Abstract
Development of an ideal wound dressing to efficiently improve the wound healing process is an important issue in wound care. The present study aims to develop a dextran/bacterial cellulose (BC) hydrogel and to evaluate its performance in wound healing applications. The assessments include material properties (morphology, thermostability and its mechanical properties), cytotoxicity, cell proliferation and wound healing. The results show that the addition of dextran affected the network structure of BC resulting in decreased decomposition temperature (339–261 °C), water content (98.7–89.2%), and tensile strength (23–0.61 MPa). However, the elongation rates were kept at approximately 33–28% in BC, 10% and 20% in dextran modified groups. Cell-based experiments showed that the dextran-modified BC hydrogel promoted enhanced cell proliferation without cytotoxicity compared to unmodified BC. Finally, the in vivo wound healing test demonstrated that dextran-modified BC hydrogel can accelerate the wound healing process and facilitate skin maturation, which suggests that dextran/BC hydrogel is a promising wound dressing for clinical applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wound healing is an intricate process in which the tissue repairs itself after injury. The process of wound healing consists of four continuous and overlapping phases: hemostasis, inflammation, proliferation and remodeling (Cohen et al. 1992). These phases reveal complex interactions involving various types of cells (platelet, fibroblast and immune cell), migration and proliferation (Ausprunk and Folkman 1977), excretion of growth factor (Werner and Grose 2003), and extracellular matrix secretion (Martin 1997). In addition, various types of wounds (incision, puncture burn, and decubitus) are caused by physical or chemical injuries leading to bleeding, varying degrees of pain, a large amount of water loss, and exudation of tissue fluid (Bryant and Nix 2015). The choice of wound healing treatment is crucial and is indicated by the particular phase of healing and wound type. In traditional treatment of wound care, bandages and gauzes are often used to provide physical protection against pathogen infection. These treatments are easy to use and are low in cost. However, these dry dressings cannot address other functions, which may limit their utility for wound healing, as they do not promote wound healing, absorb the exudate, stem bleeding, debride the wound, or ease pain (Boateng et al. 2008). Therefore, developing advanced wound dressings becomes a crucial issue.
Current studies demonstrate that treating a wound with a moist environment can enhance re-epithelization of injured skin (Winter 1962), accelerate the wound healing process (Field and Kerstein 1994) and inhibit scar formation (Woerly et al. 2004). Based on these strengths, the wet-dressing has been developed over the course of many years, and has become the main focus in wound healing research.
Bacterial cellulose (BC), produced by microorganisms such as Komagataeibacter xylinus, is used in wound healing applications (Czaja et al. 2006) due to its material properties, including the ability to maintain high water content and good biocompatibility (Lin et al. 2013a). Moreover, BC also presents a regular network structure (typically a crystalline form of cellulose Iα), which provides flexible material properties and high strength (French 2014). Numerous BC dressing studies have been conducted to improve wound healing characteristics through usage of different cultivation systems and surface modifications of BC.
The features of BC dressings have been ameliorated by modification with polysaccharides (Lin et al. 2013b), small compounds (Wei et al. 2011), proteins (Fu et al. 2013) and chemical groups (Wan et al. 2006). For instance, the nano silver-incorporated BC dressing exhibits high antimicrobial properties against both Staphylococcus aureus and Escherichia coli, which are both common pathogens in nosocomial infection (Maneerung et al. 2008). The cellulase-incorporated BC dressing, which can disintegrate on its own, could be used to avoid re-injury and pain from repeated replacement of the dressing (Hu and Catchmark 2011).
Dextran is a polymeric carbohydrate molecule composed of a glucose subunit. In previous studies, dextran has been found to exhibit non-toxic biodegradability and good biocompatibility, and is widely applied in medical applications (Van Tomme and Hennink 2007). Some studies have demonstrated that dextran can form hydrogel, a three-dimensional structure capable of absorbing large amounts of water and biological fluids (Ribeiro et al. 2013), utilizing chemical-crosslinking (Zhang et al. 2005) and photo-crosslinking (Kim et al. 1999). Dextran hydrogel has also proved that it can enhance angiogenic responses and promote skin regeneration in vivo (Sun et al. 2011). These studies reveal that the dextran-based hydrogels have great potential in wound care.
The aim of the present study was to combine BC and dextran to potentially develop a new wound dressing. The effect of fibroblast cell growth on wounds was simulated by means of cell-based experiments including cytotoxicity and cell proliferation. Finally, the efficiency of dextran-modified BC hydrogel in wound healing in vivo was assessed using an animal model.
Experimental section
Production and preparation of dextran modified bacterial cellulose hydrogel
The bacterial strain used in this study was K. xylinus ATCC 700178 (formerly Gluconacetobacter), purchased from the American Type Culture Collection (Rockville, MD, USA). For BC production, 1 mL of frozen cell suspension was thawed and added to 50 mL corn steep liquor with a fructose medium (CSL-Fru medium) in a 250-mL flask and statically cultivated at 28 °C for 5 days. A cellulose pellicle that formed on the medium surface was washed continually until it becomes cream colored, and was then stored in deionic water at 4 °C.
The crosslinking of dextran and BC was accomplished using sodium trimetaphosphate (STMP) purchased from Sigma-Aldrich (Saint Louis, MO, USA). The wet BC film was immersed in different concentrations of dextran (Sigma-Aldrich, Saint Louis, MO, USA) at 4 °C for 48 h. After washing the surface of the BC to remove the excess dextran, the BC film with dextran was dipped in STMP solution (250 mg/mL) in a vacuum oven at 50 °C for 24 h. The dextran-modified BC hydrogel was washed by using deionic water overnight, and stored at 4 °C.
Thermogravimetric analysis (TGA) and water content testing
Isothermal TGA measurements were carried out in an N2 purge (40 mL/min) in a temperature range of 40–600 °C at a heating rate of 10 °C/min. In a water content test, the wet BC samples were placed at 25 °C for 30 min to remove excess water, and their weight was recorded as W t . The dried weight of BC samples was estimated as W 0. The water content was calculated using the following equation: [(W t − W 0)/W t ] 100%, where W 0 and W t represent the weights of dried and wet BCs, respectively.
Scanning electron microscopy (SEM)
The morphology was observed by SEM at an accelerating voltage of 15 kV (JSM-5410 model, Jeol, Tokyo, Japan). The modified BC hydrogels were lyophilized and coated with a thin layer of gold nanoparticles (50–200 Å). Imaging magnification to determine the surface structure of BC samples was approximately 20,000. At least 20 fiber measurements were detected by image processing software (ImageJ, NIST, USA) in order to ensure reproducible values when measuring fiber size distributions.
Tensile strength
The tensile strength measurement of wet-modified BC hydrogels was conducted using a texture analyzer (TA-XT2 model, Texture Technologies, Westchester, NY, USA). BC samples were clipped into rectangular strips (40 × 5 × 2 mm), and the tests were carried out at 0.1 N/min force at 28 °C temperature. Tensile strength (σ) was calculated by F/A, where A is the area of the sample (measured as width × thickness) and F is the force. Elongation (ε) was calculated by ∆L/L 0, where ∆L is exerted extension from the starting point L 0. (All measurements were performed for at least five replications).
Cell based experiments
NIH/3T3 mouse fibroblasts (American Type Culture Collection, Manassas, VA, USA) were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% FBS (Biological Industries, Israel), 2 mM l-glutamine and 100 U/mL penicillin–streptomycin and cultured in an incubator at 37 °C with 5% CO2. The modified BC hydrogels were cut into a circle with a diameter size of 10 mm, and were then sterilized at 121 °C for 20 min. Finally, the modified BC hydrogels were immersed into DMEM medium at 4 °C for 24 h.
In a cytotoxicity test, 1 × 105 cell/mL NIH/3T3 cells were seeded into a 96-well plate at 37 °C with 5% CO2 for 24 h. The DMEM medium, which was used to soak the modified BC hydrogel, was used to culture the NIH/3T3 cell for 24 h. The WST-1 reagent (Roche Diagnostics, Mannheim, Germany) was diluted (10 in 190 µL), and added 200 µL in each well for 3 h. The absorbance in each well was determined at 450 nm.
The modified BC hydrogels were immersed into DMEM medium for 24 h, and put into the bottom of a 24-well plate with 1 × 105 cell/mL NIH/3T3 cell in each well. After 24, 48, and 72 h cultivation, the modified BC hydrogels were transferred to a new 24-well plate, and washed twice using PBS buffer. The diluted WST-1 reagent was added, with 500 µL in each well for 3 h, and the absorbance was determined at 450 nm for analyzing the proliferation ability of modified BC hydrogels.
Wound healing modeling
A total of 60 adult mice were used for this study. The C57BL/6 mice (male, 8 weeks old) were purchased from the National Laboratory Animal Center (Taipei, Taiwan) and maintained in the Department of Food Science Technology at National Taiwan University (Taipei, Taiwan). All procedures involving animals were approved by the Institutional Animal Care and Use Committee. The animal room was kept on a 12-h light and 12-h dark cycle with constant temperature (25 ± 2 °C) and humidity. The mice were anesthetized by intraperitoneal injection of pentobarbital (60 mg/kg), and hairs on the dorsum were shaved off. Two holes ware punched into the backs of the mice to create an injury, and a plastic fixed ring was then sewed on. After the modified BC hydrogel was used to cover the injury, the Tegaderm® (3 M, St Paul, Minn, USA) and mesh bandage were used to fix the wound dressing to the injury. Each mouse was then individually housed and fed ad libitum. The wound closure was quantified at 0, 6, 9, and 12 days by using image processing software (ImageJ, NIST, USA). The area of wound closure was calculated by the following equation: \(\left[ {W_{t} /W_{0} } \right] \times 100\%\), where \(W_{0}\) and \(W_{t}\) represent for the area of wound closure at day 0 and days post-wounding (6, 9 and 12 days), respectively. BC, a commercial wound dressing product, was used as a positive control, and the Tegaderm® film was used as the negative control group.
In the histological observations, the wounds were obtained on postoperative days 3, 7, and 14. The wounds with the plastic ring were excised, washed by PBS, and then immersed in 10, 20, and 30% sucrose solution at 24, 48, and 72 h, sequentially. After being fixed in 4% paraformaldehyde for 24 h, the wound samples were embedded in an optimal cutting temperature compound (Sakura Finetek, Torrance, CA, USA) at 4 °C, and were cut into five-micrometer sections. The wound samples were stained by hematoxylin and eosin staining (H&E staining) according to the standard procedure (Bancroft and Gamble 2008).
Statistical analysis
Statistical evaluation of all experimental data (variation from basal values) were performed using ANOVA. Post hoc comparisons with negative controls were performed with the Tukey test or the Scheffe test. Statistical analysis was conducted with IBM SPSS Statistics 20 (IBM Corporation, Armonk, NY, USA) (p < 0.05).
Results and discussion
Morphology of dextran/BC hydrogel
The BC film is a high strength, flexible and gel-like pellicle (Lin et al. 2016). After cross-linking with dextran, the BC film became thicker and harder (Fig. 1), to an extent which varies depending on the concentration of added dextran. The morphological images of dextran/BC hydrogels were observed using SEM, and are shown in Fig. 2. As the figure shows, the BC group (Fig. 2a) presented with a compact network structure, and its fiber size reached 101.7 nm. In test groups, the dextran/BC hydrogels presented rougher fiber sizes, ranging from 159 to 161 nm (10 and 20% dextran/BC hydrogels); fiber structures in 30% dextran/BC hydrogel group were not observed. Previous studies have suggested that the cross-linked networks were formed in the contact areas between BC ribbons in reaction with the glyoxal precursors (Castro et al. 2015). The formation of covalent bonds between the BC ribbons led to increased interlayer adhesion of BC fibers.
In addition, some polysaccharides such as alginic acid, carboxymethylcellulose or chitosan, crosslinked with BC, also caused the modified BC fiber size to increase, and pore size to decrease (Leitão et al. 2013). These results may indicate that as dextran is incorporated inside the BC film, it starts to bridge with BC fiber utilizing the cross-linking reagent, resulting in the increase of the BC fiber size.
Thermogravimetric analysis
The effect of dextran cross-linked with BC on thermal degradation behavior were examined by TGA as shown in Fig. 3. In the TGA analysis, BC film showed a single peak at 339 °C in the detection range from 40 to 600 °C. In addition, the 10% dextran/BC group showed a similar thermal degradable temperature at 339 °C. However, in the 20 and 30% dextran modified BC groups, the thermal temperature decreased to 271 and 216 °C, respectively. These results demonstrated that the thermostability of dextran-modified hydrogels decreased depending on increased concentration of dextran, suggesting that the cross-linked dextran existed in the modified BC hydrogel, and its content was progressively raised by adding dextran. The pure BC films with thermal degradation temperatures around 315–350 °C possess high thermostability as shown in a previous study (Lin et al. 2016). The fibers of BC are connected to each other by hydrogen binding to form a tight network structure leading to greater stability at high temperatures. Nevertheless, in the dextran-modified BC groups, the intra-hydrogen bonds between the BC fibers were broken, and connected with the hydroxyl groups of dextran by the cross-linked reagent STMP.
Based on these results, the bonds subsequently-formed from modified hydrogels may be weaker than those of the original BC hydrogel. Several studies have suggested that the cross-linking reaction may lead cellulose network structure to became denser with improved thermostability (Yang et al. 2012). However, other studies have also shown that BC cross-linked with other polysaccharides (poly (vinyl alcohol)) and compound (acrylic acid) may cause a weak-branched structure resulting in the decline of crystallinity and thermostability of the modified BC (Castro et al. 2014). This may explain why the thermostability of dextran/BC hydrogels decreased in response to increased dextran addition.
Mechanical properties and water content ability
Hydrogel is a soft, gel-like polymer, with networks that can be extensively swollen with water so that it can provide a wet environment to facilitate cell growth (by fibroblast epidermal cells, for example) on the wound, and it is highly suitable as a wound dressing (Ahmed 2015). However, hydrogel dressings can be breakable; some applications rely on the second-layer dressing to prevent the hydrogel dressing from crumbling. Developing an elastic, flexible, but sufficiently strong hydrogel dressing for clinical use is the main purpose of this research.
In our study, the mechanical property analysis used wet-state samples because it is well-known that wet dressings can accelerate the wound healing process (Winter 1962). The results (Table 1) showed that wet BC film exhibits a high tensile modulus (23 ± 0.6 MPa), whereas the 10, 20, and 30% dextran-modified BC hydrogels exhibit significantly (p < 0.05) lower tensile modulus (16 ± 2.3, 6.6 ± 0.1 and 0.61 ± 0.13 MPa), depending on the added dextran concentration. The results suggest that the cross-linking reaction with dextran contributed to the reduction of tensile strength of the modified BC hydrogels. The decreased tensile strength may due to the breaking of hydrogen bonds between cellulose fibers by acid hydrolysis, and a cross-linked reaction.
In spite of the fact that they were broken, the BC fibers bonded with STMP and dextran to form new layers. The multi-layers in dextran/BC hydrogel might theoretically increase its tensile strength, however, when compared to the original network structure of BC, the newly-formed layers were too soft to bond with each other, resulting in a reduction in the tensile strength of modified hydrogel. A previous study also showed a similar effect (Svensson et al. 2005). Concerning the results of elongation-to-break, the BC film, 10 and 20% dextran/BC hydrogel, presented similar elongation values of 33 ± 2.6, 28.5 ± 3.9 and 31.5 ± 4.9%, without significant differentiation. In contrast, the elongation value of 30% dextran/BC hydrogel presented a sudden decrease (17 ± 3.5%), suggesting that the amount of dextran in the modified hydrogel might influence its flexibility. The formation of multiple layers by a cross-linked reaction may influence the relative movement between the molecular chains and disrupt the orderly network structure of the polymer (Marrs et al. 1999). The most important material property of hydrogel dressing is that it can provide a wet environment for wound healing. BC provides high water absorption and water content ability, as the free-water is incorporated into non-crystalline microfibrils of BC (Fink et al. 1997). In the water content analysis result (Table 1), the BC group exhibited the highest water retention ability (98.7 ± 0.1%) compared to other groups. However, in the dextran modified BC groups, the water content gradually decreased from 96.7 ± 0.49 to 89.2 ± 0.7%.
A previous study showed that the wet BC film consisted of 0.9% fiber, 0.3 bound-water, and 98.8% free-water, which suggests that most of water occurring in wet BC is free water (Okiyama et al. 1992). In this study, the modified dextran can provide hydroxyl groups to assist holding the bound water. However, the ratio of bound water accounts for only a small portion of the water incorporated in dextran/BC hydrogel. By comparison, increasing the crosslinking degree of modified BC hydrogel might cause more rigid and compact polymer structure formation, leading to inhibition of free water incorporation. As a result, the addition of dextran modified with BC may increase bound water but decrease free water incorporation, resulting in an overall reduction of water content.
Cell cytotoxicity and cell proliferation
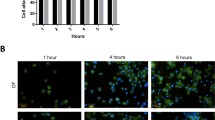
The safety of wound dressing is the top priority of this research, and even more important than its clinical efficiency. Therefore, it is necessary to ensure that dextran/BC hydrogels are available without any cytotoxicity. After immersing BC into the DMEM medium, the hydrogel-soaked medium was submitted to a cell cytotoxicity detection system (WST-1 cell viability assay) to confirm whether any cytotoxic substance may have been releases from the dextran/BC hydrogel.
Figure 4a shows that the cell viability in test groups of 10–30% dextran/BC hydrogel exhibited no significant difference compared to the control group, which means dextran/BC hydrogels do not cause cell death. Conversely, 10 and 20% dextran/BC hydrogel may slightly induce cell growth. This suggests that dextran released from the hydrogel may promote cell growth. A previous study also showed a similar result, that different molecular weights and concentrations of dextran sulfate, which were used to culture a CHO cell line, can improve its cell growth and viability (Hyoung Park et al. 2016).
In the wound healing stage, the proliferation of fibroblast cells is essential, since fibroblast cells can migrate to the wound area and secrete different types of collagens to facilitate wound healing (Ross et al. 1970); hence, the evaluation of the proliferation ability of a viable wound dressing candidate is crucial. The cell proliferation test result (Fig. 4b) showed that after 24 and 48 h incubation with medium replacement, the 20% dextran/BC hydrogel exhibited the highest proliferation ability compared to the control group and the 10% dextran/BC hydrogel group. This phenomenon was also observed in a previous study (Unnithan et al. 2012), which demonstrated that dextran-modified hydrogels exhibit higher fibroblast cell growth rates than hydrogels without dextran, signifying that dextran may present a biological function for helping cell growth.
The group of 30% dextran/BC hydrogel exhibited the lowest cell number after 24 h of cultivation. From one perspective, it presented a rough and soft surface with highly soft material properties; therefore, the NIH-3T3 cell cannot entirely adhere to the surface of hydrogel, resulting in decreased cell proliferation. Taking another viewpoint, the dextran might restrain the cell adhesion as well as its high viscosity, and the regulation of gene expression via dextran addition might be the possible reason for inhibition of cell adhesion (Li et al. 2008).
Wound healing in vivo
In the results of material property analysis and cell based experiments, the 10 and 20% dextran/BC hydrogels both presentedgood cell proliferation, high water content and suitable mechanical properties. Furthermore, the mechanical property results also demonstrated that the added dextran may cause dextran/BC to become softer and more fragile. Based on the cost considerations and mechanical property results, 10% dextran/BC hydrogel was selected for use in an in vivo experiment to measure its wound healing ability in a mouse model. Wet BC film, a commercial wound dressing product with good performance, was used as a positive control to compare with the modified hydrogel, and a dried film-Tegaderm®, which provides physical protection, was used as a negative control. After the wound was produced, the wound morphologies and wound closures were measured.
In the wound healing result (Fig. 5a), we observed that pus existed in the wounds of the negative group. However, in the group of BC and dextran/BC treatments, the wounds exhibited red-fresh color without pus formation, which suggested that BC and dextran/BC both presented good performance for preventing pathogen infection. The BC and dextran/BC hydrogels both possess nano-scale network structures that can provide physical protection to deter microorganisms from penetrating the wound (Nakagaito et al. 2005).
The dynamic healing process. a Photographs of wound healing on day 6, day 9, day 12 and day 15 and b closure quantification of wound on day 6, day 9, and day 12 under the treatment of different dressings. Values for each sample with different superscripts are significantly different (n = 3, *p < 0.05)
In the quantification of wound closure (Fig. 5b), three groups showed no differences during the early stage undergoing inflammation. After 9 days, when the wound entered the proliferation stage, the dextran/BC presented the highest ability for accelerating wound healing, owing to its high water content ability which kept the wound moist. In addition, in the cell-based experiment, results demonstrated that dextran/BC induced fibroblast cell-NIH-3T3 cell proliferation. The fibroblast cell plays important roles in the proliferation stage in vivo, such as secreting different types of collagens to accelerate wound closure (Ross et al. 1970). Therefore, the ability of dextran to induce fibroblast cell growth may explain why dextran/BC hydrogel provides wound healing ability superior to that of unmodified BC. In a 12-day treatment with dextran/BC hydrogel, the area of the wound was decreased by 40.3 ± 5.3%, which is less than the negative and BC groups (60.4 ± 7.5 and 52.8 ± 3.42%). Even though it’s difficult to measure the wound's closure in 15 days of treatment, the morphology of the wound indicated that it was almost closed in the dextran/BC treated group. These results suggest that dextran/BC hydrogel can actually improve wound healing, and it shows great potential as a wound dressing.
In histochemical staining, 3, 7, and 14 days were selected to observe the conditions of early, middle, and late stages of the wound. In the early and middle stages, the thickness of the wound was not significantly different between dressing groups (Fig. 6). However, the late stage result demonstrated that the dextran/BC hydrogel promoted significant skin maturation (p < 0.05), including a mature epithelial layer and formation of hair follicles. Therefore, we may speculate that the function of dextran in the modified BC dressing can increase fibroblast cell proliferation in the middle stage and improve skin maturation in the late stage.
A previous study indicated that polyethylene glycol diacrylate (PEGDA) -modified dextran hydrogel can enhance angiogenesis and promote skin regeneration during burn wound healing. By day 21, the PEGDA-modified dextran hydrogel treated wound revealed a mature epithelial structure (Sun et al. 2011). Another study developed a human adipose dressing and found that it can help to advance the remodeling stage of wound on day 18 through inducing fibroblast migration to the wound (Martin et al. 2015). Comparing the results of these studies, the dextran/BC hydrogel can heal the wound to regenerate skin with follicle structure within 14 days, which is faster than the previous studies, suggesting that dextran/BC hydrogel provides an excellent recovery medium for complete skin regeneration.
Conclusion
This is the first report to apply dextran/BC hydrogel as a wound dressing. Our results indicated that the modified dressing—dextran/BC hydrogel—appears to contain a high water content, rigid but flexible material properties, fine cell proliferation with non-cytotoxicity at the cell-based level, and excellent wound healing ability in vivo. The added dextran plays a key role in that it promotes fibroblast cell growth in the proliferation stage and also helps skin maturation in the remodeling stage, accelerating of the wound healing process. It is important to note that this is superior to the commercial wound dressings. Human studies are needed to further investigate the applications as a therapy for different types of wounds.
References
Ahmed EM (2015) Hydrogel: preparation, characterization, and applications: a review. J Adv Res 6:105–121
Ausprunk DH, Folkman J (1977) Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res 14:53–65
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques. Elsevier Health Sciences, New York
Boateng JS, Matthews KH, Stevens HN, Eccleston GM (2008) Wound healing dressings and drug delivery systems: a review. J Pharm Sci 97:2892–2923
Bryant RA, Nix DP (2015) Acute and chronic wounds: current management concepts. Elsevier Health Sciences, New York
Castro C et al (2014) In situ production of nanocomposites of poly (vinyl alcohol) and cellulose nanofibrils from Gluconacetobacter bacteria: effect of chemical crosslinking. Cellulose 21:1745–1756
Castro C et al (2015) In situ glyoxalization during biosynthesis of bacterial cellulose. Carbohydr Polym 126:32–39
Cohen IK, Die-gelmann RF, Lindblad WJ, Hugo NE (1992) Wound healing: biochemical and clinical aspects. Plast Reconstr Surg 90:926
Czaja W, Krystynowicz A, Bielecki S, Brown RM (2006) Microbial cellulose—the natural power to heal wounds. Biomaterials 27:145–151
Field CK, Kerstein MD (1994) Overview of wound healing in a moist environment. Am J Surg 167:S2–S6
Fink HP, Purz HJ, Bohn A, Kunze J (1997) Investigation of the supramolecular structure of never dried bacterial cellulose. In: Macromolecular symposia, vol 1. Wiley, pp 207–217
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21(2):885–896
Fu L, Zhang J, Yang G (2013) Present status and applications of bacterial cellulose-based materials for skin tissue repair. Carbohydr Polym 92:1432–1442
Hu Y, Catchmark JM (2011) In vitro biodegradability and mechanical properties of bioabsorbable bacterial cellulose incorporating cellulases. Acta Biomater 7:2835–2845
Hyoung Park J, Sin Lim M, Rang Woo J, Won Kim J, Min Lee G (2016) The molecular weight and concentration of dextran sulfate affect cell growth and antibody production in CHO cell cultures. Biotechnol Prog 32(5):1113–1122
Kim S, Won C, Chu C (1999) Synthesis and characterization of dextran-based hydrogel prepared by photocrosslinking. Carbohydr Polym 40:183–190
Leitão AF, Silva JP, Dourado F, Gama M (2013) Production and characterization of a new bacterial cellulose/poly (vinyl alcohol) nanocomposite. Materials 6:1956–1966
Li D, Dai K, Tang T (2008) Effects of dextran on proliferation and osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Cytotherapy 10:587–596. doi:10.1080/14653240802238330
Lin SP, Loira Calvar I, Catchmark J, Liu JR, Demirci A, Cheng KC (2013a) Biosynthesis, production and applications of bacterial cellulose. Cellulose 20:2191–2219. doi:10.1007/s10570-013-9994-3
Lin WC, Lien CC, Yeh HJ, Yu CM, Hsu S (2013b) Bacterial cellulose and bacterial cellulose–chitosan membranes for wound dressing applications. Carbohydr Polym 94:603–611
Lin SP, Liu CT, Hsu KD, Hung YT, Shih TY, Cheng K-C (2016) Production of bacterial cellulose with various additives in a PCS rotating disk bioreactor and its material property analysis. Cellulose 23:367–377. doi:10.1007/s10570-015-0855-0
Maneerung T, Tokura S, Rujiravanit R (2008) Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr Polym 72:43–51
Marrs H, Barton D, Jones R, Ward I, Fisher J, Doyle C (1999) Comparative wear under four different tribological conditions of acetylene enhanced cross-linked ultra high molecular weight polyethylene. J Mater Sci Mater Med 10:333–342
Martin P (1997) Wound healing- aiming for perfect skin regeneration. Science 276:75–81
Martin PM, Maux A, Laterreur V, Mayrand D, Gagné VL, Moulin VJ, Fradette J (2015) Enhancing repair of full-thickness excisional wounds in a murine model: impact of tissue-engineered biological dressings featuring human differentiated adipocytes. Acta Biomater 22:39–49
Nakagaito A, Iwamoto S, Yano H (2005) Bacterial cellulose: the ultimate nano-scalar cellulose morphology for the production of high-strength composites. Appl Phys A 80:93–97
Okiyama A, Motoki M, Yamanaka S (1992) Bacterial cellulose II. Processing of the gelatinous cellulose for food materials. Food Hydrocoll 6:479–487
Ribeiro M, Morgado P, Miguel S, Coutinho P, Correia I (2013) Dextran-based hydrogel containing chitosan microparticles loaded with growth factors to be used in wound healing. Mater Sci Eng C 33:2958–2966
Ross R, Everett NB, Tyler R (1970) Wound healing and collagen formation VI. The origin of the wound fibroblast studied in parabiosis. J Cell Biol 44:645–654
Sun G et al (2011) Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc Natl Acad Sci USA 108:20976–20981
Svensson A, Nicklasson E, Harrah T, Panilaitis B, Kaplan D, Brittberg M, Gatenholm P (2005) Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 26:419–431
Unnithan AR et al (2012) Wound-dressing materials with antibacterial activity from electrospun polyurethane–dextran nanofiber mats containing ciprofloxacin HCl. Carbohydr Polym 90:1786–1793
Van Tomme SR, Hennink WE (2007) Biodegradable dextran hydrogels for protein delivery applications. Expert Rev Med Devices 4:147–164
Wan Y, Hong L, Jia S, Huang Y, Zhu Y, Wang Y, Jiang H (2006) Synthesis and characterization of hydroxyapatite–bacterial cellulose nanocomposites. Compos Sci Technol 66:1825–1832
Wei B, Yang G, Hong F (2011) Preparation and evaluation of a kind of bacterial cellulose dry films with antibacterial properties. Carbohydr Polym 84:533–538
Werner S, Grose R (2003) Regulation of wound healing by growth factors and cytokines. Physiol Rev 83:835–870
Winter GD (1962) Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 193(4812):293–294
Woerly S, Doan VD, Sosa N, de Vellis J, Espinosa-Jeffrey A (2004) Prevention of gliotic scar formation by NeuroGel™ allows partial endogenous repair of transected cat spinal cord. J Neurosci Res 75:262–272
Yang L, Zhang HY, Yang Q, Lu Dn (2012) Bacterial cellulose-poly (vinyl alcohol) nanocomposite hydrogels prepared by chemical crosslinking. J Appl Polym Sci 126:E245–E251
Zhang R, Tang M, Bowyer A, Eisenthal R, Hubble J (2005) A novel pH-and ionic-strength-sensitive carboxy methyl dextran hydrogel. Biomaterials 26:4677–4683
Acknowledgments
The authors are very grateful to Shin-Yu Lai from Department of Anatomy and Cell Biology, School of Medicine at National Taiwan University for her assistance with the animal model experiment and H&E staining. The authors would also like to thank William Gerin, a Professor of Department of Biobehavioral Health, The Pennsylvania State University, for English editing.
Funding
This work was sponsored in part by “Aim for the Top University Plan” 102C3619 of National Taiwan University and the National Science Council, Taiwan, under Contract No. 102-2628-B-002-004-MY3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflict of interest to disclose.
Rights and permissions
About this article
Cite this article
Lin, SP., Kung, HN., Tsai, YS. et al. Novel dextran modified bacterial cellulose hydrogel accelerating cutaneous wound healing. Cellulose 24, 4927–4937 (2017). https://doi.org/10.1007/s10570-017-1448-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1448-x