 30% FR–LO were only 39 mm and 38 mm, and after 50 LCs, they were 56 mm and 50 mm, respectively.
The LOI of the pre-treated cotton fabric was only 18.4. Compared with the pre-treated cotton fabric, the LOI of the cotton fabrics treated with various concentrations of FR–LO were greatly improved. The LOI of fabrics treated with 20% and 30% FR–LO, and after 50 LCs were still greater than 26, so the fabrics were considered to have permanent durability.
The fabrics treated with ordinary ammonium organic phosphorus acid flame retardant only passed the AATCC-61 2013 1A standard, while the one treated with FR–LO had a comparatively high durability and passed the AATCC-61 2013 2A standard. This was because there were two phosphoric acid ester groups and only one ammonium phosphorus acid group, while the former flame retardants only had ammonium phosphorus acid groups and no phosphoric acid ester groups. The ammonium phosphorus acid groups that did not react with cellulose lost their ammonium ions and combined with metal ions such as Na+, Ca2+, and Mg2+ during washing, and the treated cotton fabrics quickly lost their flame retardancy; however, the phosphoric acid esters in the FR–LO did not lose their flame retardancy, and the cotton fabrics treated with FR–LO had excellent durability that was as good as Proban and Pyrovatex CP (Supplementary Material, Table S1). No formaldehyde was used to synthesize FR–LO, and the cotton fabrics treated with the FR–LO did not release formaldehyde during use, making FR-LO a promising candidate for replacing Proban and Pyrovatex CP.
TGA
The thermal performance of pre-treated cotton and cotton treated with 30% FR–LO in nitrogen atmosphere was measured using TGA. The mass loss curves, the derivative thermogravimetry analyses (DTG) curves under a nitrogen atmosphere, and the corresponding data are shown in Fig. 5 and Table 5. The initial decomposition temperature of a material is generally considered to be the temperature at which 10% weight loss occurs. It could be seen from Fig. 5 that the pre-treated cotton and cotton treated with 30% FR–LO started to decompose at 349.7 °C, and 248.1 °C, respectively. Both of these substances underwent water evaporation, in which the cellulose lost its free and bound water before degradation. Pre-treated cotton rapidly degraded from 349.7–416.0 °C, and the most rapid weight loss occurred at 361.2 °C. Within this temperature range, the cellulose degraded to levoglucose, flammable hydrocarbons, carbonyl gases, CO, and CO2 (Thach-Mien et al. 2014; Wan et al. 2019a, 2019b). At 700 °C, the residual weight was only 8.61%. The cotton fibers treated with 30% FR–LO underwent three-step degradation similar to the pretreated cotton fibers in which they degraded rapidly from 248.1–291.2 °C, and the fastest weight loss rate occurred at 283.2 °C. At 700 °C, the residual weight was still 37.24%. After FR–LO treatment, the thermal cracking process of cotton fabric changed significantly. The decomposition temperature of cotton treated with 30% FR–LO was lower than that of the pre-treated cotton because FR–LO easily decomposed into phosphoric acid at lower temperatures, and the presence of phosphoric acid promoted the char formation (Xu et al. 2019; Zheng et al. 2018), This is the reason for the difference in TGA spectrum. Therefore, the residual weight of cotton treated with 30% FR–LO was much higher than that of untreated cotton, showing that FR–LO endowed the cotton fabrics with flame retardancy.
TGA-FTIR
The pyrolysis products of pre-treated cotton and cotton treated with 30% FR–LO were analyzed by TGA-FTIR to explore the flame retardancy mechanism of FR–LO. Three- and two-dimensional spectra of the gaseous volatiles of pre-treated cotton and cotton treated with 30% FR–LO under a nitrogen atmosphere are presented in Fig. S4 and Fig. 6. The data corresponding to the characteristic absorption peaks of each sample are listed in Table 6. Both figures showed that the absorption peaks of most of gases released by treated cotton were much weaker than those of pre-treated cotton, except those at 2304 cm−1 (CO2) and 1513 cm−1 (N–H). The N–H stretching vibration was found in the gas released by the treated cotton but not in pre-treated cotton, which suggests that FR–LO decomposed and released NH3 at high temperatures. In contrast to CO2 and NH3, which are non-combustible gases, treated cotton released far fewer combustible gases such as hydrocarbons (C–H), carbonyl compounds (C = O), and ether compounds (C–O–C) at high temperatures than pre-treated cotton. The results showed that FR–LO mainly exhibited a condensed phase flame retardancy mechanism, which decomposed phosphorous-containing substances before accelerating the dehydration of cellulose. This reduced the release of combustible gases and diluted combustible gases by releasing ammonia gas (Liu et al. 2020).
Microscale combustion calorimetry
The thermal properties of the cotton fabrics treated with different concentrations of FR–LO were examined by microscale combustion calorimetry. The corresponding parameters, such as the total heat release (THR), peak heat release rate (PHRR), and temperature of maximum HRR (Tmax) are presented in Table 7 and Fig. 7. The PHRR of pre-treated cotton fabric during combustion was 365.4 W/g, and 19.1 kJ/g of heat was released during the entire combustion process. However, the PHRR values of cotton treated with 15%, 20%, and 30% FR–LO were only 107.3 W/g, 91.2 W/g, and 53.8 W/g, respectively. The THR values were also much lower than those of untreated cotton, with values of 8.8 kJ/g, 5.5 kJ/g, and 3.9 kJ/g, respectively. The values of PHRR and THR decreased upon increasing the FR–LO concentrations. The Tmax values of cotton fabrics treated with FR–LO were lower than those of untreated cotton, which was consistent with the TGA results.
Physical properties
To explore the stiffness and tensile strengths of the cotton fabrics treated FR–LO, the bending lengths and the tensile strengths of samples were tested, and the results are listed in Table 8. The bending lengths of the cotton fabrics increased upon increasing the FR–LO concentration, and at 30% FR–LO, the warp and weft bending lengths increased by 36% and 28%, respectively. The results indicated that the cotton fabrics treated by FR–LO were somewhat harder than pre-treated cotton, but they were still soft enough to be used in textiles. The tensile strengths of the cotton fabrics decreased upon increasing the FR–LO concentration. When 30% FR–LO was used, the warp and weft tensile strengths decreased by 33.1% and 28.4%, respectively, and the tensile strengths also decreased. Since FR–LO is neutral, the physical properties of the cotton fabrics treated with FR–LO did not significantly change. In addition, the bending lengths of the samples after 50 LCs were shorter than that of the treated samples and the tensile strengths of the samples after 50 LCs were stronger.
XRD
In order to determine the effect of FR–LO on the crystal structure of cellulose, XRD analysis was used to test it, and the result is presented in Fig. 8. The peaks of raw cotton at 15.15° correspond to the (1–10) plane; the peaks at 16.88° are attributed to the (110) plane; the peaks at 22.88° are attributed to the (200) plane; and the peaks at 34.69° correspond to the (004) plane of cellulose I. After pretreatment with sodium hydroxide, the diffraction peak strength of the corresponding cellulose I decreases. In addition, new diffraction peaks appeared, the peak at 22.53° is probably a composite of the cellulose I (200) reflection and the cellulose II (020) reflections. The increased peak height at about 20 deg from I to II is due to adding some (1 1 0) cellulose II intensity to the (012) and (102) cellulose I peak. The peak shape and curve trend of the cotton treated with FR–LO is similar to that of pre-treated cotton, but the corresponding strength are all lower than that of pre-treated cotton. There was less cellulose in the sample as perhaps the primary cause of the lower peak compared with the pre-treated cotton. This result suggests that sodium hydroxide will cause the changed the allomorph of cellulose crystal while FR-LO will not.
XPS
XPS test results of pre-treated cotton and cotton treated with 30% FR–LO are shown in Fig. 9, 10 Table 9. As shown in Fig. 9, only C and O elements were detected in the pre-treated cotton while C, O, N and P elements were detected in the cotton treated with 30% FR–LO, which was consistent with EDS test. Further analysis of the P2p peak of cotton before and after finishing revealed that there was no P2p peak in the pre-treatment cotton, but there were two peaks in the cotton treated with 30% FR–LO, PO22− at 133.4 eV and P(= O) –O–C at 134.8 eV, respectively. The results showed that phosphorous in flame retardants could react with cellulose to form P = O and P–O–C bonds, which was consistent with the results of FT–IR analysis.
Conclusion
In this work, a novel flame retardant with only one reactive ammonium phosphorus acid group and other phosphoric acid ester groups was synthesized without formaldehyde. The LOI of the cotton fabrics treated with 30% FR-LO reached 43.4 and remained at 29.7 after 50 LCs according to AATCC 61–2013 2A standard, and the treated cotton fabrics exhibited very high flame retardancy and excellent durability. The results of VFT and SEM results showed that the treated cotton maintained an intact structure and formed char after combustion. Thermal performance measurements such as TGA, TGA-FTIR and MCC showed that cotton treated with FR–LO decomposed in advance at high temperature and released only a small amount of combustible gases and heat compaared with pre-treated cotton. Cotton treated with FR–LO had a slightly higher hardness and a lower tensile strength than pre-treated cotton. Replacing ammonium phosphorus acid groups with phosphate ester groups in the cotton fabric flame retardants greatly increased the durability of the treated cotton fabrics.
References
Alongi J, Carletto RA, Di Blasio A, Carosio F, Bosco F, Malucelli G (2013) DNA: a novel, green, natural flame retardant and suppressant for cotton. J Mater Chem A 1:4779–4785. https://doi.org/10.1039/c3ta00107e
Alongi J, Di Blasio A, Cuttica F, Carosio F, Malucelli G (2014) Bulk or surface treatments of ethylene vinyl acetate copolymers with DNA: Investigation on the flame retardant properties. Eur Polym J 51:112–119. https://doi.org/10.1016/j.eurpolymj.2013.12.009
Atakan R, Bical A, Celebi E, Ozcan G, Soydan N, Sarac AS (2019) Development of a flame retardant chemical for finishing of cotton, polyester, and CO/PET blends. J Ind Text 49:141–161. https://doi.org/10.1177/1528083718772303
Costes L, Laoutid F, Brohez S, Dubois P (2017) Bio-based flame retardants: When nature meets fire protection. Mater Sci Eng R Rep 117:1–25. https://doi.org/10.1016/j.mser.2017.04.001
Decher G, Hong JD (1991) Buildup of ultrathin multilayer films by a self-assembly process.1. Consecutive adsorption of anionic and cationic bipolar amphiphiles on charged surfaces. Makromol Chem Macromol Symp 46:321–327. https://doi.org/10.1002/masy.19910460145
Di Blasi C, Branca C, Galgano A (2007) Flame retarding of wood by impregnation with boric acid-pyrolysis products and char oxidation rates. Polym Degrad Stab 92:752–764. https://doi.org/10.1016/j.polymdegradstab.2007.02.007
Fabia J, Gawlowski A, Rom M, Slusarczyk C, Brzozowska-Stanuch A, Sieradzka M (2020) PET fibers modified with cloisite nanoclay. Polymers. https://doi.org/10.3390/polym12040774
Feng Y, He C, Wen Y, Ye Y, Zhou X, Xie X, Mai Y-W (2017) Improving thermal and flame retardant properties of epoxy resin by functionalized graphene containing phosphorous, nitrogen and silicon elements. Compos A 103:74–83. https://doi.org/10.1016/j.compositesa.2017.09.014
Fu Q, Medina L, Li Y, Carosio F, Hajian A, Berglund LA (2017) Nanostructured wood hybrids for fire-retardancy prepared by clay impregnation into the cell wall. ACS Appl Mater Interfaces 9:36154–36163. https://doi.org/10.1021/acsami.7b10008
Horrocks AR (2013) Textile flammability research since 1980-Personal challenges and partial solutions. Polym Degrad Stab 98:2813–2824. https://doi.org/10.1016/j.polymdegradstab.2013.10.004
Hu X, Yang H, Jiang Y, He H, Liu H, Huang H, Wan C (2019) Facile synthesis of a novel transparent hyperbranched phosphorous/nitrogen-containing flame retardant and its application in reducing the fire hazard of epoxy resin. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2019.120793
Huang S, Zhong L, Li S, Liu M, Zhang Z, Zhang F, Zhang G (2019) A novel monosodium-glutamate-based flame retardant containing phosphorus for cotton fabrics. Cellulose 26:2715–2728. https://doi.org/10.1007/s10570-018-02241-8
Jia Y, Hu Y, Zheng D, Zhang G, Zhang F, Liang Y (2017) Synthesis and evaluation of an efficient, durable, and environmentally friendly flame retardant for cotton. Cellulose 24:1159–1170. https://doi.org/10.1007/s10570-016-1163-z
Jiang S-D, Tang G, Chen J, Huang Z-Q, Hu Y (2018) Biobased polyelectrolyte multilayer-coated hollow mesoporous silica as a green flame retardant for epoxy resin. J Hazard Mater 342:689–697. https://doi.org/10.1016/j.jhazmat.2017.09.001
Kashiwagi T, Du FM, Douglas JF, Winey KI, Harris RH, Shields JR (2005) Nanoparticle networks reduce the flammability of polymer nanocomposites. Nat Mater 4:928–933. https://doi.org/10.1038/nmat1502
Klemm D, Heublein B, Fink HP, Bohn A (2005) Cellulose: Fascinating biopolymer and sustainable raw material. Angew Chem Int Ed 44:3358–3393. https://doi.org/10.1002/anie.200460587
Li Y-C et al (2010) Flame retardant behavior of polyelectrolyte-clay thin film assemblies on cotton fabric. ACS Nano 4:3325–3337. https://doi.org/10.1021/nn100467e
Li S, Huang S, Xu F, Zhao T, Zhang F, Zhang G (2020) Imparting superhydrophobicity and flame retardancy simultaneously on cotton fabrics. Cellulose 27:3989–4005. https://doi.org/10.1007/s10570-020-03041-9
Liang S, Neisius NM, Gaan S (2013) Recent developments in flame retardant polymeric coatings. Prog Org Coat 76:1642–1665. https://doi.org/10.1016/j.porgcoat.2013.07.014
Liu S, Wan C, Chen Y, Chen R, Zhang F, Zhang G (2020) A novel high-molecular-weight flame retardant for cotton fabrics. Cellulose 27:3501–3515. https://doi.org/10.1007/s10570-020-03020-0
Lukawski D, Grzeskowiak W, Lekawa-Raus A, Widelicka M, Lisiecki F, Dudkowiak A (2020) Flame retardant effect of lignin/carbon nanotubes/potassium carbonate composite coatings on cotton roving. Cellulose 27:7271–7281. https://doi.org/10.1007/s10570-020-03270-y
Molaba TP, Chapple S, John MJ (2016) Aging studies on flame retardant treated lignocellulosic fibers. J Appl Polym Sci. https://doi.org/10.1002/app.44175
Nazir R, Gaan S (2020) Recent developments in P(O/S)-N containing flame retardants. J Appl Polym Sci. https://doi.org/10.1002/app.47910
Salmeia KA, Gaan S, Malucelli G (2016a) Recent advances for flame retardancy of textiles based on phosphorus chemistry. Polymers. https://doi.org/10.3390/polym8090319
Salmeia KA, Jovic M, Ragaisiene A, Rukuiziene Z, Milasius R, Mikucioniene D, Gaan S (2016b) Flammability of cellulose-based fibers and the effect of structure of phosphorus compounds on their flame retardancy. Polymers. https://doi.org/10.3390/polym8080293
Song P, Cao Z, Fu S, Fang Z, Wu Q, Ye J (2011) Thermal degradation and flame retardancy properties of ABS/lignin: Effects of lignin content and reactive compatibilization. Thermochim Acta 518:59–65. https://doi.org/10.1016/j.tca.2011.02.007
Sun L et al (2020) Preparation of a novel flame retardant containing triazine groups and its application on cotton fabrics. New J Chem 44:7386–7394. https://doi.org/10.1039/c9nj06268h
Tarbuk A, Grancaric AM, Leskovac M (2014) Novel cotton cellulose by cationisation during the mercerisation process-part 1: chemical and morphological changes. Cellulose 21:2167–2179. https://doi.org/10.1007/s10570-014-0245-z
Thach-Mien N, Chang S, Condon B, Slopek R, Graves E, Yoshioka-Tarver M (2013) Structural effect of phosphoramidate derivatives on the thermal and flame retardant behaviors of treated cotton cellulose. Ind Eng Chem Res 52:4715–4724. https://doi.org/10.1021/ie400180f
Thach-Mien N, Chang S, Condon B (2014) The comparison of differences in flammability and thermal degradation between cotton fabrics treated with phosphoramidate derivatives. Polym Adv Technol 25:665–672. https://doi.org/10.1002/pat.3268
Tian P, Lu Y, Wang D, Zhang G, Zhang F (2019) Solvent-free synthesis of silicon-nitrogen-phosphorus flame retardant for cotton fabrics. Cellulose 26:6995–7007. https://doi.org/10.1007/s10570-019-02554-2
van der Veen I, de Boer J (2012) Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88:1119–1153. https://doi.org/10.1016/j.chemosphere.2012.03.067
Wan C, Liu M, Tian P, Zhang G, Zhang F (2019a) Renewable vitamin B5 reactive N-P flame retardant endows cotton with excellent fire resistance and durability. Cellulose. https://doi.org/10.1007/s10570-019-02886-z
Wan C, Tian P, Liu M, Zhang G, Zhang F (2019b) Synthesis of a phosphorus-nitrogen flame retardant endowing cotton with high whiteness and washability. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2019.111738
Xie K, Gao A, Zhang Y (2013) Flame retardant finishing of cotton fabric based on synergistic compounds containing boron and nitrogen. Carbohydr Polym 98:706–710. https://doi.org/10.1016/j.carbpol.2013.06.014
Xu F, Zhong L, Xu Y, Zhang C, Wang P, Zhang F, Zhang G (2019) Synthesis of three novel amino acids-based flame retardants with multiple reactive groups for cotton fabrics. Cellulose 26:7537–7552. https://doi.org/10.1007/s10570-019-02599-3
Zhang T, Liu W, Wang M, Liu P, Pan Y, Liu D (2017) Synthesis of a boron/nitrogen-containing compound based on triazine and boronic acid and its flame retardant effect on epoxy resin. High Perform Polym 29:513–523. https://doi.org/10.1177/0954008316650929
Zhang Z, Dong C, Liu J, Kong D, Sun L, Lu Z (2019) Preparation of a synergistic reactive flame retardant based on silicon, phosphorus and nitrogen and its application to cotton fabrics. Cellulose. https://doi.org/10.1007/s10570-019-02900-4
Zhao P, Liu S, Xiong K, Wang W, Liu Y (2016) Highly flame retardancy of cotton fabrics with a novel phosphorus/nitrogen/silicon flame-retardant treating system. Fibers Polym 17:569–575. https://doi.org/10.1007/s12221-016-5316-3
Zheng D, Zhou J, Wang Y, Zhang F, Zhang G (2018) A reactive flame retardant ammonium salt of diethylenetriaminepenta(methylene-phosphonic acid) for enhancing flame retardancy of cotton fabrics. Cellulose 25:787–797. https://doi.org/10.1007/s10570-017-1543-z
Zhu W, Yang M, Huang H, Dai Z, Cheng B, Hao S (2020) A phytic acid-based chelating coordination embedding structure of phosphorus-boron-nitride synergistic flame retardant to enhance durability and flame retardancy of cotton. Cellulose 27:4817–4829. https://doi.org/10.1007/s10570-020-03063-3
Funding
This work was supported by the “Natural Funds for Chongqing” (cstc2019jcyj-msxmX0412) and the “National Natural Science Foundation of China” (Grant No.21905233).
Author information
Authors and Affiliations
Contributions
SL: Software, data curation, writing—original draft, writing—review and editing, visualization, project administration. PW: Validation. YC: Formal analysis. CW: Investigation. GZ: Conceptualization, methodology, resources, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, S., Chen, Y., Wan, C. et al. A novel high durability flame retardant with phosphoric acid ester groups and a reactive ammonium phosphorus acid group for cotton fabrics. Cellulose 28, 2479–2493 (2021). https://doi.org/10.1007/s10570-020-03648-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03648-y