Abstract
A flame retardant hexachlorocyclotriphosphazene (HCCP) diethylenetriamine ammonium phosphoric acid and phosphoric acid ester (HPDPP) with –N=P–(N)3–, –P(=O)(OCH3)2, and reactive –P(=O)(O−NH4+)2 groups was synthesized for cotton fabrics. The results showed that the cotton fabrics treated with HPDPP had high flame retardance and durability. The vertical flammability test (VFT), thermogravimetric (TG) analysis, thermogravimetric-Fourier infrared spectrometer (TG-FTIR) and cone calorimetry tests showed that cotton fabrics treated with HPDPP had high flame resistance, displaying condensed phase flame retardance mechanism. The Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), scanning electron microscope (SEM) and energy dispersive spectrometer (EDS) implied that HPDPP molecular could enter the inner amorphous space and graft on cotton fibers. The mechanical properties of cotton fabrics treated with HPDPP were well sustained. Moreover, the limiting oxygen index (LOI) of cotton fabrics treated with 40% HPDPP reached 41.3%, and after 50 laundering cycles (LCs), it reached 29.7% according to the AATCC 61-2013 3A washing standard (vigorous washing), which suggested the flame retardance and durability of treated cotton fabrics were improved significantly. And the EDS of washed cotton fabrics showed that the cotton fabrics treated with HPDPP combined the lowest metal ions compared with cotton fabrics treated with flame retardants with only reactive ammonium phosphoric acid group, because of the fact that besides the necessary reactive ammonium phosphoric groups, the phosphoric acid ester groups can not combine Ca2+ and Mg2+ etc. Thus, introducing –N=P–(N)3– groups and phosphoric acid ester groups is an efficient method to significantly increase the durability of the treated fabrics.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton is omnipresent in our day-to-day life, especially in garments because of its soft hand, excellent hygroscopicity and antistatic properties. However, because of its high flammability and low limiting oxygen index (LOI), cotton materials represent a potential fire risk for goods and life. Therefore, imparting flame retardance to cotton fabrics is very important (Lokhande et al. 2022; Mayer-Gall et al. 2015). Proban and pyrovatex CP are two of the most widely used durable flame retardants for cotton fabrics (Thi Hong Khanh and Huong, 2019), because they can endow good flame retardance and remarkable durability (Salmeia et al. 2016a). However, the cotton fabrics treated with these two flame retardants can release formaldehyde during use, which endangers people’s health and pollutes the environment (Barbalini et al. 2020; Vishwakarma et al. 2021).
Many reports introduced novel reactive groups into flame retardant molecules to replace the reactive P–CH2OH and N–CH2OH groups formed by formaldehyde in proban and pyrovatex CP, to react with cellulose to form covalent bonds between flame retardant and cellulose. These novel reactive groups mainly include olefin (Xu et al. 2022), monochlorotriazine (Chen et al. 2022; Zhou et al. 2022). However, because of the bulk polymerization and less efficient reaction of olefin group with the hydroxyl groups of cellulose (Podkościelna et al. 2022; Sun et al. 2022), the cotton fabrics finished with these P-containing olefin-based flame retardants only had a poor flame retardancy (Manfredi et al. 2018). Simultaneously, for flame retardants with reactive monochlorotriazine group, the finished cotton fabrics only showed weak flame retardance because of the low grafting efficiency between the monochlorotriazine group and cellulose (Salmeia et al. 2016b). Therefore, the preparation of formaldehyde-free and durable flame retardants is still the focus of extensive research.
In recent years, biomass materials got more attention for preparing of flame retardants for cotton fabrics because of the advantages of using eco-friendly materials. Several reports extracted biomaterials such as DNA (Ortelli et al. 2019; Yu et al. 2021), phytic acid (Li et al. 2022a), and a hydrophobic protein to be directly used as flame retardants (Leong et al. 2021; Tawiah et al. 2019a). Some biomass materials can be used to synthesize flame retardants, such as amino acid (Chen et al. 2021), protein (Bosco et al. 2013), banana pseudostem (Kambli et al. 2018), polysaccharides (Li et al. 2022b), and lignosulfonates (Angelini et al. 2019; Li et al. 2020; Padhi et al. 2022; Tawiah et al. 2019b). In fact, most of the treated cotton fabrics were not durable because of the lack of reactive groups in the molecules to react with cellulose to form covalent bonds (Fan et al. 2022; Khan et al. 2023; Wang et al. 2022).
In previous studies, some flame retardants with reactive ammonium phosphoric acid groups (–P(=O)(O−NH4+)2) were developed for cotton fabrics. They were grafted onto the cotton fabrics through the P–O–C covalent bonds (Zheng et al. 2016; Jia et al. 2017; Ali et al. 2023), which increased the flame retardance greatly and the durability to some extent (Feng et al. 2017; Tian et al. 2019; Wan et al. 2020). The treated cotton fabrics can only pass the AATCC 61-2013 1A washing standard 50 laundering cycles (LCs) (careful/gentle hand washing), and can not pass the more restrictive AATCC 61-2013 2A washing standard 50LCs (home machine washing) (Liu et al. 2020). This should be that only C6 hydroxyl groups in the cellulose will react with the flame retardant, and the crystallinity of the cotton fiber is almost 70% which can not react with flame retardant. Thus, there are not enough reaction sites on the cotton fibers to react with the reactive ammonium phosphoric acid groups of the flame retardant. Consequently, a large number of reactive ammonium phosphoric acid groups on the flame retardant do not react with cellulose, and during the washing process, the NH4+ in (–P(=O)(O−NH4+)2) groups in the flame retardants grafted on cotton fibers are easily exchanged with Ca2+ and Mg2+ etc. to form Ca (PO3)– and Mg (PO3)– etc. groups (Ding et al. 2022a; Liu et al. 2022; Zhao et al. 2022a). The reactive ammonium phosphoric acid groups will change to phosphoric acid and polymetaphosphoric acid at high temperature, which can promote the char formation efficiently to show flame retardance (Ding et al. 2022b; Zhao et al. 2022b). However, the Ca (PO3)– and Mg (PO3)– etc. groups only change to CaHPO4 and MgHPO4 etc. at high temperature, which can not efficiently promote the dehydration and char formation, leading to a significant decrease in the flame retardance of the treated cotton fabrics (Xu et al. 2021).
In this study, a novel flame retardant for cotton fabrics is synthesized based on hexachlorocyclotriphosphazene (HCCP). The latter reacts with diethylenetriamine (DETA) to introduce the –N=P–(N)3– groups, and introduced –PO(OH)2 and –PO(OCH3)2 groups through formaldehyde (which changes into –CH2– group and impossibly generates formaldehyde) in the flame retardant molecules, –PO(OH)2 groups can react with cellulose to form P–O–C covalent bonds. As all the phosphorus elements in the HCCP ring form –N=P–(N)3– groups in the HPDPP flame retardant, which are very stable and can not hydrolyze to combine metal ions, and the phosphoric acid ester groups (–PO(OCH3)2) are also relatively stable. Therefore, the ratio of phosphorus groups directly combining Ca2+ etc. metal ions or hydrolyzing to combine Ca2+ etc. metal ions significantly decreased. It can be expected that the cotton fabrics treated with this flame retardant based on HCCP can have high durability and pass the more restrictive AATCC 61-2013 3A washing standard 50 LCs.
Experiments
Materials
The pure cotton (164.8 g/m2) was purchased from a textile market in Chongqing Chaotianmen (Chongqing, China). The hexachlorocyclotriphosphazene (HCCP) was provided by Sigma Aldrich Trading Co. (Shanghai, China). The diethylenetriamine (DETA), phosphate, dicyandiamide, dimethyl phosphite, and urea were provided by Tianjin Yongda Chemical Reagent Co. Ltd (Tianjin, China). The formaldehyde, sodium hydroxide, ethyl acetate, and isopropyl alcohol were provided by Chongqing Chuandong Chemical Co., Ltd (Chongqing, China).
HPDPP flame retardant
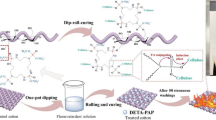
HCCP has very reactive phosphoryl halides (N–P–Cl/N=P–Cl) groups that can easily react with the –NH2 group. The HPDPP was synthesized through three steps. DETA (17 g, 0.1722 mol), HCCP (10 g, 0.0287 mol), and ethyl acetate (110 mL) were first agitated in a 250 mL round bottom flask which was put in an ice water bath for 30 min, and then reacted at room temperature for 20 min. After the reaction finished, the ethyl acetate was separated using a rotary evaporator to yield a white powder. Afterwards, the white powder, phosphate (9.41 g, 0.1148 mol), dimethyl phosphite (23.68 mL, 0.2583 mol), and formaldehyde (39.24 mL, 0.3731 mol) were added to the beaker, which was kept at 65 °C in a water bath to react for 1 h. Finally, urea was put into the beaker, heated at 140 °C in an oil bath to disintegrate it and adjust the pH of the solution to 5–6, yielding a yellow viscous product. The latter was then purified with isopropyl alcohol and dried. Finally, a flame retardant hexachlorocyclotriphosphazene diethylenetriamine phosphoric acid ester ammonium phosphoric acid (HPDPP) was obtained. Because there were many permutations and combinations for (CH3O)2P(=O)– and (HO)2P(=O)– groups introduced to the flame retardant molecules, every similar component should have similar flame retardance. Scheme 1 only presented a possible production of flame retardant compound.
Preparation of treated cotton fabrics
The cotton fabrics were immersed in a 20% NaOH solution for 5 min, and washed with 2% acetic acid solution and tap water to reduce their crystallinity and thus increase the reaction sites. These pre-treated cotton samples then served as control cottons.
The HPDPP was dissolved with deionized water to prepare flame retardant solutions with mass concentrations of 20%, 30%, and 40%. 6% dicyandiamide was added to the flame retardant solutions and used as a catalyst. The pre-treated cotton fabrics were dipped into the solutions with different concentrations of flame retardant at a bath ratio of 1:20, and soaked in a water bath at 70 °C for 1 h. Afterwards, the impregnated cotton fabrics undergone two dipping and rolling processes, and they were cured at 180 °C for 5 min. Finally, the samples were rinsed with running water and dried at 120 °C in an oven to reach a consistent weight in order to obtain the HPDPP treated cotton fabrics. Table 1 showed the weight gains (WG) of cotton fabrics treated with different concentrations of HPDPP solution, where FRC-20, FRC-30, and FRC-40 represented cotton fabrics treated with 20%, 30%, and 40% HPDPP solution, respectively. Scheme 2 showed the synthesis of the HPDPP flame retardant and its grafting reaction with cotton fibers, respectively.
The treated fabrics WG was calculated as:
where W0 and W1 were the weights of the cotton fabrics before and after HPDPP finishing, respectively.
Characterization
1H NMR, and 31P NMR spectra were used to determine the chemical structure of the flame retardants using a 400 MHz nuclear magnetic resonance instrument (AVANCE III, Switzerland).
The VFTs of the samples were conducted using a YG815B vertical fabric flame retardant tester provided by Nantong Sansi Electromechanical Science & Technology Co. Ltd. (Jiangxi, China), according to the ASTM D6413-2015 standard. The LOIs were determined using a JL-JF-5 Fully Automatic Oxygen Index Tester provided by Beijing Beiguang Precision Instrument & Equipment Co. Ltd (Beijing, China), according to the ASTM D2863-2017 standard. The washing fastness of the cotton fabrics treated with HPDPP was determined according to the AATCC 61-2013 3A washing standard. 0.15% sodium dodecyl sulfate was configured as a detergent with running water, The washing temperature was 71 °C, and the cylinder filled with 50 mL of washing liquid and 100 stainless steel balls (The diameter of the stainless steel ball was 6 mm). This method was used to evaluate the washing process under violent conditions. It was important to mention that one specimen was similar to that produced by five home machine washes at a temperature of 60 ± 3 °C. Note that the tester was provided by Wuxi Textile instrument Co. Ltd. (Jiangsu, China).
Thermogravimetric (TG) analysis (Pyris 1-PerkinElmer Co. Ltd., USA) was used to analyze the thermal degradation behavior of the control and treated cottons under nitrogen and air atmosphere. The tests conducted in the temperature range of 40–700 °C with a heating rate of 20 K/ min. A thermogravimetric-Fourier infrared spectrometer (TG-FTIR) was used to examine the gas release from the control and treated cotton during pyrolysis with nitrogen at 40–700 °C, a temperature increases of 20 K/ min. The combustion heat release behaviors of the cotton fabrics measured using an FTT 0007 cone calorimeter (6810, China) at 35 kW/m2 heat flux based on the ASTME1354 standard.
The surface micromorphologies of the cotton fibers and carbon residue were observed by scanning electron microscopy (SEM, Phenom ProX, Phenom Scientific Co. Ltd., Netherlands) with Energy dispersive spectroscopy (EDS), at an acceleration voltage of 10 kV. Fourier transform infrared spectroscopy (FTIR, PE Co. Ltd, USA) was used to assess the structures of the cotton fibers with a Spectrum GX spectrometer in the range of 4000 cm−1–500 cm−1. The crystallinity was analyzed by X-ray diffraction (XRD) at scattering angles ranging between 5° and 60° with a step size of 0.02° using a Rigaku XD-3wide-angle instrument operating at 35 kV and 20 mA (Beijing Purkinje General Instrument Co. Ltd., Beijing, China).
The mechanical properties of the fabrics were tested using a YM065A electronic fabric strength tester according to the ASTM D5035-11 (2019) standard (Nantong Hongda Experiment Instruments Co. Ltd., China). A YG(B)022D automatic fabric stiffness tester provided by Wenzhou Darong Textile Machinery Co. Ltd., China, was used to study the material stiffness with a moving speed of 120 mm/min and a test angle of 45°, according to the ASTM D1388-14 standard.
The content of formaldehyde remaining in the fabric after treatment was measured using a TU-1810 UV–visible spectrophotometer provided by Beijing General Analysis Instrument Co. Ltd., China, according to the standard outlined in the GB/T 2912.1–2009 Water Extraction Method.
Results and discussion
NMR characterization of HPDPP
The NMR spectrum of HPDPP flame retardant is shown in Fig. 1. 1H NMR (D2O, 400 MHz) δ (ppm): 4.79 (D2O), 3.89 (6 H, H5), 3.60 (54 H, H6), 3.53(18 H, H7), 3.34 (30 H, H2, H10, H11), 2.95 (24 H, H3, H4, H8, H9), 1.15 (5 H, H1); 31P NMR (D2O, 400 MHz) δ(ppm): 8.41 (P1), 6.14 (P2), 0.29 (P3), 0.07 (P4), − 10.51 (P5). These data indicate that HPDPP was successfully synthesized.
Structural characterization of cotton samples
Figure 2a presents the FTIR spectra of HPDPP, FRC-40, and control cotton. The peaks at 3181 cm−1 and 1401 cm−1 in the spectrum of HPDPP correspond to the vibration absorption of –NH4+. The peaks at 1470 cm−1, 912 cm−1, and 767 cm−1 are attributed to the C–N, P–N, and P–C groups, respectively. The control cotton and FRC-40 spectra show the characteristic absorption peaks. More precisely, the wide peak at 3453 cm−1 is attributed to the O–H stretching vibration, and the absorption peaks at 2905 cm−1 and 1170 cm−1 are attributed to the –CH2 and C–O–C stretching vibrations, respectively. In the spectra of FRC-40 and HPDPP, the appearance of new peaks at 1656 cm−1, 1187 cm−1, and 1039 cm−1 is attributed to the N–H, P=N , and P–O–C stretching vibration (Liu et al. 2022b), respectively. Combining the durability of FRC-40, it suggested that HPDPP grafted onto the cotton fibers through P–O–C covalent bonds.
The diffraction peaks of the samples are presented in Fig. 2b. The small diffraction peak at 12.36° correspond to the (1–10) crystal plane of cellulose II. The diffraction peaks at 14.91°, 16.8°, 22.86°, and 34.74° correspond to the (1–10), (110), (200), and (004) crystal planes of cellulose I, respectively (French 2014). The shoulder at 20.34° corresponds to the (012) and (102) reflections from cellulose I. Compared with control cotton, the characteristic diffraction peak intensity of the HPDPP treated cotton fabrics are clearly reduced. This implies that HPDPP entered the amorphous space of the cotton fibers, and reacted with cellulose, which affected its crystal structure to some extent.
Surface morphology and elemental analysis
The SEM images showing the surface morphology of the untreated cotton fibers and HPDPP treated cotton fibers, are presented in Fig. 3. It can be seen that the surface of the pure cotton fibers has some wrinkles and the fibers have some curls. The FRC-40 fibers have a little swelling, which should be caused by the HPDPP molecules enter into the amorphous space of the cotton fibers. After burning (Fig. 3j–l), the surface of the HPDPP treated cotton fabrics after the AATCC 61-2013 3 A washing standard 50 LCs retains the complete structure. The treated cotton fibers retain the structural integrity of the fabric despite of the partial breakage, which suggests that the HPDPP acts as a condensed phase flame retardancy mechanism.
Table 2 presents the elemental contents of cotton fabrics treated with ammonium salt of diethylenetriaminepenta (methylenephosphonic acid) flame retardant (ADTPMPA)(Zheng et al. 2018) and ammonium salt of pentaethylenehexamine octa (methylene-phosphoric acid) flame retardant (APHOMPA)(Chen et al. 2020) with only ammonium phosphoric acid groups, HPDPP with –N=P–(N)3–, phosphoric acid ester, and ammonium phosphoric acid groups, after AATCC 61-2013 1A/3A washing standard 50 LCs (washing intensity: 1A < 3 A). After AATCC 61-2013 1A/3A washing standard 50 LCs, the total metal contents of cotton fabrics treated with flame retardants with phosphoric acid ester and ammonium phosphoric acid groups are much lower than those of the cotton fabrics treated with flame retardants only with ammonium phosphoric acid groups. These are because during the washing process, the NH4+ in the unreacted –P=O (O−NH4+)2 groups in flame retardant was easily exchanged by Ca2+, Mg2+, and Na+ etc. to form Ca (PO3)– and Mg (PO3)–, Na2 (PO3)– etc. groups, while these groups can not generate phosphoric acid and polymetaphosphoric acid at high temperature to promote the dehydration of cotton fabrics into char, so the flame retardants containing only this –P=O (O−NH4+)2 groups can only pass the AATCC 61-2013 1A washing standard 50 LCs (Careful/Gentle hand washing). However, all the phosphorus elements in the HCCP ring formed –N=P–(N)3– groups in the HPDPP flame retardant, which are very stable and can not combine the metal ions, and the phosphoric acid ester groups are also relatively stable. Therefore, the ratio of phosphorus groups directly combining Ca2+ etc. metal ions or hydrolyzing to combine Ca2+ etc. metal ions significantly decrease. As a result, the durability of cotton fabrics treated with HPDPP flame retardant increased greatly.
HPDPP thermal degradation behavior analysis
The impact of the HPDPP on the cotton fabrics thermal decomposition behavior is measured using a TG analyzer in N2 and air atmosphere. The TG and DTG curves are presented in Fig. 4a–d. Table 3 shows the initial decomposition temperature (Tonset), the maximum decomposition temperature (Tmax), and the residue at 700 °C. Under nitrogen atmosphere, the thermal decomposition process can be divided into three stages. The control cotton and FRC-40 have respectively a small weight loss at 40 to 322.9 °C and 40 to 260.2 °C, which corresponds to the evaporation of water due to moisture absorption(Nguyen et al. 2020). Thermal degradation occurs from 322.9 to 382.9 °C for control cotton and from 260.2 to 304.9 °C for FRC-40 in the second stage. For control cotton, it mainly decomposed into flammable gases, resulting the high weight loss. For treated cotton, the flame retardant decomposed to generate phosphoric acid and polymetaphosphoric acid to promote the char formation of cellulose, and decreased the generation of flammable gases compared with control cotton, resulting the low weight loss, and showing flame retardance. Such phenomena had been frequently reported in other phosphorus-containing flame retardant epoxy thermosets (Feng et al. 2018; Gholivand et al. 2022; Huo et al. 2020; Nguyen et al. 2020). At the third stage, when increasing the temperature to 700 °C, the residue further decreases. For FRC-40, the residue is 33.39% at 700 °C, while for control cotton, almost no residue is left. These results show that HPDPP changes the pyrolytic pathway and significantly improves the flame retardance of cotton fabrics.
The degradation trend of cotton fabrics in the air environment are nearly identical to those in N2. Only in the third stages, due to the fact that oxygen in air can oxidize the residue, the weight of the samples more rapidly decreases compared with those under nitrogen environment. However, the FRC-40 still has a residue of 10.78% after high temperatures. These results imply that the cotton fabrics treated with HPDPP have high flame retardance.
TG-FTIR analyses
TG-FTIR is used to investigate the differences of the pyrolysis volatile gases of control cotton and FRC-40 at high temperature. Figure 5 show the 3-dimensional (3D) TG-FTIR spectra of the cotton samples pyrolysis process. Six FTIR spectra of typical pyrolysis volatilization products versus time are also listed in Fig. 6. It can be seen that the peak absorption of pyrolytic volatiles reached around 14.4 min and 9.7 min for the control cotton and FRC-40, respectively. These pyrolysis volatilization products were mainly divided into inflammable gases and flammable volatiles. The H2O (3567 cm−1) absorption peaks reduced and CO2 (2356 cm−1) absorption peaks enhanced compared with the control cotton. The absorption intensities of carbonyl compounds (1713 cm−1) significantly decreased, and the absorption peaks of hydrocarbons (2975 cm−1) and ethers (1067 cm−1) almost disappeared. The new ammonia (1507 cm−1) absorption peak in the modified cotton was attributed to the thermal decomposition of the elemental N-containing flame retardant at high temperatures (Lu et al. 2023). Therefore, this indicated that treated cotton decomposed earlier than control cotton at high temperature, and released much less combustible gaseous substances in the pyrolysis process than the control cotton, which showed the flame retardant changed the pyrolysis pathway. It was consistent with TG results that the flame retardant promoted the cellulose to form lots of char residue to get flame retardance.
Cone calorimetry
To simulate the burning of the textile in a real fire, cone calorimetry tests were conducted on control cotton and FRC-40. The heat release rate (HRR) and total heat release (THR) versus time profiles upon exposure to heat flux at 35 kW/m2 are shown in Fig. 7. Table 4 presents some of the key relevant parameters for assessing the fire hazard of cotton fabric materials. After ignited, the control cotton burned rapidly with a peak heat release rate (pHRR) and THR value of 156.7 kW/m2 and 4.2 MJ/m2, respectively. As for FRC-40, the pHRR and THR are significantly lower than control cotton with 23.8 kW/m2 and 2.5 MJ/m2, respectively. The ratio of pHRR to TpHRR is the flame growth rate (FGR), which relates the material size to thermal reaction ability, the higher its value, the greater the fire hazard. Moreover, the total smoke production (TSP) of FRC-40 increase, which can attributed to the HPDPP that promoted dehydration of cotton fabric into char, and inhibited the oxidative degradation of cotton at high temperature, resulting in incomplete oxidative decomposition of cellulose, which exhibits reduced pHRR and THR, as well as increased smoke emission. Compared with control cotton, the CO2/CO ratio of FRC-40 decreases significantly, which means the combustion efficiency decreased gradually. This indicates that the formation of a char layer makes treated cotton fabrics burn less adequately (Piao et al. 2022). In addition, it can be clearly seen from Table 4 that the FRC-40 has a much lower probability of catching a fire, which can not be ignited, but the TTI of the control cotton fabric is 10 s, with 9.5% and 2.5% residue remaining at the end of the test, respectively. Thus, the fire safety performance of treated cotton fabrics is the best, performing excellent flame retardancy (Luo et al. 2022).
VFT and LOI analysis
Figure 8a and Table 1 show the vertical flame test graphs and relative data for cotton fabrics, respectively. For the control cotton, the cotton fabric is easily ignited, there is almost no char residue left after burning, just a little ash exists. In addition, the after-flame time and after-glow time are respectively 12 and 16 s in Table 1. However, in the case of treated cotton fabrics, the samples do not continuously burn removed of the fire source, and they retain an intact char frame structure. It can be observed from Fig. 8a and Table 1 that the concentration of the HPDPP solution increases, the char length of the HPDPP treated cotton fabrics is steadily reduced, FRC-40 is just 42 mm, and FRC-40-50LCs is 50 mm, which is significantly less than the standard (150 mm). In conclusion, the cotton fabrics treated with HPDPP are greatly improved with self-extinguishing behavior. Also, they do not exhibit after-glow time and after-flame time upon vertical flammability tests.
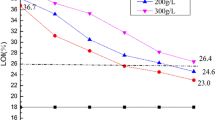
The LOI is the volume fraction concentration of oxygen in a mixture of oxygen and nitrogen when the polymer is just able to support its combustion. The control cotton LOI is approximately 17.7%. Figure 8b shows the LOIs of the HPDPP treated cotton fabrics after different laundering cycles. The LOI of FRC-40 can reach 41.3%, and it decreases to 29.7% after 50 LCs according to the AATCC 61-2013 3A washing standard. The LOI of FRC-30 is 26.5%, and that of FRC-20 is 26.1% after 30 LCs. In comparison, the APHOMPA treated cotton fabrics after 50 LCs is still 28.8% when using the AATCC 61-2013 1A washing standard (Chen et al. 2020), while the HPDPP treated cotton fabrics can meet the more restrictive requirements of the AATCC 61-2013 3A washing standard and could be considered as durable flame retardant fabrics.
The high durability of the cotton fabrics treated with HPDPP is consistent with the analyses presented in Sect. 1. Because all the phosphorus elements in the HCCP ring of HPDPP are only combined with the N element, the –N=P–(N)3– structure is very stable and does not hydrolyze. In addition, although there are some phosphoric acid groups in HPDPP, only a small proportion of them will hydrolyze and combine metal ions, which results in reducing the flame retardance. However, many tests showed that the cotton fabrics treated with HPDPP have high durability and flame retardancy.
Samples mechanical properties analysis
The tensile strength and bending length of the cotton fabrics with different add-on levels were tested to evaluate the impact of HPDPP on the inherent properties of cotton fabrics (Table 5). As the concentration of the HPDPP solution increases, the tensile strength of the treated cotton fabrics gradually decreases. Compared with control cotton, the tensile strengths of treated cotton fabrics show a decrease in the warp and weft directions, respectively. These phenomena should be the –P = (O−NH4+)2 groups generate –P=O(OH)2 groups in the grafting process (180 °C for 5 min), which generate acids to damage the cotton fabrics. The bending length is proportional to the soft hand of cotton materials, and the bending lengths of the treated cotton fabrics only increased slightly compared with control cotton, which does not affect the use of the cotton fabric in the textile market.
Formaldehyde content
The formaldehyde content of treated cotton fabrics is measured according to the GB/T2912.1-2009 standard. Figure 9 shows pictures of extracts from the control cotton and cotton fabrics treated with HPDPP after color development with acetylacetone. It can be seen that the cotton fabrics treated with HPDPP extract do not display any substantial color development. The residual formaldehyde content in the cotton fabrics is less than 20 mg/kg and can be assessed as “not detected”. This is because the formaldehyde finally changed to –CH2, and it is impossible for –CH2 to return to formaldehyde.
Conclusion
In this paper, HPDPP with –N=P–(N)3–, phosphoric acid ester, and reactive ammonium phosphoric acid groups is successfully synthesized for cotton fabrics to obtain a high durable reactive flame retardant without formaldehyde. The treated cotton fabrics have extremely excellent flame retardancy and durability. In addition, the 30% and 40% HPDPP treated cotton fabrics could pass the AATCC 61-2013 3A washing standard 50 LCs. The TG and cone calorimetry tests shows a condensed phase flame retardance mechanism. The obtained cotton samples have high durability due to the formation of P–O–C covalent bonds between the flame retardant and cellulose, the –N=P–(N)3– group deriving from HCCP and the phosphoric acid ester group can not hydrolyze to combine metal ions. The results show that HPDPP can be considered as a highly durable flame retardant. Finally, the HPDPP flame retardant molecule introducing –N=P–(N)3–, phosphoric acid ester groups, and reactive ammonium phosphoric acid groups can significantly improve the durability of cotton fabrics.
Data availability
The data are available.
References
Ali W, Zilke O, Danielsiek D, Salma A, Assfour B, Shabani V, Caglar S, Phan HM, Kamps L, Wallmeier R, Feng Y, Textor T, Gutmann JS, Mayer-Gall T (2023) Flame-retardant finishing of cotton fabrics using DOPO functionalized alkoxy- and amido alkoxysilane. Cellulose. https://doi.org/10.1007/s10570-022-05033-3
Angelini S, Barrio A, Cerruti P, Scarinzi G, Garcia-Jaca J, Savy D, Piccolo A, Malinconico M (2019) Lignosulfonates as fire retardants in wood flour-based particleboards. Int J Polym Sci 2019:1. https://doi.org/10.1155/2019/6178163
Barbalini M, Bartoli M, Tagliaferro A, Malucelli G (2020) Phytic acid and biochar: an effective all bio-sourced flame retardant formulation for cotton fabrics. Polymers 12(4):811. https://doi.org/10.3390/polym12040811
Bosco F, Carletto RA, Alongi J, Marmo L, Di Blasio A, Malucelli G (2013) Thermal stability and flame resistance of cotton fabrics treated with whey proteins. Carbohydr Polym 94(1):372–377. https://doi.org/10.1016/j.carbpol.2012.12.075
Chen Y, Wang D, Liu S, Lu Y, Zhang G, Zhang F (2020) A novel P–N-based flame retardant with multi-reactive groups for treatment of cotton fabrics. Cellulose 27(15):9075–9089. https://doi.org/10.1007/s10570-020-03387-0
Chen Y, Liu S, Wan C, Zhang G (2021) Facile synthesis of a high efficiency and durability L-citrulline flame retardant for cotton. Int J Biol Macromol 166:1429–1438. https://doi.org/10.1016/j.ijbiomac.2020.11.022
Chen Q, Zhang J, Li J, Sun J, Xu B, Li H, Gu X, Zhang S (2022) Synthesis of a novel triazine-based intumescent flame retardant and its effects on the fire performance of expanded polystyrene foams. Polym Degrad Stab 203:110079. https://doi.org/10.1016/j.polymdegradstab.2022.110079
Ding D, Liu Y, Lu Y, Liao Y, Chen Y, Zhang G, Zhang F (2022a) Highly effective and durable P–N synergistic flame retardant containing ammonium phosphate and phosphonate for cotton fabrics. Polym Degrad Stab. https://doi.org/10.1016/j.polymdegradstab.2022.109964
Ding D, Liu Y, Lu Y, Chen Y, Liao Y, Zhang G, Zhang F (2022b) A formaldehyde-free P–N synergistic flame retardant containing phosphonate and ammonium phosphate for cotton fabrics. J Nat Fibers. https://doi.org/10.1080/15440478.2022.2091711
Fan S, Lu X, Li H, Du X, Huang X, Ma Y, Wang J, Tao X, Dang Z, Lu G (2022) Efficient removal of organophosphate esters by ligand functionalized MIL-101 (Fe): modulated adsorption and DFT calculations. Chemosphere 302:134881. https://doi.org/10.1016/j.chemosphere.2022.134881
Feng Y, He C, Wen Y, Ye Y, Zhou X, Xie X, Mai YW (2017) Improving thermal and flame retardant properties of epoxy resin by functionalized graphene containing phosphorous, nitrogen and silicon elements. Compos Part A Appl Sci Manufac 103:74–83. https://doi.org/10.1016/j.compositesa.2017.09.014
Feng Y, He C, Wen Y, Ye Y, Zhou X, Xie X, Mai YW (2018) Superior flame retardancy and smoke suppression of epoxy-based composites with phosphorus/nitrogen co-doped graphene. J Hazard Mater 346:140–151. https://doi.org/10.1016/j.jhazmat.2017.12.019
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21(2):885–896. https://doi.org/10.1007/s10570-013-0030-4
Gholivand K, Faraghi M, Barzegari A, Fallah N, Dusek M, Eigner V (2022) Synthesis, characterisation, investigation of flame retardancy properties and thermal stability of two new phosphoramides with cotton fabric. J Anal Appl Pyrol 165:105568. https://doi.org/10.1016/j.jaap.2022.105568
Huo S, Yang S, Wang J, Cheng J, Zhang Q, Hu Y, Ding G, Zhang Q, Song P (2020) A liquid phosphorus-containing imidazole derivative as flame-retardant curing agent for epoxy resin with enhanced thermal latency, mechanical, and flame-retardant performances. J Hazard Mater 386:121984. https://doi.org/10.1016/j.jhazmat.2019.121984
Jia Y, Hu Y, Zheng D, Zhang G, Zhang F, Liang Y (2017) Synthesis and evaluation of an efficient, durable, and environmentally friendly flame retardant for cotton. Cellulose 24(2):1159–1170. https://doi.org/10.1007/s10570-016-1163-z
Kambli ND, Samanta KK, Basak S, Chattopadhyay SK, Patil PG, Deshmukh RR (2018) Characterization of the corn husk fibre and improvement in its thermal stability by banana pseudostem sap. Cellulose 25(9):5241–5257. https://doi.org/10.1007/s10570-018-1931-z
Khan AUH, Naidu R, Dharmarajan R, Fang C, Shon H, Dong Z, Liu Y (2023) The interaction mechanisms of co-existing polybrominated diphenyl ethers and engineered nanoparticles in environmental waters: a critical review. J Environ Sci (China) 124:227–252. https://doi.org/10.1016/j.jes.2021.10.018
Leong WI, Lo OLI, Cheng FT, Cheong WM, Seak LCU (2021) Using recombinant adhesive proteins as durable and green flame-retardant coatings. Synth Syst Biotechnol 6(4):369–376. https://doi.org/10.1016/j.synbio.2021.10.005
Li P, Liu C, Xu YJ, Jiang ZM, Liu Y, Zhu P (2020) Novel and eco-friendly flame-retardant cotton fabrics with lignosulfonate and chitosan through LbL: flame retardancy, smoke suppression and flame-retardant mechanism. Polym Degrad Stab 181:109302. https://doi.org/10.1016/j.polymdegradstab.2020.109302
Li C-B, Wang F, Sun R-Y, Nie W-C, Song F, Wang Y-Z (2022a) A multifunctional coating towards superhydrophobicity, flame retardancy and antibacterial performances. Chem Eng J 450:138031. https://doi.org/10.1016/j.cej.2022.138031
Li SQ, Tang RC, Yu CB (2022b) Flame retardant treatment of jute fabric with chitosan and sodium alginate. Polym Degrad Stab 196:109826. https://doi.org/10.1016/j.polymdegradstab.2022.109826
Liu S, Wan C, Chen Y, Chen R, Zhang F, Zhang G (2020) A novel high-molecular-weight flame retardant for cotton fabrics. Cellulose 27(6):3501–3515. https://doi.org/10.1007/s10570-020-03020-0
Liu J, Zhang X, Liu S, Lei C (2022) Char structure and charring mechanism of phosphazene-based epoxy resin during combustion. Polym Degrad Stab 200:109927. https://doi.org/10.1016/j.polymdegradstab.2022.109927
Liu Y, Ding D, Lu Y, Chen Y, Liao Y, Zhang G, Zhang F (2022b) Efficient and durable cotton fabric surface modification via flame retardant treatment. Coll Surf A. https://doi.org/10.1016/j.colsurfa.2022.129005
Lokhande KD, Bhakare MA, Bondarde MP, Dhumal PS, Some S (2022) Bio-derived efficient flame-retardants for cotton fabric. Cellulose 29(6):3583–3593. https://doi.org/10.1007/s10570-022-04478-w
Lu Y, Lu Y, Yang Y, Liu Y, Ding D, Chen Y, Zhang G (2023) Synthesis and application of a novel high durable cotton flame retardant rich in P[sbnd]N covalent bonds and ammonium phosphate groups. Chem Eng J. https://doi.org/10.1016/j.cej.2022.140422
Luo X, Li Z, Shen J, Liu L, Chen H, Hu Z, Krucinska I, Yao J (2022) A facile strategy to achieve efficient flame-retardant cotton fabric with durable and restorable fire resistance. Chem Eng J 430:132854. https://doi.org/10.1016/j.cej.2021.132854
Manfredi A, Carosio F, Ferruti P, Alongi J, Ranucci E (2018) Disulfide-containing polyamidoamines with remarkable flame retardant activity for cotton fabrics. Polym Degrad Stab 156:1–13. https://doi.org/10.1016/j.polymdegradstab.2018.07.028
Mayer-Gall T, Knittel D, Gutmann JS, Opwis K (2015) Permanent flame retardant finishing of textiles by allyl-functionalized polyphosphazenes. ACS Appl Mater Interfaces 7(18):9349–9363. https://doi.org/10.1021/acsami.5b02141
Nguyen HK, Sakai W, Nguyen C (2020) Preparation of a novel flame retardant formulation for cotton fabric. Materials. https://doi.org/10.3390/ma13010054
Ortelli S, Malucelli G, Blosi M, Zanoni I, Costa AL (2019) NanoTiO2 @DNA complex: a novel eco, durable, fire retardant design strategy for cotton textiles. J Coll Interface Sci 546:174–183. https://doi.org/10.1016/j.jcis.2019.03.055
Padhi SSP, Jimenez Bartolome M, Nyanhongo GS, Schwaiger N, Pellis A, van Herwijnen HWG, Guebitz GM (2022) Role of surface enhancement in the enzymatic cross-linking of lignosulfonate using alternative downstream techniques. ACS Omega 7(27):23749–23758. https://doi.org/10.1021/acsomega.2c02421
Piao J, Ren J, Wang Y, Feng T, Wang Y, Liu W, Dong H, Chen W, Jiao C, Chen X (2022) Green P–N coating by mechanochemistry: efficient flame retardant for cotton fabric. Cellulose 29(4):2711–2729. https://doi.org/10.1007/s10570-022-04436-6
Podkościelna B, Matuszewska A, Stefaniuk D, Ruminowicz-Stefaniuk M, Ciołek B, Jaszek M (2022) Interactions between biofiller-modified polymeric composites and wood-rotting fungi in terms of their biotechnological applications. Ind Crops Prod 186:115125. https://doi.org/10.1016/j.indcrop.2022.115125
Salmeia KA, Gaan S, Malucelli G (2016a) Recent advances for flame retardancy of textiles based on phosphorus chemistry. Polymers. https://doi.org/10.3390/polym8090319
Sun B, Yuan Z, Li K, Tang W, Xu J, Zheng H, Li G, Liu Y (2022) Remarkable toughness reinforcement of elastomer with cellulose microgel: employing polymer matrix and cellulose nanofiber network as the continuous phase and energy dissipation agent. Ind Crops Prod 186:115227. https://doi.org/10.1016/j.indcrop.2022.115227
Tawiah B, Yu B, Yang W, Yuen RKK, Fei B (2019) Flame retardant poly (lactic acid) biocomposites based on azo-boron coupled 4,4′-sulfonyldiphenol and its combination with calcium lignosulfonate—crystalline and mechanical properties. Polym Adv Technol 30(9):2207–2220. https://doi.org/10.1002/pat.4649
Thi H, Khanh VU, Huong NTHI (2019) Influence of crosslinking agent on the effectiveness of flame retardant treatment for cotton fabric. Ind Textila 70(5):413–420. https://doi.org/10.35530/IT.070.05.1610
Tian P, Lu Y, Wang D, Zhang G, Zhang F (2019) Synthesis of a new N–P durable flame retardant for cotton fabrics. Polym Degrad Stab 165:220–228. https://doi.org/10.1016/j.polymdegradstab.2019.04.024
Vishwakarma A, Reddy VJ, Kandola BK, Kumar V, Dasari A, Chattopadhyay S (2021) Egg white protein-hypophosphorous acid-based fire retardant single bilayer coating assembly for cotton fabrics. Cellulose 28(16):10689–10705. https://doi.org/10.1007/s10570-021-04208-8
Wan C, Liu M, Tian P, Zhang G, Zhang F (2020) Renewable vitamin B5 reactive N–P flame retardant endows cotton with excellent fire resistance and durability. Cellulose 27(3):1745–1761. https://doi.org/10.1007/s10570-019-02886-z
Wang Y, Xie T, Zhang J, Dang B, Li Y (2022) Green fabrication of an ionic liquid-activated lignocellulose flame-retardant composite. Ind Crops Prod 178:114602. https://doi.org/10.1016/j.indcrop.2022.114602
Xu F, Zhang G, Wang P, Dai F (2021) A novel ε-polylysine-derived durable phosphorus-nitrogen‐based flame retardant for cotton fabrics. Cellulose 28(6):3807–3822. https://doi.org/10.1007/s10570-021-03714-z
Xu F, He X, Hou Z, Cao X, Yang X, Ma Z, Cai J, Niu Y (2022) Effect of environment-friendly intercalated coal and coal gangue on flame‐retardant, antistatic, and mechanical properties of poly(vinyl chloride)/acrylonitrile–styrene–acrylate composites. J Vinyl Addit Technol. https://doi.org/10.1002/vnl.21935
Yu S, Xia Z, Kiratitanavit W, Kulkarni S, Kumar J, Mosurkal R, Nagarajan R (2021) Facile microwave assisted flame retardant treatment for cotton fabric using a biobased industrial byproduct: phytic acid. Cellulose 28(16):10655–10674. https://doi.org/10.1007/s10570-021-04191-0
Zhao P, Xu F, Chen Y, Huang T, Zhang G (2022a) A novel durable flame retardant for cotton fabrics based on diethylenetriamine. Polym Degrad Stab 195:109796. https://doi.org/10.1016/j.polymdegradstab.2021.109796
Zhao P, Xu F, Huang T, Wang P, Zhang G (2022b) A novel durable flame retardant with phosphonate groups and reactive ammonium phosphorus acid groups for cotton fabrics. J Nat Fibers. https://doi.org/10.1080/15440478.2022.2072444
Zheng D, Zhou J, Zhong L, Zhang F, Zhang G (2016) A novel durable and high-phosphorous-containing flame retardant for cotton fabrics. Cellulose 23(3):2211–2220. https://doi.org/10.1007/s10570-016-0949-3
Zheng D, Zhou J, Wang Y, Zhang F, Zhang G (2018) A reactive flame retardant ammonium salt of diethylenetriaminepenta(methylene-phosphonic acid) for enhancing flame retardancy of cotton fabrics. Cellulose 25(1):787–797. https://doi.org/10.1007/s10570-017-1543-z
Zhou X, Qiu S, Chu F, Xu Z, Hu Y (2022) An integrated intumescent flame retardant of bismaleimide from novel maleimide-functionalized triazine-rich polyphosphazene microspheres. Chem Eng J 450:138083. https://doi.org/10.1016/j.cej.2022.138083
Funding
This work was supported by the Open Project Program (BTBUFR21-2) from Petroleum and Chemical Industry Engineering Laboratory of Non-halogen Flame Retardants for Polymers, Beijing Technology and Business University, China.
Author information
Authors and Affiliations
Contributions
Material preparation, data collection and analysis were performed by Qian Tang. Software, formal analysis, and investigation were performed by YY and YL. Methodology and validation were performed by SD and YC. Resources, writing, review, and editing were performed by GZ.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Ethical approval and consent to participate
Comply with ethical standards.
Consent for publication
The manuscript has been approved for publication by all authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, Q., Yang, Y., Lu, Y. et al. A highly durable reactive flame retardant with –N=P–(N)3– and phosphoric acid ester groups for cotton fabrics. Cellulose 30, 10533–10550 (2023). https://doi.org/10.1007/s10570-023-05528-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05528-7