Abstract
A bio-based P–N synergistic flame retardant ammonium salt of d-Panthenol-tri (methylphosphonic acid) (ADPTMPA) was synthesized using a formaldehyde-free and solvent-free strategy for the highly efficient preparation of durable flame-retardant cotton. The ADPTMPA structure was verified by 1H NMR, 13C NMR and 31P NMR. FT-IR test revealed successful grafting of the flame retardant onto the cotton through P–O–C covalent bonding. The morphologies and structures of pure cotton and treated cotton were characterized by SEM and XRD, and the elemental compositions of cotton samples were analyzed by EDX. Volatile pyrolysis products of treated cotton were analyzed by TG-IR. Flame retardancy and washability of treated cotton were analyzed by limiting oxygen index (LOI) and vertical flammability tests (VFT). The LOIs of 40 wt% ADPTMPA treated cotton before and after 50 laundering cycles was determined as 46.5% and 35.8%, respectively. VFT showed the treated cotton exhibited no smoldering and continuous burning. All carbon lengths of treated cottons were less than 60 mm. The heat release rate and total heat release of treated cotton were decreased by 91.34% and 58.01% compared with pure cotton, respectively. There was 29.42 wt% carbon residual remaining when the treated cotton was exposed to heat flow of 35 kw/m2. The treated cotton exhibited excellent flame retardancy and washability. Additionally, the mechanical properties and whiteness of the treated cotton were well-maintained.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton cellulose is a kind of natural biomacromolecule with many excellent characteristics such as heat resistance, alkali resistance, and soft deformability (Lessan et al. 2011). However, cotton fabric possesses significant flammability characteristics, with combustion temperature in the range of 360–425 °C and limiting oxygen index (LOI) value of only 18% (Li et al. 2010). Many fire accidents have resulted from the burning of cotton fabric, so it is extremely urgent to develop eco-friendly flame retardants for treatment of cotton.
In recent years, the development of flame retardant has paid more attention to environmental issues. Halogen-free and environmental friendly flame retardants are of significant interest. In particular, natural biomass materials such as lignin (Ding et al. 2014), chitosan (Liu et al. 2017), starch (Choi et al. 2018), cyclodextrin (Veerappagounder et al. 2016), and itaconic acid (Hill et al. 2017) have been used to prepare various flame retardants with environmentally friendly, renewable, and biodegradable properties. Recently, Basta et al. used phosphorylated glucose to culture glucose bacilli and obtained bacterial cellulose which was used to manufacture flame retardant paper (Basta and El-Saied 2009). Qian Wei et al. prepared flame retardant cotton by treating with tea saponin using a coating method (Qian et al. 2015). S. Basak et al. designed a banana pseudostem sap to treat jute textiles and demonstrated improved jute flame retardant performance (Basak et al. 2018). These natural materials can replace traditional materials that are less degradable to alleviate problems related to the energy crisis and environmental pollution. Another area of active research and development is the use of biological materials as flame retardants, such as deoxyribonucleic acid (DNA), protein, and phytic acid. Phytic acid has a high phosphate content and can be combined with nitrogen and silicon compounds to improve the flame retardancy of cotton (Alongi and Malucelli 2012). Alongi et al. grafted DNA onto cotton fiber, and shows the LOI of the modified cotton fiber increased from 18 to 28% to reach the flame retardant standard (Alongi et al. 2014). Bosco et al. soaked fabric in a solution containing serum protein to form a coating to further improve the mechanical properties of the fabric and this coating can isolate oxygen in favour of the residual carbon rate increase of flame-retardant fabric (Bosco et al. 2013). However, these natural flame retardant materials either exhibit sub-optimal flame retardancy or have poor washable performance, limiting wide application.
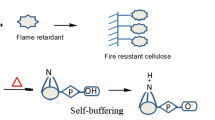
d-panthenol is widely distributed in plants and animals, with high amounts in foods such as milk and soybean milk.d-panthenol can be converted into pantothenic acid, which is involved in the metabolism of sugar, fat, and protein in the form of coenzyme (CoA) in the human body. In this work, the renewable d-panthenol (provitamin B5) was tested as a sustainable material and reacted with phosphoric acid under formaldehyde-free and solvent-free condition to obtain a novel N–P synergistic flame retardant, ADPTMPA. ADPTMPA was applied onto cotton cellulose, providing excellent flame retardancy and outstanding washability to the treated cotton fiber material. The treatment of the cotton is low-cost, eco-friendly, and safe to use. The mechanical properties and whiteness of the treated cotton are well sustained. This ADPTMPA flame retardant should be easily scaled for industrial application to treat combustible cotton textile to decrease fire risk.
Experimental
Materials
Cotton fabric was obtained from Chaotianmen Market (Chongqing, China), with a density of 126.89 g/m2.d-Panthenol (scientific name: dextro-N- (α-γ-dihydroxy-β, β-dimethylbutyryl) β-aminopropanol) (98%) was purchased from Aladdin Chemistry Industry Co. Ltd. (Shanghai, China). Urea and phosphoric acid H3PO4 (analytical reagent grade) were supplied by Chengdu Kelong Chemical Reagents Co. Ltd. (Chengdu, China). Dicyandiamide was acquired from Chongqing Chuandong Chemical Industry Co. Ltd. (Chongqing, China). All used reagents were analytical grade and used without further purification.
Methods
Preparation of flame retardant
To prepare the flame retanrdant, 0.05 mol (10.26 g) of d-Panthenol 1 and 0.15 mol (14.70 g) of phosphoric acid 2 were mixed in a 250 mL beaker equipped with mechanical stirring and a thermometer. The esterification reaction was conducted at 110 °C for 2.5 h to obtain a viscous white liquid intermediate 3. Then, 0.30 mol (18.00 g) urea 4 was added into solution 3 with constant stirring at 170 °C for 2 h to obtain a white fluid 5. The fluid product became solid when cooled to room temperature. Product 5 was washed with ethanol for purification, and then dried in an oven at 80 °C. The product ADPTMPA 5 was gained with a yield of 90.62%. The synthesis of ADPTMPA is displayed in Fig. 1.
The molecule structure of ADPTMPA 5 was determined using 1H NMR, 13C NMR, and 31P NMR spectral analysis. The detailed attribution is as follows:
1H NMR (D2O, 600 MHz) δ(ppm): 4.71(D2O), 3.69 (s, H1), 0.79 (s, H3), 3.86 (s, H4), 2.94 (t, H6), 1.90 (m, H7), 1.60 (m, H8), 3.60 (t, H9); 13C NMR (D2O, 600 MHz) δ (ppm):62.62 (t, C1), 163.75 (s,C2), 16.32 (q, C3), 68.42 (d, C4), 163.75 (s, C5), 36.95 (t,C7), 27.16 (t, C8), 59.91 (t, C9); 31P NMR (D2O, 600 MHz) δ(ppm): 0.98, 0.70 and 0.20.
Coating of cotton fabric
The prepared flame retardant ADPTMPA was dissolved in distilled water at concentrations of 20 wt%, 30 wt%, and 40 wt% ADPTMPA solution. Next, 10 wt% dicyandiamide 6 was added to the above solutions as a catalyst to accelerate the grafting reaction between ADPTMPA and cotton cellulose. Pure cotton was cleaned and dried and then samples were impregnated in the prepared solutions using a bath ratio of 1:20 (cotton weight: volume of the solution) at 70 °C for 2 h in a constant temperature oscillator. Subsequently, the treated cotton fabric was heated in a continuous setting dryer at 180 °C for 5 min, and the material was then rinsed with distilled water and finally dried to a constant weight at 110 °C in an oven. The grafting reaction of ADPTMPA and cotton is shown in Fig. 2.
The weight gain rate (WG wt%) of ADPTMPA added to cotton fabric was calculated according to Eq. (1):
Wa and Wb represent the weights of ADPTMPA-treated cotton and pure cotton, respectively.
Characterization
The structure of the ADPTMPA flame retardant was confirmed by 1H NMR, 13C NMR, and 31P NMR spectra (Bruker AV III 128, USA, 600-MHz) using deuterium oxide (D2O) as the solvent and tetramethylsilane (TMS) as an internal standard agent. Fourier transform infrared (FT-IR) spectra of ADPTMPA, treated cotton, and pure cotton were determined using a Spectrum GX apparatus (PE Co., USA) using KBr pressed-disk technique at a scanning range of 500–4000 cm-1with a resolution of 4.0 cm−1.
LOI testing was evaluated on a M606B digital oxygen index apparatus according to the ASTM D2863-2000 standard. Vertical flammability test was performed on a YG815B apparatus according to the ASTMD6413-99 standard.
The washing resistance of the treated cotton was analyzed using a soaping fastness tester (Roaches Co., England) in accordance with the AATCC 61-2006 standard. The test was performed using a water temperature of 49 °C, and a wash time of 45 min.
The surface morphologies of pure cotton, treated cotton, and the residue of treated cotton after combustion were observed using a Hitachi S-4800 scanning electron microscope (SEM) apparatus (Netherlands) at the 10 kV beam voltage. Chemical compositions of the treated cotton samples before and after burning were evaluated with a JEOL-6300F energy dispersive X-ray (EDX).
Thermogravimetric (TG) analysis was performed using a Pyris 1 thermal analyzer (PerkinElmer, USA). Pure cotton and treated cotton samples were separately tested in nitrogen and air atmosphere with a heating rate of 20 K/min from 40 to 700 °C.
The crystal structure was investigated by Rigaku XD-3 X-ray diffraction (XRD). Pure cotton and treated cotton samples were tested with CuK radiation, with measured voltage and current of 36 kV and 20 mA, respectively. XRD analysis used a diffraction angle of 2°–52° and step size of 0.02° (λ = 0.154 nm).
The cone calorimetry test was conducted in accordance with international standard ASTME1354 under a radiation heat flux of 35 kW/m2. The following parameters were evaluated: time to ignition (TTI, s), time to flameout (TTF, s), total heat release (THR, kW/m2), heat release rate (HRR, kW/m2), peak heat release rate (PHRR, kW/m2), peak heat release rate time (TPHRR, s), fire growth rate (FGR, kW/(m2 s)), the ratio of CO2/CO, and the final percentage of residue (%).
TG-IR analysis was carried out on a thermogravimeter (Pyris 1, PerkinElmer, USA) connected to an infrared spectrometer (Nicolet 6700). The analysis was conducted from 40 to 700 °C under N2 atmosphere. The heating rate was 20 K/min, and the scanning range was 500–4000 cm-1 with a resolution of 4.0 cm -1.
Breaking strength was determined using an electronic fabric tension tester HD026N according to the ASTM5035-2006 standard. Whiteness was measured using a Datacolor650 whiteness tester in accordance with the AATCC110-2000 method. Air permeability was measured using an air permeability tester according to the standard TS 391 EN ISO 9237, with a constant pressure drop of 100 Pa (20 cm2 test area). The bending rigidity was evaluated by using a YG (B) 022D-type automatic fabric stiffness tester in accordance with the ASTMD1388-96 (2002) standard. All samples were measured three times and their average values were determined.
Results and discussion
FT-IR spectra
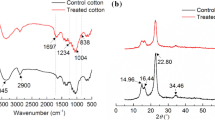
Different chemical bonds or functional groups of the samples were determined by FT-IR analysis. Figure 3 reveals the molecular structural changes of the pure and treated cotton by the FT-IR spectra. The spectra of pure cotton and treated cotton showed peaks at 3385 cm-1, 2904 cm-1, and 1695 cm-1 in spectra of both the treated and untreated samples, and were attributed to the absorption of O–H (from H2O), C–H (from aliphatic hydrocarbon), and C=O groups, respectively (Wei et al. 2019). In addition, some new peaks appeared in the spectra of treated cotton, at 1555 cm-1 and 1374 cm-1, which were ascribed to N–H and C–N bonds, respectively, from the ADPTMPA flame retardant (Liu et al. 2018b). The absorption peak at 1228 cm-1 was attributed to the stretching vibration of P=O, and the weak peak at 849 cm-1 was assigned to the stretching vibration of P–OH group (Coleman et al. 2011; Viornery et al. 2002). The peak at 1021 cm-1 was ascribed to the stretching vibration of P–O–C covalent bond (Huang et al. 2019). Together, the spectral results verify the successful grafting of the flame retardant ADPTMPA onto the cotton fabric.
Flame retardant property and durability
The LOI and VFT were measured to assess the flame retardancy and durability properties of the cotton samples. Table 1 shows the relationships of flame-retardant concentration, weight gain rate, and LOI value of cotton. The LOI of pure cotton was measured as 18.6%, and the LOI values of treated cotton increased with the increase of ADPTMPA concentration (from 20 to 40 wt%). For concentrations of flame retardant of 20 wt%, 30 wt%, and 40 wt%, the WGs of the treated cotton were 17.42%, 21.80%, and 25.10%, and the corresponding LOI values of the treated cottons were 42.4%, 44%, and 46.5%, respectively. These results indicated improved flame retardancy of the treated cottons. After 30 LCs, the LOI value of cotton fabric treated with 20 wt% flame retardant decreased to 31.6% with a WG of 9.28%, which was still sufficient to meet the national flame retardant standards, so should be regarded as semi-durable flame retardant fabric. For concentrations of flame retardant of 30 wt% and 40 wt%, the LOI values of treated cotton were 33.2% with WG of 11.78%, and 35.8% with WG of 13.49%, respectively, after 50 LCs. These results suggest that the ADPTMPA-treated cotton materials exhibited achieved excellent durability and superior flame-retardancy.
The decreased LOI value after washing reflects partial hydrolysis at a higher temperature due to the polarity of the P–O–C covalent bonds. The phosphonic acid produced by hydrolysis would produce sodium salts, magnesium salts, and calcium salts during the washing process, which could reduce the flame retardancy of the treated cotton after repeated washing.
To further explore the flame retardancy and washability of the treated cotton fabrics, VFT were performed for the pure cotton and treated cotton samples after different LCs, as shown in Fig. 4 and Table 2. After ignition, pure cotton burned vigorously for 14 s and smoldered for 5 s, and was completely incinerated with a small amount of gray and broken residual carbon. In contrast, all treated cotton samples burned slowly and extinguished immediately after removing the flame, and formed a complete and continuous carbon frame. The higher the ADPTMPA concentration used, the better the resulting flame retardancy. The data presented in Table 2 show that cotton fabrics treated with flame retardant concentrations of 20 wt%, 30 wt%, and 40 wt% do not exhibit the after-flame and smoldering phenomenon, and had char lengths of 46 mm, 40 mm, and 36 mm respectively. The char length of the 20 wt% treated cotton after 30 LCs was 60 mm, and the char lengths of the 30 wt% and 40 wt% treated cotton samples had char lengths of 57 mm and 55 mm, respectively, after 50 LCs, sufficient to meet the international requirements of flame retardancy and washing resistance.
The VFT results were consistent with the successful grafting of the flame retardant ADPTMPA onto cotton fabric. The phosphoric acid decomposition product from ADPTMPA can promote rapid dehydration and carbonization at high temperature on the surface of cotton fabric to form a dense carbon layer which can be used as thermal insulation layer to delay heat transfer, and the formation of a carbon skeleton can effectively reduce combustible volatiles that are generated by the decomposition of cotton fibers (Yan et al. 2017). A small amount of nitrogen element from ADPTMPA decomposes at high temperature to produce ammonia gas. This non-combustible gas can help to isolate the flame, prevent oxygen transmission, and dilute other flammable gases (Liu et al. 2018a).
Thermal stability
The thermal degradation property of the prepared cotton fabrics was characterized using TG and DTG (derivative thermogravimetry). The TG and DTG curves of pure cotton and treated cotton in nitrogen and air atmosphere are shown in Fig. 5a–d, respectively, and the TG data are exhibited in Table 3.
In air atmosphere, the T10% is at 290.34 °C, and the mass of pure cotton began to rapidly decrease at 290.34 °C, and the temperature at the first degradation stage increased from 290.34 (T10%) to 360.29 °C (Tmax) with a mass loss about 69%, with aliphatic carbon or volatile products mainly generated in this pyrolysis process (Jiang et al. 2019). The temperature then increased from 360.29 to 503.83 °C with approximately a 17% mass loss, corresponding to the conversion of some aliphatic carbons to aromatic carbon and the production of CO and CO2 during the carbonization of cellulose (Wang et al. 2018c). Only 1.24 wt% residue of pure cotton remained at 700 °C. The starting cracking temperature of treated cotton was observed at 244.93 °C, much higher than the 45 °C for pure cotton. The treated cotton exhibited a rapid mass decrease from 244.93 to 306.23 °C (T10%-Tmax), with about 33% mass loss. At 700 °C, 28.49 wt% of the sample was retained. Under nitrogen atmosphere, the T10% of pure cotton was 312.89 °C, and the main pyrolysis temperature range was from 300 to 400 °C with a 76% mass loss. The pure cotton can depolymerize from sugar-based units into volatile products (mainly containing levoglucosan, furan, and furan derivatives) or undergo glycosyl unit dehydration to form thermally stable aromatic char (Carosio et al. 2015). The amount of residual carbon for the pure cotton sample was 14.88 wt% at 700 °C. The T10% of treated cotton was 232.15 °C, much improved compared to that of pure cotton, consistent with the gradual depolymerization of the glycosyl units in the fiber. From 232.15 to 302.61 °C, the mass of the treated cotton rapidly decreased, leading to a 34% mass loss. Finally, 40.03 wt% residual carbon remained at 700 °C.
These results indicated improved thermal stability of the treated cotton compared to the pure cotton. The decomposition temperature of treated cotton is advanced attributed that the flame retardant decomposed at a lower temperature, producing phosphoric acid to decrease the decomposition temperature of cotton. The ADPTMPA containing P and N decomposes to form phosphoric acid and polyphosphoric acid, which can accelerate the degradation and promote the carbonization of cotton fiber. In addition, nitrogen element from ADPTMPA can generate non-combustible ammonia gas (Levchik et al. 1996; Xu et al. 2019). This gas blocks the supply of oxygen and removes most decomposition heat to greatly reduce the surface temperature of the treated cotton. The synergy of the nitrogen and phosphorus interferes with the combustion of the cotton fabric, leading to thorough suppression of combustion.
Cone calorimetry
Cone calorimetry was used to analyze the combustion process of materials in real fire scenarios (Hicyilmaz et al. 2019). The relevant data are presented in Table 4. The HRR (Fig. 6a) and THR (Fig. 6b) of treated cotton were significantly reduced compared with those of pure cotton. The smaller HRR value is, the slower the combustion. The HRR curve of the treated cotton appeared relatively flat, indicating that treated cotton was heated, and then the cellulose macromolecules decomposed at a slow and steady rate. As can be seen from the data presented in Table 4, the TTI and TTF of pure cotton were 7 s and 25 s, respectively. Treated cotton was not ignited. The PHRR value of the treated cotton was 16.15 kW/m2 and that of pure cotton was 186.55 kW/m2, indicating the treatment resulted in a 91.34% decrease. The TPHRR was delayed from 22 s (pure cotton) to 32 s (treated cotton). In addition, the THR value decreased from 2.31 MJ/m2 (pure cotton) to 0.97 MJ/m2 (treated cotton), indicating the release of less heat from the treated cotton in the combustion process, thus inhibiting further combustion and improving the safety of cotton fabric. CO2 and CO are the main components of the combustion gas. The lower the CO2/CO ratio is, the lower the combustion efficiency of the treated cotton is. The CO2/CO ratio of the pure cotton was 78.05, while that of treated cotton was only 18.40, indicating that the treated cotton exhibited good flame retardant performance. The safety of materials is often assessed by FGR value, and in this study this was calculated according to Eq. (2).
The FGR value of the treated cotton (0.51 kW m-2 s-1) was 93.98% lower than that of pure cotton (8.48 kW m-2 s-1). The lower the FGR value, the slower the rate of fire spread and the better the safety. The amount of residual increased from 1.15 wt% for the pure cotton to 29.42 wt% for the treated cotton. This is mainly due to the decomposition of the ADPTMPA flame retardant at high temperature to produce phosphoric acid substances, which accelerated the transformation of cellulose fiber into carbon, thereby reducing the combustion decomposition of fibers and the production of flammable volatile gases to prevent the burning of the cotton fabric (Wu et al. 2017).
TG-IR analysis of volatile pyrolysis products
TG-IR was used to detect the thermally degraded volatile products of pure and treated cotton under nitrogen atmosphere. The total TG-IR data are presented in Fig. 7 and show that the curves for the pure and treated cotton curves exhibited similar main peak positions, indicating that the volatile products of thermal degradation are basically the same. The obvious absorption peaks are as follows: the peak at 3588 cm-1 was attributed to the absorption of –OH from H2O (Ghanadpour et al. 2015; Wang et al. 2019). The peak at 2855 cm-1 corresponded to the C–H stretching vibration absorption peak of aliphatic compounds, and 2317 cm-1 was assigned to the absorption peak of CO2 (Qin et al. 2019). The peak at 1727 cm-1 was attributed to the absorption of carbonyl-containing compounds and 1084 cm-1 was assigned to the absorption peak of C–O–C groups (Chen et al. 2017; Wang et al. 2018b). The intensity of absorption peaks of pure cotton and treated cotton at 3588 cm-1, 855 cm-1, and 2317 cm-1 showed little change, while the peaks for treated cotton at 1727 cm-1 and 1084 cm-1 were obviously weaker than those of pure cotton, implying that treatment with ADPTMPA altered the thermal degradation process of cellulose, resulting in a significant reduction in the amounts of ethers and carbonyl-containing compounds.
The absorption peak intensities of the thermally degraded volatile products of pure cotton and treated cotton are shown in Fig. 7. The absorption intensities of the incombustible gases H2O and CO2 are displayed in Fig. 7a and c. The absorption intensities of aliphatic, carbonyl-containing compounds, and ether compounds are shown in Fig. 7b, d and e respectively. The absorption intensities of combustible compounds in treated cotton were lower than those in pure cotton, which suggested that lower amounts of flammable aliphatic, carbonyl, and ether compounds were released from treated cotton compared to the amounts released from pure cotton.
XRD patterns
XRD spectral analysis was applied to compare differences of the crystal structures of pure cotton and treated cotton. The XRD spectra of treated cotton and pure cotton were basically the same, as shown in Fig. 8. There are strong diffraction peaks at 14.47°, 16.48°, 22.58°, and 59° in both spectra, which correspond to the (1–10), (110), (200), and (004) lattice planes of cellulose I, respectively (Lu et al. 2018a). No new diffraction peaks appeared in the XRD spectrum of treated cotton, consistent with no significant effect of ADPTMPA treatment on the main crystal structure of cotton cellulose. However, the intensity of all the diffraction peaks were slightly weakened in the treated cotton, which attributed to the reaction between ADPTMPA and cotton reducing the relative content of the crystallinity of cotton fiber, resulting in weaker intensities of characteristic peaks for treated cotton fiber (Ford et al. 2012).
SEM morphology
SEM was next applied to provide insight into the microstructure of the fiber surface. Figure 9 shows the SEM images of the surface morphology of pure cotton and treated cotton samples before and after combustion at different magnifications. Figure 9a and e show that the fiber surface of the pure cotton sample is smooth, the fiber is folded and flat, and there is large spacing between the fibers. Figure 9b and f show that the cotton fiber has retained the flat and curly appearance after 40 wt% ADPTMPA treatment. The surface of the treated cotton fiber becomes smoother than that of pure cotton, and the filaments appear more plump and exhibit an inward curl. Because ADPTMPA has a low molecular weight and good water solubility, it can easily permeate into the cotton fiber to graft with cotton cellulose and make the cellulose expand (Tian et al. 2019). The surface morphologies in Fig. 9b, c, f, g are substantially similar, indicating that the flame retardant cotton exhibits good wash fastness. Figure 9d and h show that the treated cotton did not shrink or break obviously after combustion, but retained the overall morphology structure of the fiber. Additionally, the surface of the fiber was covered with an expanded carbon frame. This is because the phosphoric acid produced by pyrolysis of the flame retardant promotes the dehydration of the cellulose fiber into carbon, and the fiber retains the intact carbon frame. In addition, a small amount of bubbles are apparent on the surface of the fibers, because the flame retardant contains N element, which can convert into non-flammable gas (NH3) during the combustion process. The gas continuously strikes the carbon surface of the fiber, causing swelling of the carbon layer and bubbles to form, which prevent the efficient transfer of heat and flammable gas to the surface of the cotton fabric, thus effectively improving the overall flame retardancy of cotton fabric (Feng et al. 2017).
EDX analysis of samples
EDX was next used to evaluate the element compositions and contents of micro-samples of the material. The data presented in Figs. 10 and 11 show that the ADPTMPA-treated cotton samples contain C, O, N, and P elements, both before and after burning. Figure 12 shows the atomic percentages were 20.20% of N and 2.68% of P for the treated cotton, and P decreased to 17.56% and 1.86%, respectively, after 50 LCs. After combustion of the treated cotton samples, the relative content of N (19.91%) slightly decreased and the relative content of P (4.50%) increased, indicating that burning promoted the conversion of C, O, and N into gases such as H2O, CO2 and NH3 which can escape from the cotton, resulting in a decrease of N and a relative increase of P.
Physical and mechanical properties
Breaking strength, bending rigidity, air permeability, and whiteness were measured for pure and treated cotton, and the results are summarized in Table 5.
The breaking strength of treated cotton was reduced to some extent compared with that of pure cotton. The warp breaking strength of 40 wt%-treated cotton was decreased by 30.72%, and the weft was decreased by 33.89%. This suggests baking the cellulose at 180 °C for 5 min have damaged the crystalline region of the cellulose fiber. After 50 LCs, the breaking strength of treated cotton was slightly recovered because some of the flame retardant was eluted from the treated cotton fiber, providing the opportunity for repair of the crystallization zone destroyed during the flame retardant finishing process. Although the breaking strength of treated cotton was reduced, this has only little effect on its application in the textile field.
The bending rigidity of treated cotton increased with increased concentration of the flame retardant. Compared with the pure cotton, the bending rigidity of the 20 wt%-treated cotton increased by 14.29% in the warp direction and increased by 14.05% in the weft direction, indicating little effect of the treatment on bending rigidity and retention of the softness of natural cotton. After 50 LCs, the bending rigidity of the treated cotton fabric was slightly reduced.
In addition, it can be seen from Table 5 that with increased concentration of the flame retardant, the gas permeability of the treated cotton fabric decreased. The penetration of flame retardant molecules into the interior of the cotton fibers caused expansion of the fibers and narrowing of the interspace between cotton fibers, resulting in a decrease in gas permeability (Lu et al. 2018b). The gas permeability of cotton treated with 40 wt% ADPTMPA flame retardant was 316 mm/s, which is about 11.48% lower than that of pure cotton. This result suggested retention of the breathability of the treated cotton. After 50 LCs, the gas permeability of the treated cotton increased slightly.
With increased concentration of the flame retardant, whiteness gradually decreased. The whiteness of pure cotton was 90.8% and the whiteness values of the cotton fabric treated with 20 wt%, 30 wt%, and 40 wt% ADPTMPA flame retardant were 86.5%, 84.3%, and 82.6%, respectively. The whiteness performance of the treated cotton remained high due to the presence of nitrogen and self-buffering properties of the flame retardant (Wang et al. 2018a). The slight decrease in the whiteness of treated cotton was due to the oxidation of cellulose at high temperature.
Conclusions
ADPTMPA, a novel eco-friendly and renewable P–N synergistic flame retardant, was successfully synthesized under solvent-free and formaldehyde-free conditions. The ADPTMPA structure was characterized using NMR and FT-IR. The treated cotton fabrics exhibited excellent flame retardancy with LOI of 46.5% and outstanding washability with LOI of 35.8% after 50 LCs. There was little change in the crystalline area of the treated cotton. The char formation of treated cotton fabric was greatly improved, with residues of 28.49 wt% and 42.03 wt% under air and nitrogen atmospheric conditions, respectively. Use of ADPTMPA can promote the release of non-combustible gases and inhibit the release of combustible gases. The HRR, THR and the rate of CO2/CO of treated cotton were reduced significantly, and the residue increased, suggesting outstanding flame retardancy and thermostability of the treated cotton. The morphology of the carbon residue after burning was well-preserved. Treatment with the flame retardant had little effect on the breaking strength and bending rigidity of treated cotton. The whiteness and gas permeability of treated cotton were well sustained.
References
Alongi J, Malucelli G (2012) State of the art and perspectives on sol–gel derived hybrid architectures for flame retardancy of textiles. J Mater Chem 22:21805–21809. https://doi.org/10.1039/c2jm32513f
Alongi J, Milnes J, Malucelli G, Bourbigot S, Kandola B (2014) Thermal degradation of DNA-treated cotton fabrics under different heating conditions. J Anal Appl Pyrol 108:212–221. https://doi.org/10.1016/j.jaap.2014.04.014
Basak S, Samanta KK, Chattopadhyay SK, Saxena S, Narkar R (2018) Banana pseudostem sap and boric acid- A new green intumescent for making self-extinguishing cotton fabric. Indian J Fibre Text Res 43:36–43
Basta AH, El-Saied H (2009) Performance of improved bacterial cellulose application in the production of functional paper. J Appl Microbiol 107:2098–2107. https://doi.org/10.1111/j.1365-2672.2009.04467.x
Bosco F, Carletto RA, Alongi J, Marmo L, Di Blasio A, Malucelli G (2013) Thermal stability and flame resistance of cotton fabrics treated with whey proteins. Carbohyd Polym 94:372–377. https://doi.org/10.1016/j.carbpol.2012.12.075
Carosio F, Fontaine G, Alongi J, Bourbigot S (2015) Starch-based layer by layer assembly: efficient and sustainable approach to cotton fire protection. ACS Appl Mater Interfaces 7:12158–12167. https://doi.org/10.1021/acsami.5b02507
Chen X, Wang W, Jiao C (2017) A recycled environmental friendly flame retardant by modifying para-aramid fiber with phosphorus acid for thermoplastic polyurethane elastomer. J Hazard Mater 331:257–264. https://doi.org/10.1016/j.jhazmat.2017.02.011
Choi K, Seo S, Kwon H, Kim D, Park YT (2018) Fire protection behavior of layer-by-layer assembled starch-clay multilayers on cotton fabric. J Mater Sci 53:11433–11443. https://doi.org/10.1007/s10853-018-2434-x
Coleman RJ, Lawrie G, Lambert LK, Whittaker M, Jack KS, Grondahl L (2011) Phosphorylation of alginate: synthesis, characterization, and evaluation of in vitro mineralization capacity. Biomacromol 12:889–897. https://doi.org/10.1021/bm1011773
Ding N, Wang XF, Tian YM, Yang L, Chen HZ, Wang ZC (2014) A renewable agricultural waste material for the synthesis of the novel thermal stability epoxy resins. Polym Eng Sci 54:2777–2784. https://doi.org/10.1002/pen.23838
Feng YJ, Zhou Y, Li DK, He S, Zhang FX, Zhang GX (2017) A plant-based reactive ammonium phytate for use as a flame-retardant for cotton fabric. Carbohyd Polym 175:636–644. https://doi.org/10.1016/j.carbpol.2017.06.129
Ford ENJ, Rawlins JW, Mendon SK, Thames SF (2012) Effect of acid value on the esterification mechanism of maleinized soybean oil with cotton. J Coat Technol Res 9:637–641. https://doi.org/10.1007/s11998-012-9420-z
Ghanadpour M, Carosio F, Larsson PT, Wagberg L (2015) Phosphorylated cellulose nanofibrils: a renewable nanomaterial for the preparation of intrinsically flame-retardant materials. Biomacromol 16:3399–3410. https://doi.org/10.1021/acs.biomac.5b01117
Hicyilmaz AS, Altin Y, Bedeloglu A (2019) Polyimide-coated fabrics with multifunctional properties: flame retardant, UV protective, and water proof. J Appl Polym Sci. https://doi.org/10.1002/app.47616
Hill V, Howell B, Daniel Y (2017) Oligomeric flame retardants for polymeric materials from itaconic acid Abstracts of Papers of the American Chemical Society 253
Huang S, Zhong L, Li SN, Liu MS, Zhang Z, Zhang FX, Zhang GX (2019) A novel monosodium-glutamate-based flame retardant containing phosphorus for cotton fabrics. Cellulose 26:2715–2728. https://doi.org/10.1007/s10570-018-02241-8
Jiang ZM, Li H, He YW, Liu Y, Dong CH, Zhu P (2019) Flame retardancy and thermal behavior of cotton fabrics based on a novel phosphorus-containing siloxane. Appl Surf Sci 479:765–775. https://doi.org/10.1016/j.apsusc.2019.02.159
Lessan F, Montazer M, Moghadam MB (2011) A novel durable flame-retardant cotton fabric using sodium hypophosphite, nano TiO2 and maleic acid. Thermochim Acta 520:48–54. https://doi.org/10.1016/j.tca.2011.03.012
Levchik SV, Levchik GF, Balabanovich AI, Camino G, Costa L (1996) Mechanistic study of combustion performance and thermal decomposition behaviour of nylon 6 with added halogen-free fire retardants. Polym Degrad Stab 54:217–222. https://doi.org/10.1016/s0141-3910(96)00046-8
Li YC et al (2010) Flame retardant behavior of polyelectrolyte-clay thin film assemblies on cotton fabric. ACS Nano 4:3325–3337. https://doi.org/10.1021/nn100467e
Liu LX, Pan Y, Wang Z, Hou YB, Gui Z, Hu Y (2017) Layer-by-layer assembly of hypophosphorous acid-modified chitosan based coating for flame-retardant polyester-cotton blends. Ind Eng Chem Res 56:9429–9436. https://doi.org/10.1021/acs.iecr.7b02303
Liu H, Zhang B, Han J (2018a) Improving the flame retardancy and smoke suppression properties of polyurethane foams with SiO2 microcapsule and its flame-retardant mechanism polymer-plastics. Technol Eng 57:1139–1149. https://doi.org/10.1080/03602559.2017.1373398
Liu Y, Wang QQ, Jiang ZM, Zhang CJ, Li ZF, Chen HQ, Zhu P (2018b) Effect of chitosan on the fire retardancy and thermal degradation properties of coated cotton fabrics with sodium phytate and APTES by LBL assembly. J Anal Appl Pyrol 135:289–298. https://doi.org/10.1016/j.jaap.2018.08.024
Lu Y, Jia Y, Zhou Y, Zou J, Zhang G, Zhang F (2018a) Straightforward one-step solvent-free synthesis of the flame retardant for cotton with excellent efficiency and durability. Carbohyd Polym 201:438–445. https://doi.org/10.1016/j.carbpol.2018.08.078
Lu Y, Jia Y, Zhou Y, Zou J, Zhang G, Zhang F (2018b) Straightforward one-step solvent-free synthesis of the flame retardant for cotton with excellent efficiency and durability. Carbohydr Polym 201:438–445. https://doi.org/10.1016/j.carbpol.2018.08.078
Qian W, Li XZ, Wu ZP, Liu YX, Fang CC, Meng W (2015) Formulation of intumescent flame retardant coatings containing natural-based tea saponin. J Agric Food Chem 63:2782–2788. https://doi.org/10.1021/jf505898d
Qin HL, Li XF, Zhang XL, Guo ZG (2019) Preparation and performance testing of superhydrophobic flame retardant cotton fabric. New J Chem 43:5839–5848. https://doi.org/10.1039/c9nj00307j
Tian P, Lu Y, Wang D, Zhang G, Zhang F (2019) Solvent-free synthesis of silicon–nitrogen–phosphorus flame retardant for cotton fabrics. Cellulose. https://doi.org/10.1007/s10570-019-02554-2
Veerappagounder S, Nalankilli G, Shanmugasundaram OL (2016) Study on properties of cotton fabric incorporated with diammonium phosphate flame retardant through cyclodextrin and 1,2,3,4-butane tetracarboxylic acid binding system. J Ind Text 45:1204–1220. https://doi.org/10.1177/1528083714555780
Viornery C et al (2002) Surface modification of titanium with phosphonic acid to improve bone bonding: characterization by XPS and ToF-SIMS. Langmuir 18:2582–2589. https://doi.org/10.1021/la010908i
Wang DF, Zhong L, Zhang C, Zhang FX, Zhang GX (2018a) A novel reactive phosphorous flame retardant for cotton fabrics with durable flame retardancy and high whiteness due to self-buffering. Cellulose 25:5479–5497. https://doi.org/10.1007/s10570-018-1964-3
Wang S, Du ZL, Cheng X, Liu YS, Wang HB (2018b) Synthesis of a phosphorus- and nitrogen-containing flame retardant and evaluation of its application in waterborne polyurethane. J Appl Polym Sci. https://doi.org/10.1002/app.46093
Wang Z, Shu X, Zhu H, Xie L, Cheng S, Zhang Y (2018c) Characteristics of biochars prepared by co-pyrolysis of sewage sludge and cotton stalk intended for use as soil amendments. Environ Technol. https://doi.org/10.1080/09593330.2018.1534891
Wang S, Du X, Deng S, Fu X, Du Z, Cheng X, Wang H (2019) A polydopamine-bridged hierarchical design for fabricating flame-retarded, superhydrophobic, and durable cotton fabric. Cellulose 26:7009–7023. https://doi.org/10.1007/s10570-019-02586-8
Wei DD, Dong CH, Chen ZH, Liu J, Li Q, Lu Z (2019) A novel cyclic copolymer containing Si/P/N used as flame retardant and water repellent agent on cotton fabrics. J Appl Polym Sci. https://doi.org/10.1002/app.47280
Wu Q, Zhang Q, Zhao L, Li SN, Wu LB, Jiang JX, Tang LC (2017) A novel and facile strategy for highly flame retardant polymer foam composite materials: transforming silicone resin coating into silica self-extinguishing layer. J Hazard Mater 336:222–231. https://doi.org/10.1016/j.jhazmat.2017.04.062
Xu F, Zhong L, Xu Y, Zhang C, Zhang FX, Zhang GX (2019) Highly efficient flame-retardant and soft cotton fabric prepared by a novel reactive flame retardant. Cellulose 26:4225–4240. https://doi.org/10.1007/s10570-019-02374-4
Yan HQ, Zhao L, Fang ZP, Wang H (2017) Construction of multilayer coatings for flame retardancy of ramie fabric using layer-by-layer assembly. J Appl Polym Sci. https://doi.org/10.1002/app.45556
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wan, C., Liu, M., Tian, P. et al. Renewable vitamin B5 reactive N–P flame retardant endows cotton with excellent fire resistance and durability. Cellulose 27, 1745–1761 (2020). https://doi.org/10.1007/s10570-019-02886-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02886-z