Abstract
Monolithic titania (TiO2) aerogel was fabricated by using bacterial cellulose (BC) as the bio-template and preceramic polymer as titanium resource, via freeze-drying and two-step calcination process. As-prepared TiO2 aerogel BCTi-air-2 h possessed low bulk density (0.04 g/cm3) and moderate mechanical strength. SEM images showed that TiO2 aerogel was composed of 3D reticulate TiO2 nanofibers. The anatase crystalline structure of TiO2 with crystal size around several nanometers was revealed by XRD measurements. N2 adsorption/desorption analysis indicated that TiO2 aerogel possessed high specific area (115 m2/g) and macroporous structure, which contributed to the high photocatalytic activity. Most importantly, the monolithic state will ease the usage, recycling, and regeneration of TiO2 aerogel as photocatalyst.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, bacterial cellulose (BC) attracts more and more attentions not only because of ever-increasing environmental concerns but also because of its unique structure and properties (Foresti et al. 2017; Chen et al. 2013; Lin et al. 2017; Wu et al. 2013). It is an extracellular product of bacteria (normally Acetobacter xylinum), possessing excellent mechanical strength, large specific surface area (SSA), and three-dimensional (3D) interconnected porous structure (Svensson et al. 2005; Wu and Cheng 2017; Hsieh et al. 2008). In the catalyst area, BC can be used as an ideal template or catalyst support, because it can simultaneously provide fast mass transfer ability and large space for chemical reactions. In addition, its monolithic state will ease the usage, recycling, and regeneration of catalyst.
Titania (TiO2) as photocatalyst has been extensively studied, owing to the exceptional performance on wastewater treatment and degradation of volatile organic compounds (VOC) (Liu et al. 2010). Photocatalytic activity of TiO2 is expected to be effectively enhanced by taking advantage of unique structure of BC. Previously, Zhang and Qi (2005) used BC as a sacrificial template to prepare mesoporous TiO2 networks consisting of anatase nanowires, but fine structure of BC was destroyed during fabrication process. Dal’Acqua et al. (2015) prepared Au/TiO2/BC composites by directly mixing TiO2/Au nanoparticles and BC, but 3D interconnected porous structure of BC also collapsed. Shi et al. (2016) and Liu et al. (2017) fabricated mesoporous SiO2/(WO3)x/TiO2/BC composite aerogels by using small-molecule precursors of TiO2 as starting materials, combined with the solvothermal method in the presence of BC. Although these composite aerogels retained fine microstructure of BC, carbohydrate nature of BC makes composite aerogels vulnerable under the harsh environments, such as high temperature, humidity, UV light, and acid/base conditions, resulting in poor durability and performance. Moreover, the complete and uniform coating of TiO2 on the surface of cellulose fibers was difficult to achieve because of poor film-forming properties of TiO2 nanoparticles and small-molecule precursors of TiO2 (Luo and Huang 2015). After removal of BC by calcination, monolithic material could not be obtained due to the collapse and shrinkage of 3D interconnected porous structure.
To solve the above problem, preceramic polymers instead of TiO2 nanoparticles and small-molecule precursors can be used. They possess precise composition and excellent processability. Through curing and calcination, they can be converted to various types of ceramics such as SiO2, SiC, SiCO, Si3N4, ZrO2, TiO2, HfC, TaC, ZrC/SiC, and HfB2/HfC/SiC/C composite ceramics (Colombo et al. 2010; Cai et al.2013a, b; Lu et al. 2015; Yu et al. 2017). In our previous work, a thin layer of N-doped TiO2 catalyst was successfully coated on the surface of quartz fabrics by the preceramic polymer route (Yu et al. 2017). In light of excellent film-forming properties of preceramic polymers, complete and uniform coating is possible to form on the surface of cellulose fibers and transform to corresponding ceramic nanofibers through calcination, eventually leading to the prefect replication of fine structure of BC.
Herein, this study aims at fabricating the monolithic TiO2 aerogel with the well-preserved 3D interconnected porous structure of BC, by using BC as the bio-template and preceramic polymer as the starting material. A novel fabrication route was developed, and corresponding mechanism was interpreted. The bulk density, morphology, crystalline structure, pore diameter distribution, SSA, and photocatalytic activity of samples were systematically investigated.
Experimental section
Materials
Purified BC sheets with a thickness of 1.5 cm biosynthesized by Acetobacter xylinum were kindly offered by Ms C.Y. Zhong (Hainan Yeguo Foods Co., Ltd., Haikou, China). Preceramic polymer aqueous solution (solid content ≈ 16 wt%, titanium content ≈ 9.4 wt%) was obtained from Tunable Materials Co., Ltd., Suzhou, China. Synthesis of preceramic polymer was described elsewhere in detail (Li et al. 2017). The above raw materials and other reagents were used as received without further treatment.
Preparation of monolithic composite aerogel
First, BC hydrogel was immersed into 0.03 g/mL (concocted according to the solid content) of preceramic polymer solution, and then kept for 48 h to ensure that preceramic polymer was homogeneously dispersed into BC hydrogel. Afterwards, the hydrogel containing preceramic polymer (BCTi hydrogel) was transferred to a plastic container and frozen in liquid N2 for 15 min, followed by freeze-drying process for 48 h and curing at 200 °C for 2 h. As-obtained sample (BCTi-cured) was kept in a desiccator to avoid adsorption of moisture.
Preparation of monolithic TiO2 aerogel
To fabricate monolithic TiO2 aerogel, two steps of calcination process were employed. First, the monolithic composite aerogel BC-cured was heated in a tube furnace under argon (Ar) atmosphere with a programmed temperature (RT → 300 °C/1 h → 450 °C/2 h with a heating rate of 1 °C/min). As-obtained sample was referred to as BCTi-Ar. Second, BCTi-Ar was calcined again in the tube furnace under air atmosphere with a programmed temperature (RT → 450 °C with a heating rate of 1 °C/min). According to different dwelling time at 450 °C, the prepared samples were referred to as BCTi-air-1 h, BCTi-air-2 h, BCTi-air-3 h, respectively.
Photocatalytic activity investigation
Photocatalytic activity of TiO2 aerogel for degradation of methyl orange was tested under UV light illumination. TiO2 aerogel (30 mg) was put into a quartz cuvette fulfilled with a 30 mL of methyl orange aqueous solution (15 mg/L). The cuvette was placed in the dark for 30 min to ensure the complete adsorption of methyl orange, and then illuminated by a mercury lamp (500 W). The degradation of methyl orange was monitored by UV–vis spectra (Shimadzu-UV-2600 spectrophotometer).
Characterization and instruments
Freeze-drying procedure was operated on a freeze dryer (LGJ-10E, Four-Ring Science Instrument Plant Beijing Co., Ltd., Beijing, China). Scanning electron microscope (SEM, HITACHI SU8020, Hitachi, Ltd., Tokyo, Japan) was used to observe the morphology of samples. X-ray diffraction (XRD) measurements were performed on a powder diffractometer (Rigaku D/MAX 2500, Rigaku Co., Tokyo, Japan) using a Cu/Ka radiation (40 kV, 200 mA, λ = 1.54056 Å). The specimens were continuously scanned from 3° to 90° (2θ) at a speed of 8°/min. N2 adsorption/desorption isotherms were measured at 77 K on a surface area and porosity analyzer (Micromeritics ASAP 2020, Micromeritics Instrument Co., Norcross, GA, USA).
Results and discussion
Route and mechanism for fabrication of monolithic TiO2 aerogel
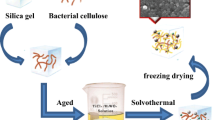
When preceramic polymer was directly treated by freeze-drying, samples severely cracked. After the introduction of BC, composite aerogel with intact shape can be obtained by freeze-drying, suggesting that BC had a strengthening effect on preceramic polymer matrix. However, TiO2 aerogel still easily cracked when composite aerogel was simply calcined in air. Through many efforts, monolithic TiO2 aerogel with intact shape was successfully prepared by an optimized route as shown in Fig. 1.
In the first step, preceramic polymer aqueous solution with the suitable concentration (around 0.03 g/mL) need to be prepared, because higher concentration will result in coalescence of microstructure, while lower concentration will cause dramatic shrinkage. Then, BC hydrogel was immersed into this solution and held for 48 h to ensure the homogeneous dispersion of preceramic polymer in the hydrogel. Ice crystals were generated and gradually grew during freezing in liquid N2. Meanwhile, preceramic polymer was precipitated from the solution and expelled to the boundary between cellulose fibers and ice crystals, leading to the formation of a thin layer of coating on the surface of cellulose fibers. After freezing procedure, 3D reticulate cellulose nanofibers coated with preceramic polymers would exist in ice crystals like impurity. Subsequently, ice crystals were removed by sublimation in freeze-drying process to avoid the collapse of fine structure of pristine BC, leaving preceramic polymer to attach to the surface of cellulose nanofibers. After curing at 200 °C preceramic polymer was fixed on the surface of cellulose nanofibers through chemical reactions between preceramic polymer and hydroxyl groups of cellulose fibers. The bulk density (Table 1) of yellow BCTi-cured aerogel (Fig. 2) was 0.03 g/cm3, obviously higher than that (0.01 g/cm3) of BC aerogel due to the incorporation of preceramic polymer.
To get monolithic TiO2 aerogel, two-step calcination process was employed. In the first calcination process, Ar was used as protecting and carrier gas, and composite aerogel was carbonized at 450 °C for 2 h to form the core–shell structure (Fig. 1). The inner layer was composed of carbon nanofibers which derived from the carbonization of cellulose nanofibers (Wu et al. 2013). The outer layer was composed of coating of TiO2 nanoparticles and free carbon which came from carbonization of preceramic polymer. Carbon nanofibers will enhance the strength of aerogel and aid the maintaining of 3D reticulate structure. Without the strengthening and supporting effects of carbon nanofibers, the decomposition of BC and preceramic polymer during calcination process will result in collapse and crack of aerogel. This explained why monolithic TiO2 aerogel could not be obtained by the direct calcination in air. After the first calcination, most of shrinkage had occurred, and the shrinkage in the second calcination process will be alleviated. During the second calcination process, residual carbon was gradually removed. At the same time, TiO2 nanoparticles were sintered and connected with each other to endow monolithic TiO2 aerogel with moderate mechanical strength for handling.
Finally, through immersion, freeze-drying, two-step calcination process, the pure monolithic TiO2 aerogel BCTi-air (Fig. 2) with a low bulk density of 0.04 g/cm3 and moderate mechanical strength was successfully fabricated.
Morphology observation
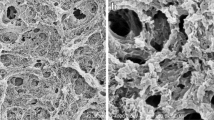
The homogeneous 3D interconnected porous structure of BC aerogel was observed in SEM images (Fig. 3). Through freeze-drying and curing procedures, the complete and uniform coating on the surface of cellulose nanofibers was shown, due to the excellent film-forming properties of preceramic polymers. After treatment at 450 °C for 2 h in Ar, 3D interconnected porous structure was well preserved, attributed to the strengthening and supporting effects of carbon nanofibers in BCTi-Ar. After the second calcination, fine structure of BC still remained in BCTi-air-1 h, BCTi-air-2 h, and BCTi-air-3 h. Based on these observations, it was confirmed that TiO2 aerogel had perfectly replicated the unique structure of BC.
Crystalline structure evolution
Main characteristic peaks of BC were shown at 2θ = 14.8°, 16.3°, and 22.7° (Fig. 4), which were assigned to cellulose I structure (Zhang et al. 2015). After curing at 200 °C, the wide peaks belonging to anatase phase (PDF card no. 73-1764) appeared, indicating that TiO2 crystals was generated. In BCTi-Ar, similar peaks were displayed, revealing that crystal size was kept unchanged. The residual carbon derived from the first calcination process may function as impurity to inhibit growth of TiO2 crystals. Once residual carbon was removed, characteristic peaks of anatase phase in BCTi-air-1 h became sharper, indicating growth of TiO2 crystals. With increase of dwelling time at 450 °C, TiO2 crystals further grew, as suggested by the intensified XRD signals. For BCTi-air-2 h, the crystal size was calculated as 6.8 nm according to the Scherrer equation. As aforementioned, all of BCTi-air aerogels possessed typical crystalline structure of TiO2 with anatase as the single phase.
N2 adsorption/desorption analysis
N2 adsorption/desorption results of BC, and BCTi samples were shown in Fig. 5 and Table 1. BC displayed type II isotherms, indicating the existence of macropores which can be also observed in SEM images (Fig. 3). 3D reticulate nanofibrous structure were responsible for the large SSA (102 m2/g) of BC. After incorporation of preceramic polymer, SSA of BCTi-cured was greatly increased to 291 m2/g which was higher than that (220 m2/g) of previous reports (Foresti et al. 2017), because preceramic polymer coating would provide much more fine structure. A slight decrease was shown for BCTi-Ar, caused by pyrolysis of preceramic polymer. After removal of residual carbon, SSA was dramatically decreased to 115 m2/g, which was higher than that (61 m2/g) of reported pure TiO2 aerogel derived from BC template (Zhang and Qi 2005), mainly because of the disappearance of fine structure derived from residual carbon. Moreover, with the increase of dwelling time at 450 °C, SSA was further decreased because of the shrinkage of skeleton and the growth of TiO2 crystals. N2 adsorption/desorption analysis revealed that monolithic TiO2 aerogel possessed 3D interconnected macroporous structure and large SSA. The former will give TiO2 aerogel with fast mass transfer ability, and the latter will provide large space for chemical reactions.
Photocatalytic activity study
Photocatalytic degradation of methyl orange under UV light was performed to evaluate photocatalytic activity of TiO2 aerogel. For BCTi-air-1 h, BCTi-air-2 h, and BCTi-air-3 h, most of methyl orange was degraded within 12 min (Fig. 6). Among them, BCTi-air-2 h demonstrated the highest photocatalytic activity. In the case of BCTi-1 h-air, although SSA was the highest, trace amount of residual carbon may exist, deteriorating photocatalytic activity of TiO2 crystals (Fig. 4). Whereas, for BCTi-air-3 h, the inferior photocatalytic activity was caused by decrease of SSA. Although the monolithic TiO2 aerogel demonstrated a little lower photocatalytic efficiency compared to nanopowder P25 (commercial TiO2 nanopowder, commonly used as the benchmark), in contrast, the monolithic state of TiO2 aerogel will facilitate separation and regeneration, and eventually ease practical applications.
Conclusions
In this work, monolithic TiO2 aerogel was successfully fabricated, by using BC as the bio-template and preceramic polymer as titanium resource, via the freeze-drying and two-step calcination process. As-obtained TiO2 aerogel possessed 3D reticulate nanofibrous structure, low bulk density, moderate mechanical strength, and high photocatalytic activity under UV light. These merits suggest that it may have great potential applications for photocatalyst. Moreover, the TiO2 aerogel may serve as a platform, on which sensors, electrode materials, and other catalyst systems can be engineered by doping.
References
Cai T, Qiu W, Liu D, Han W, Ye L, Zhao A, Zhao T (2013a) Synthesis of soluble poly-yne polymers containing zirconium and silicon and corresponding conversion to nanosized ZrC/SiC composite ceramics. Dalton Trans 42:4285–4429
Cai T, Qiu W, Liu D, Han W, Ye L, Zhao A, Zhao T (2013b) Synthesis, characterization, and microstructure of hafnium boride-based composite ceramics via preceramic method. J Am Ceram Soc 96:1999–2004
Chen L, Huang Z, Liang H, Guan Q, Yu S (2013) Bacterial-cellulose-derived carbon nanofiber@MnO2 and nitrogen-doped carbon nanofiber electrode materials: an asymmetric supercapacitor with high energy and power density. Adv Mater 25:4746–4752
Colombo P, Mera G, Riedel R, Sorarù GD (2010) Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics. J Am Ceram Soc 93:1805–1837
Dal’Acqua N, Mattos AB, Krindges I, Pereira MB, Barud HS, Ribeiro SJL et al (2015) Characterization and application of nanostructured films containing Au and TiO2 nanoparticles supported in bacterial cellulose. J Phys Chem C 119:340–349
Foresti ML, Vázquez A, Boury B (2017) Applications of bacterial cellulose as precursor of carbon and composites with metal oxide, metal sulfide and metal nanoparticles: a review of recent advances. Carbohydr Polym 157(Supplement C):447–467
Hsieh YC, Yano H, Nogi M, Eichhorn SJ (2008) An estimation of the Young’s modulus of bacterial cellulose filaments. Cellulose 15:507–513
Li Y, Pan X, Yang Z (2017) A method for preparation of the titania precursor and its solution. CN 2017113044355
Lin SP, Kung HN, Tsai YS, Tseng TN, Hsu KD, Cheng KC (2017) Novel dextran modified bacterial cellulose hydrogel accelerating cutaneous wound healing. Cellulose 24:4927–4937
Liu G, Wang L, Yang H, Cheng H, Lu G (2010) Titania-based photocatalysts-crystal growth, doping and heterostructuring. J Mater Chem 20:831–843
Liu D, Shi F, Liu J, Hu S, Yu L, Liu S et al (2017) Synthesis of SiO2–WxTiO2 composite aerogels via solvothermal crystallization under the guidance of bacterial cellulose followed by freeze drying method. J Sol Gel Sci Technol 84:42–50
Lu Y, Ye L, Han W, Sun Y, Qiu W, Zhao T (2015) Synthesis, characterization and microstructure of tantalum carbide-based ceramics by liquid polymeric precursor method. Ceram Int 41:12475–12479
Luo Y, Huang J (2015) Hierarchical-structured anatase-titania/cellulose composite sheet with high photocatalytic performance and antibacterial activity. Chem Eur J 21:2568–2575
Shi F, Yu T, Hu S, Liu J, Yu L, Liu S (2016) Synthesis of highly porous SiO2–(WO3)x·TiO2 composite aerogels using bacterial cellulose as template with solvothermal assisted crystallization. Chem Eng J 292(Supplement C):105–112
Svensson A, Nicklasson E, Harrah T, Panilaitis B, Kaplan DL, Brittberg M et al (2005) Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 26:419–431
Wu CN, Cheng KC (2017) Strong, thermal-stable, flexible, and transparent films by self-assembled TEMPO-oxidized bacterial cellulose nanofibers. Cellulose 24:269–283
Wu Z, Li C, Liang H, Chen J, Yu S (2013) Ultralight, flexible, and fire-resistant carbon nanofiber aerogels from bacterial cellulose. Angew Chem 125:2997–3001
Yu H, Ye L, Zhang T, Zhou H, Zhao T (2017) Synthesis, characterization and immobilization of N-doped TiO2 catalysts by a reformed polymeric precursor method. RSC Adv 7:15265–15271
Zhang D, Qi L (2005) Synthesis of mesoporous titania networks consisting of anatase nanowires by templating of bacterial cellulose membranes. Chem Commun 21:2735–2737
Zhang B, Azuma J, Uyama H (2015) Preparation and characterization of a transparent amorphous cellulose film. RSC Adv 5:2900–2907
Acknowledgments
This work is financially supported by the grants of the China Postdoctoral Science Foundation (No. 2017M612655). The authors also gratefully acknowledge the financial support from the Pearl River Talent Scheme (No. 2016ZT06C322).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, Bx., Yu, H., Zhang, Y. et al. Bacterial cellulose derived monolithic titania aerogel consisting of 3D reticulate titania nanofibers. Cellulose 25, 7189–7196 (2018). https://doi.org/10.1007/s10570-018-2073-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-2073-z