Abstract
This study investigated the possibility of substituting petroleum-based polymers with biopolymers for films and paper coatings. Arabinoxylan (AX) was extracted from distillers’ grains, a low-value corn ethanol byproduct, and modified through crosslinking with glutaraldehyde (GA) which was made into films and paper coatings. The effects of degree of substitution (DS) on film and coating properties of GA cross-linked AX, referred to as GAX, were investigated. The GAX films had markedly higher tensile strength, approximately 3 times higher than the unmodified AX films at low DS, with higher DS causing a negative effect on the film tensile strength. Compared to unmodified AX coating, paper coated with GAX also had significantly higher tensile index, presumably due to high adhesion between the coating and paper interface. When used as a coating binder with calcium carbonate pigments, GAX showed comparable performance to polyvinyl alcohol, a common industrial binder, demonstrating the potential to be substituted for the petroleum-based paper coating binder.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Paper is one of the oldest man-made materials and made from wood or non-wood lignocellulosic materials. The fibrous structure imparts paper surface with porousness and roughness, which can be minimized but not eliminated by sizing and fiber choice (Barhoum et al. 2014). The abundant hydroxyl groups on the cellulose surface can also impart paper with high hydrophilicity (high ink absorbency). Consequently, pigment coatings are widely applied to fill in the cavities and cover the paper surface to produce a tight and smooth surface, reduce ink adsorption, improve paper’s optical properties and enhance its barrier properties (Barhoum et al. 2014; Laudone et al. 2006). Some common pigment coatings include calcium carbonate, bentonite clay, titanium dioxide, silica, and talc (Barhoum et al. 2014; Laudone et al. 2006). Pigment coatings are inorganic compounds and need additives, typically polymers, to bind them together and onto the paper surface. Some widely used binders include polymers of styrene butadiene, styrene butadiene acrylonitrile, styrene acrylic and vinyl acetate (Fardim 2002). These non-biodegradable polymers are mostly synthesized from petroleum and thus raise problems for sustainability, environment and paper recycling (Aulin and Lindström 2011). Therefore, finding sustainable polymer such as bio-polymer coating binders is of great interest.
The biopolymers widely studied for producing films and coatings may be categorized into three major classifications including polysaccharides, proteins and lipids (Guilbert et al. 1997). Among them, polysaccharides are the most abundant bio-polymers including cellulose, starches, hemicelluloses and chitosan (Aulin and Lindström 2011). Films from polysaccharides usually have good oil barrier properties, but are typically hydrophilic (Aulin and Lindström 2011). Produced from cellulose, nano-fibrillated cellulose readily forms strong and transparent films (Aulin et al. 2010; Wang et al. 2013; Zhu et al. 2011). Unmodified and modified (oxidized, cationized, hydroxyalkylated, etc.) starch is used widely as paper sizing agents (Maurer 2009). Modified cellulose or starch is also used in the paper coating as co-binders or thickeners (Fardim 2002; Maurer 2009). Chitosan finds its promising use for medical applications due to its antibacterial properties (Aulin and Lindström 2011). Hemicelluloses also form film readily, but the film is usually weak and highly hygroscopic without modification (Hansen and Plackett 2008). On the other hand, different from cellulose and starch, which already have important uses in material, food or bio-energy industries (Xiang et al. 2015), hemicelluloses have still been underutilized (Xiang et al. 2014a; Hartman et al. 2006a; Xing et al. 2011) and therefore the studies on their practical applications may improve hemicelluloses’ industrial potentials.
To exploit the industrial potential of hemicelluloses and improve the economy of corn ethanol industries, our group has investigated the potential utilizations of the hemicelluloses in distillers’ grains (DG), a corn ethanol byproduct. We have converted the hemicelluloses in DG into furfural (Xiang and Runge 2014), or utilized them as emulsifying agents (Xiang et al. 2014b). In a previous study, we extracted the DG hemicelluloses by alkaline solution and processed them into films and paper coatings (Xiang et al. 2014a). However, the films suffered from low mechanical strength and were highly hygroscopic.
Different from cellulose and starch, which are homogeneous polymers with high molecular weight consisting exclusively of D-glucopyranose, hemicelluloses are heterogeneous polymers with low molecular weight consisting of different types of monomers (d-xylose, l-arabinose, d-galactose, d-mannose, etc.) (Ebringerová and Heinze 2000). The hemicelluloses found in corn and thus DG are mainly arabinoxylans (AX) (Xiang et al. 2014a; Gáspár et al. 2007). AX has a backbone of β-(1-4)-d-xylopyranose units being substituted at C-2 or/and C-3 position with side-chains consisting of one or more monomers of α-l-arabinofuranose, d-galactopyranose and d-glucuronic acid (Scheme 1) (Xiang et al. 2014a; Ebringerová and Heinze 2000).
Proposed structure of corn AX (adapted from Ebringerová and Heinze 2000)
Previous studies have chemically modified hemicelluloses to improve the properties of their films or coatings (Lindblad and Albertsson 2005; Ebringerová et al. 2005). Similar to cellulose modification, the etherification of hemicelluloses with epoxides or alkyl, carboxymethyl and benzyl halides, and the acylation of hemicelluloses with carboxylic acid derivatives are usually achieved in alkaline or N,N-dimethyl formamide (DMF)/LiCl solutions with or without catalysts (Lindblad and Albertsson 2005; Ebringerová et al. 2005). Ethylation of AX was done by reaction with ethyl iodine in DMF (Saghir et al. 2009). Carboxymethylated xylan/AX can be prepared by reaction with sodium monochloroacetate in alkaline solution (Alekhina et al. 2014; Petzold et al. 2006; Saghir et al. 2008). With increasing degree of substitution (DS), carboxymethylated xylan have shown reduced oxygen permeability and increased water vapor permeability, but decreased mechanical strength (Alekhina et al. 2014). The films made of benzylated O-acetylgalactoglucomannan hemicelluloses have demonstrated significantly increased oxygen permeability and decreased water adsorption ability compared to unmodified hemicelluloses (Hartman et al. 2006b). By reacting xylan with propylene oxide in alkaline solution, hydroxyalkylation of xylan can be achieved (Jain et al. 2000; Laine et al. 2013). Hydroxypropyl xylan has been studied as a paper coating binder, but it had lower oxygen and water vapor permeability compared to commercial biopolymer coatings (Laine et al. 2013).
When considering functionalizing the AX, aldehydes were considered, as they are able to form acetals when reacting with hydroxyl groups, and thus it was hypothesized that introducing aldehyde groups onto hemicelluloses might be able to crosslink the hemicelluloses with themselves to make strong films or with fiber-based surfaces, such as fiber or wood, to impart coatings with great adhesion. Glutaraldehyde (GA) is a dialdehyde compound (Scheme 2), which is widely used to crosslink biopolymers with hydroxyl or amine groups. Studies have been reported on reacting GA with starch (El-Tahlawy et al. 2007; Lv et al. 2012), protein (Bigi et al. 2001; Matsuda et al. 1999), guar gum (Gliko-Kabir et al. 1999; Soppirnath and Aminabhavi 2002), and chitosan (Monteiro and Airoldi 1999; Arguelles-Monal et al. 1998). However, very few studies have been done on crosslinking hemicelluloses with GA.
Modified hemicelluloses having a high DS have been found to negatively impact film properties (Alekhina et al. 2014). Therefore, controlling the DS was expected to be a key variable for improving hemicelluloses properties. However, there are few studies conducted on how the material properties of modified hemicelluloses are affected by low level of DS. Thus, the effect of low DS on the modified hemicelluloses material properties was also of interest for investigation in this study.
Experimental section
Fractionation of DG arabinoxylan
Distillers’ grains (DG) samples were obtained from Didion Milling Inc. (Cambria, WI, USA). AX was extracted from DG and purified according to the procedure described previously (Xiang et al. 2014b). Briefly, DG was pre-extracted by acetone for 12 h in a Soxhlet extractor to remove fats and organic impurities. The DG was then reacted in 3 % (w/v) NaOH solution at 50 °C at 1:10 solid to liquid ratio (w/v) for 3 h. The separated alkali-soluble part was adjusted to pH 5.5, purified with bentonite clays, and slowly poured into three volume of 95 % ethanol with constant stirring. The precipitated solid was washed several times with 70 % ethanol, freeze-dried, and ground to give a powder form of AX.
The extracted AX was in a white powder form and consisted of (based on the oven-dried weight) 19 % arabinan, 28 % xylan, 5 % galactan, 6 % glucan, 2 % mannan, 5 % uronic acid, and 12 % crude protein. Number average molecular weight (M n) of the AX was 62 kDa, weight average molecular weight (M w) was 450 kDa, and the polydispersity index (M w/M n) was 7.2. The compositions and molecular weights of the AX were consistent with and detailed discussions can be seen in our previous studies (Xiang et al. 2014a, b).
Modification of DG arabinoxylan
The AX powder was dissolved in deionized (DI) water to make a 5 % (w/v) solution and the pH was adjusted to 3.0. Different amounts of GA (25 % in water, BP2548-1, Fisher Scientific, PA, USA) were then added to the solution leading to GA/arabinoxylose unit (AXU) mole ratios of 0.01, 0.05, 0.1, 0.5, 1, 2, 3. AXU stands for AX mono sugar unit, which has a molar mass of 132 g/mol assuming the AX consists of only arabinose and xylose since the content of other minor units is very small (Xiang et al. 2014a, b). The mixture was maintained at 30 °C with stirring for 2 h. The GA modified arabinoxylan (GAX) was precipitated by pouring the mixture into three volumes of 95 % ethanol and was washed three times with 70 % ethanol. Three discrete samples were made for each modification to allow for statistical intervals to be calculated.
Characterization of modified DG arabinoxylan
The DS of a GAX sample was defined as the mole of GA reacted per mole of AXU. The samples were coded as GAXn, where the number n denotes the DS.
The free aldehyde content of GAX sample was determined based on Yu et al. (2010) by using hydroxylamine hydrochloride. In parallel, the aldehyde content in the solution after GAX precipitation was also measured to determine the unreacted GA content. By subtracting the amount of unreacted GA from the amount of GA added to the reaction, the amount of reacted GA and thus the DS of the GAX sample were determined.
13C NMR spectra of AX and GAX samples were acquired on a Bruker Biospin (Billerica, MA, USA) AVANCE 700 MHz spectrometer fitted with a cryogenically-cooled 5-mm TXI gradient probe with inverse geometry (proton coils closest to the sample). Finely ball-milled samples (~50 mg) were swelled in D2O (1 mL). The spectra were recorded after 8000 normal scans and processed using Topspin 3.1 software (Bruker, Billerica, MA, USA).
Preparation and characterization of modified arabinoxylan films
Approximately 0.8 g of GAX was dispersed in 8 mL of DI water with magnetic stirring at 350 rpm for 30 min at 90 °C to make a 10 % (w/v) suspension. The suspension was poured out evenly on a 101 mm × 83 mm glass plate covered with non-stick polytetrafluoroethylene tape ensuring even thickness of the film, which was measured to be approximately 0.11 mm. The suspension was then allowed to dry at room temperature for 24 h. The formed film was equilibrated in a climatic chamber at 30 °C and 40 % relative humidity (RH).
Three films from each modification (with different GA/AXU ratios) were made and each film was cut into 6 specimens with a 10 mm width. The tensile strength of the specimens was evaluated on a MTS® Insight tensile testing machine (MTS Systems Corp, Eden Prairie, MN USA). The tensile test had a data acquisition rate of 10.0 Hz and a test speed of 10 mm/min and used to determine peak stress, strain at break, and modulus of elasticity (MOE).
Fourier transform infrared (FT-IR) spectra were obtained at 25 °C after a total of 10 scans using a Spectrum 100 FT-IR spectrometer (Perkin Elmer, Waltham, MA, USA) equipped with a universal ATR sampling accessory.
Contact angles were measured at 25 °C using DI water drops by a Dataphysics OCA 15 Optical Contact Angle Measuring System (Dataphysics, Garden City, NY, USA). Three measurements at random places of the tested surface were conducted. Surface tension energy was calculated according to Balkenende et al. (1998) from the following equation:
where θ is the contact angle (°), γS is the surface tension energy of the film surface (mJ/m2), and γL is the surface tension energy of the liquid (DI water was used in our case, so γL = 72.8 mJ/m2).
Paper coated by modified arabinoxylan
Prior to coating, the rheological properties of AX and GAX coatings were investigated. The dynamic (absolute) viscosity of AX and GAX coatings of 10, 5, 2.5 and 1.25 % (w/w) (suspension in water) was determined at 25 °C by using a rotational viscometer (Fungilab Adv Series, New York, USA) at rotational speeds of 1, 2.5, 5, 10, 20, 50 rpm.
For the paper coated with only AX or GAX, 10 % (w/w) coating suspensions were prepared with pH maintained at about 3. For GAX as paper coating binders, 40 % (w/w) suspension was prepared, and the solid contained 10 % GAX and 90 % calcium carbonate. For comparison, polyvinyl alcohol (PVA) was also mixed with calcium carbonate as paper coating binder at the same formulation.
Prior to coating, printing paper (386 mm × 286 mm) were dried in an oven at 105 °C for 30 min and placed in a desiccator (0 % RH) for 24 h. Both sides of the paper were coated once or twice to control the coating weight to about 6 and 12 g/m2. The coatings were applied on the paper manually on a bench top coater using RDS Mayer rod 7, hot air dried at ~200 °C for 1 min and further oven dried at 105 °C for 30 min. The oven dried coated paper samples were placed in a desiccator (0 % RH). After 24 h, the samples were weighed immediately to ascertain the coating weight. Each modification or formulation had two coated paper samples prepared.
Prior to tensile testing, each coated paper samples were cut into 12 specimens with a width of 25 mm and conditioned at 40 % RH and 30 °C for 24 h. The dried tensile strength measurement used the same method for film tensile strength test, while the wet tensile strength measured the specimens after soaking the middle part in water for 10 s. Peak load (N) at break was recorded. Tensile index (TI) (N m/g) was calculated according to TAPPI method T 494 om-01:
where P is the peak load at break (N); W is the width of the specimen (m); ρ is the coating grammage (g/m2). Significant differences of TI between samples were identified using Tukey HSD test (P < 0.05) in JMP Pro 11 software (SAS Institute Inc. Cary, NC, USA).
Scanning electron microscopy (SEM) (Hitachi S-3200) was used to study the coating-paper interface structure. The coated sample was frozen in a −80 °C freezer overnight and cut with a never used razor blade. The cross section of the cut paper was mounted perpendicularly, sputter coated with gold by a Sputter Coater (SeeVac Auto Conductavac IV) and scanned by the SEM.
TAPPI brightness, color, and opacity were measured by TAPPI methods T452 om-98, T524 om-02 and T 425 om-06, respectively, on a Technidyne Brightimeter (Micro S5, New Albany, IN, USA).
Results and discussion
Characterization of modified arabinoxylan
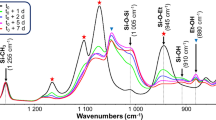
Aldehyde can form acetals with alcohols under acidic condition (Loudon 2009). The proposed structure of glutaraldehyde crosslinked arabinoxylan (GAX) can be seen in Scheme 2. The side chains of AXU substituted by GA have several possibilities (Brown et al. 2012; Matsuda et al. 1999; Walt and Agayn 1994). The GA can have one aldehyde group react with AX hydroxyl groups to form acetals or hemiacetals leaving the other as a free aldehyde group, or have both aldehydes react crosslinking two AX molecules together (Scheme 2). Additionally, more complicated structures might exist. Under acidic condition or high concentration, GA can polymerize with itself through forming hemihydrate multimers or aldol condensation reaction forming α,β-unsaturated aldehydes (Matsuda et al. 1999; Monteiro and Airoldi 1999; Loudon 2009; Walt and Agayn 1994). Thus, a GAX side chain can contain more than one GA molecules (Scheme 2). The evidences of those structures can be seen by comparing GAX and unmodified AX FTIR (Fig. 1) and 13C NMR spectra (Fig. 2).
Associated with Scheme 2, GAX FTIR spectra showed a peak at ~1735 cm−1 corresponding to the C=O in the free aldehyde group (Fig. 1). The peak at ~1550 cm−1 should be an evidence of GA polymerizing with itself forming unsaturated aldehyde C=C bonds. The peak at ~1268 cm−1 should correspond to the C–O bonds of acetals (Tipson et al. 1959). The 13C NMR spectra (Fig. 2) of GAX were in consistent with the FTIR spectra. The peak at 211.1 ppm corresponds to the carbons on the free aldehyde groups. The peaks at 130.4 and 128.7 ppm corresponded to the ethylenic carbons connected to the aldehyde group of the unsaturated aldehyde structure (Monteiro and Airoldi 1999). The peaks of GA units’ hemiacetal or acetal carbons (in both the polymerized GA structure and GA-AX structures) might overlap with the peaks between 90 and 110 ppm of the acetal bonds connecting AXU, but some slightly enhanced peaks from GAX spectrum can be seen at 108.3, 102.1 and 99.4 ppm. The peak at 14.7 ppm might indicate the allylic carbons connected to the double bonds of the unsaturated aldehydes. The peaks between 20 and 35 ppm should correspond to other alkane carbons in GA units.
We also presented in Fig. 3 the DS, as GA reacted mol/mol of AXU, as well as the amount of free aldehyde in GAX samples. The DS of GAX ranged from 0.03 to 2.0. However, the free aldehyde contents were very low ranging from 8 to 35 μmol/mol AXU consistent with the very weak aldehyde peak (1735 cm−1 and 211.1 ppm) in the FTIR and 13C NMR spectra. The DS increased proportionally to the amount of GA added for the reaction; however, the aldehyde content had a maximum value of about 35 μmol/mol AXU at 0.5 mol of GA added per mole AXU and then decreased, which agreed with the decreasing intensity of the aldehyde peak from FTIR spectra (Fig. 1). Previous studies have found that, at high GA concentration, GA units tend to polymerize with themselves to form cyclic structures (Walt and Agayn 1994). Therefore, the decreasing amount of free aldehyde group suggests the added GA units promotes self-polymerizing into cyclic structures (Scheme 2) that leads to the observed decrease of free aldehyde content.
Modified arabinoxylan film properties
The film mechanical and surface properties are very essential for applying the GAX films as packaging and coating materials. The peak stress, strain at break and MOE for the AX and GAX films were showed in Fig. 4. The unmodified AX film had a peak stress of about 26.5 MPa, a strain at break of about 1.6 %, and a MOE of about 2100 MPa. Previous studies have shown that the unmodified AX from other sources (e.g. corn hull, barley husk and rye endosperm) had peak stresses of about 50 MPa, strains at break of 2–8 %, and MOEs of 1300–2500 MPa (Höije et al. 2005; Mikkonen and Tenkanen 2012; Stevanic et al. 2011; Zhang and Whistler 2004). The lower peak stress and strain at break of DG AX compared to other sources may be due to the presence of protein, which is film forming but may interrupt the stronger AX films (Aydt et al. 1991). After GA crosslinking, when the GA/AXU mole ratio for the reaction reached 0.1, peak stress of the GAX film increased almost 3 times to a maximum of 70.7 MPa, while keeping adding GA until GA/AXU mole ratio became 3.0, the peak stress first decreased to about 57 MPa and then became constant (Fig. 4a). The changes of strain at break with DS of GAX films had a very similar pattern to the peak stress (Fig. 4b), which predicted that the MOE of all the GAX films were very similar since MOE is calculated by divided the stress by strain. The MOE results from the mechanical test reading were indeed similar among AX and GAX films (Fig. 4c). The MOE results might also indicated that all the AX and GAX films had similar stiffness or softness.
The contact angle of water on the GAX film surface was measured after the water dropped on the surface for 60 s and shown in Fig. 5a. The surface tension energy of the GAX film surface was calculated based on the contact angle and shown in Fig. 5b. The surface tension of GAX films did not change significantly at low DS compared to unmodified AX film with contact angle of ~95° and surface tension energy of ~15 mJ/m2. However, when the GA/AXU mole ratio of GAX film reached 3.0, the contact angle decreased to ~65° and surface tension energy increased to ~36 mJ/m2. The sudden change of surface tension of GAX film at high DS may be explained by that, at high DS probably with a high level of crosslinking, some GA oligomers might be trapped within the polymer matrix through hydrogen bonding, and when the water drops on to the GAX film surface, those GA oligomers quickly dissolve into water lowering the surface tension. Another reason may be that GAX samples do not dissolve in water, but GAX at high DS disperses in water more easily than at low DS, which could be due to the breakage of intermolecular hydrogen bonding. Consequently, the water may be easier to penetrate into the free space within the films of GAX1.95 resulting lower contact angle.
The amount of GA added for the reaction directly reflected the DS of GAX as the two variables displayed a linear relation (Fig. 3). Therefore, the effects of GA crosslinking on the mechanical and surface tension behaviors of GAX films postulated that, increasing the DS enhances the film strength, but the maximum film strength appeared at low DS and continuing to increase the DS might not further enhance the GAX film tensile strength. Additionally, the GA crosslinking does not alter the stiffness and surface tension of the GAX film. However, at high level of crosslinking, GAX film surface tension was greatly lowered. These findings suggest that maintaining the crosslinking of AX with GA at low DS might give improved material properties to the GAX films and better economy to the process. It also added evidence to that high DS of modifications on hemicelluloses have negative effects on its material properties (Alekhina et al. 2014).
Modified arabinoxylan coated paper properties
As previously discussed, typical paper coatings are a mixture of coating binder and pigment. However, to initially evaluate GAX as a binder, it is reasonable to evaluate its properties without any pigment interactions. The coated paper surface property affects its printing quality and thus was also evaluated. These evaluations would result in finding an optimized GAX modification (with different DS) suitable for coating binder test with pigments.
Viscosity of coating suspensions
The coating suspension viscosity is critical both to the fluid transport during processing as well as having strong impact on coated paper properties (Ghassemzadeh et al. 2001) and thus was investigated before the coating process. The dynamic viscosity of AX and GAX coating suspension of 5 and 10 % (w/w) at different rotational speed was shown in Fig. 6. At 10 % (w/w) coating suspension, AX demonstrated a very strong non-Newtonian fluid behavior by showing an evident shear thinning phenomenon that its viscosity decreased with increasing rotational speed (shear rate) (Nishiwaki-Akine and Watanabe 2014). GAX coating suspension at a low DS of 0.03 still showed a moderate shear shinning phenomenon, while at high DS, the GAX coating suspensions behaved very close to Newtonian fluid that the shear shinning phenomenon almost disappeared. When the coating suspensions were diluted from 10 to 5 % (w/w), all the GAX coating suspensions, without regard to their DS, started to demonstrate Newtonian fluid properties with their viscosities constant at different rotational speed. However, the unmodified AX coating suspension at 5 % still demonstrated non-Newtonian shear thinning behavior. Diluted coating suspensions at 2.5 and 1.25 % (w/w) displayed Newtonian fluid properties for both the GAX and AX coating suspensions. Additionally, all the AX and GAX coating suspensions with the same solid contents had similar viscosity values at high shear rate (rotational speeds greater than 20 rpm), and the solid content affected the viscosity significantly. For example, at 50 rpm rotational speed, both the AX and GAX coating suspensions had viscosities of ~1500 mPa s at 10 % (w/w) solid content, ~90 mPa s at 5 %, ~15 mPa s at 2.5 % and ~7 mPa s at 1.25 % (Table 1). Therefore, the GA crosslinking basically changed the AX high solid content suspension from a non-Newtonian fluid to a Newtonian fluid. It has been reported that intra-molecular crosslinking was dominant over inter-molecular crosslinking for GA crosslinked PVA with molecular weight (M w) > 115 kDa and DS < 2 (Aharoni 1977). The M w of the DG AX in our study was ~400 kDa. Consequently, it is very likely that, due to our relatively low GA crosslinking level and high M w of the AX molecules, the intra-molecular crosslinking might be more prevalent than the inter-molecular crosslinking, which reduced the AX molecule radius of gyration and thus caused the suspension display Newtonian fluid properties (Aharoni 1977; Gebben et al. 1985).
Coating structure
The coating structure on paper samples coated with 10 % (w/w) AX or GAX suspensions were investigated by taking SEM images (Fig. 7) both as planar surfaces and cross-sections. The paper cross-sectional pictures revealed that the GAX coating had improved adhesion to paper surface compared to the unmodified AX coating. The interface separation of AX coating and paper surface may be seen clearly (Fig. 7d), while the interface between GAX coating and paper surface cannot be seen at all (Fig. 7f). Additionally, it was clear that the coating did not penetrate into the paper fiber matrix suggesting that we can eliminate the effects of coating penetration on the paper mechanical strength. It has been reported that coating suspension with viscosity higher than 20 mPa s had limited penetration to the paper (Ghassemzadeh et al. 2001). Thus, the high viscosity of our 10 % coating suspensions (~1500 mPa s) might be the reason for the coating not being penetrated into the paper fiber structure.
Tensile index of coated paper
The coated paper was tested for both dry and wet TI. The dry TI was compared with the weighted average, which is the theoretical TI by adding the film (coating) and paper strength together without considering the interaction between them. Statistical differences between samples were also evaluated by Tukey HSD test. All the tested samples including AX and GAX coated paper had actual dry TI much larger than the weighted average and larger than that of the plain paper (Fig. 8a). This result postulated that the unmodified and modified AX all had interactions or connections with the paper fibers combining them into a stronger composite than each of its components. Comparing between the GAX and AX samples statistically, all the GAX coated paper had higher dry TI at 6 g/m2 coating weight, while GAX0.03 and GAX0.17 had higher dry TI at 12 g/m2 coating weight. Among the GAX coated paper, GAX0.03 had the highest TI statistically (86.1 N m/g at 6 g/m2 and 83.5 N m/g at 12 g/m2 coating weight). With the DS increased, the TI slightly decreased at the same coating weight, which had the similar trend as the film strength changed with DS (Fig. 4). By excluding the effects of coating penetration on paper mechanical strength as shown by the SEM picture (Fig. 7), it postulated that, the GAX coating, especially at low DS, had very strong chemical interactions with paper fibers probably including hydrogen bonding and covalent bonding. It was hypothesized that there possibly were acetals or/and hemiacetals formed between GAX and the paper fiber cellulose. Additionally, at the same modification, the dry TI at 6 g/m2 coating weight was lower than at 12 g/m2 for some samples, which was probably due to the thicker coating layer preventing the outer surface of GAX from interacting with paper fiber.
For wet paper, all the coated paper had wet TI higher than the plain paper. Compared to the unmodified AX coated paper statistically, the GAX0.03 coated paper had lower wet TI, GAX0.17 and GAX0.56 had similar wet TI, and GAX1.95 had significant higher wet TI at the same coating weight (Fig. 8b). With the GAX coating DS increased, the wet TI increased at the same coating weight. With the increasing DS, the increasing wet TI of GAX coated paper might suggest an increasing amount of covalent bonds, most probably acetal or hemiacetal bonds, between GAX aldehyde groups and paper fiber hydroxyl groups.
However, the results obtained were somewhat contradictory, as the higher DS of GAX coatings corresponded to higher wet TI but lower dry TI. It was speculated that even though GAX1.95 coating might have more covalent bondings with paper, the GAX0.03 coating might still have even stronger overall hydrogen bonding interactions with the paper fibers giving it the highest dry tensile strength.
Wetting behavior of coated paper surface
The hydrophobicity and absorption behavior of coated paper surface have a large impact on printing qualities. If the paper surface is too hydrophilic and wetting too fast, it might cause low printing density and high ink penetration; if the paper surface is too hydrophobic and wetting too slow, it could lower the printing density too (Barhoum et al. 2014). The contact angle changes in a 3 min period were recorded for AX and GAX coated paper in Fig. 9. Compared to other GAX samples at higher DS, even though GAX0.03 and AX samples had higher hydrophobicity, their contact angle values decreased faster with time indicating a faster wetting rate. GAX1.95 sample having the lowest hydrophobicity might be due to its film sample also having the lowest hydrophobicity. However, the hydrophobicity of other coated samples might not necessarily follow the trend as shown by the film contact angle measurements. As shown in the SEM pictures (Fig. 7a, c, e), the coated paper fibers were not entirely covered with coating and thus affected the wetting behaviors. Therefore, besides the film hydrophobicity, the coating structures might also have important effects on paper hydrophobicity and absorption behavior (Barhoum et al. 2014). Overall, the GAX0.03 coated paper had the highest hydrophobicity, and might prevent the water-based ink from spreading too fast over the surface and thus could improve the printing resolution.
Modified arabinoxylan as paper coating binder
As discussed earlier, sample GAX0.03 imparted the coated paper with the highest hydrophobicity and dry TI among the modified AX samples. Thus, GAX0.03 was selected to be tested as coating binder by mixing with calcium carbonate and compared with commercial PVA. The ingredient of the paper coating with calcium carbonate was 40 % (w/w) solids and the solid contained 10 % binders and 90 % calcium carbonate, which is a typical commercial paper coating recipe with PVA (Barhoum et al. 2014; Laudone et al. 2006). The coating weight of GAX coating was higher than the PVA coating suggesting GAX coating had better adhesion to paper (Table 2). The dry TI and wet TI of GAX coated paper were lower but comparable to PVA coated paper. However, GAX coating gave the paper with a more hydrophobic surface and might give the printing with more brilliant appearance. The opacity and whiteness of GAX and PVA coated paper were very close. The brightness of the GAX coated paper was a little lower than desired but should be improved easily by bleaching the DG AX with hydrogen peroxide (Sun et al. 2004). The GAX coating with calcium carbonate should have an alkaline pH and acetal bonds may not be formed. Therefore, hemiacetals and hydrogen bonds may be the major interactions between the coating and paper fiber surface. However, beyond calcium carbonate, common paper coating pigments also include bentonite clay, titanium dioxide, silica, and talc (Barhoum et al. 2014; Laudone et al. 2006). Most of them should not affect the acidic pH of the coating solution and thus acetals may possibly be formed resulting better paper surface adhesion for other types of pigments. Additionally, GA toxicity or contact irritancy may be of concern (Leinster et al. 1993). However, when cross-linked with AX, or dried into films no free GA should be present, greatly diminishing safety concerns, though a process should confirm that no unreacted GA is present. In general, the GAX0.03 can act as a potential substitute for the commercial petroleum-based PVA paper coating binder.
Conclusions
Crosslinking AX extracted from DG by GA was based on the formation of acetal bonds between AX hydroxyl groups and GA aldehyde groups under acidic condition. The crosslinked AX, free aldehyde groups and polymerized GA units contributed to the structural variety of the GAX. The DS of GAX was proportionally to the amount of GA added.
Films made of GAX displayed tensile strength approximately 3 times higher than that of the unmodified AX film at low DS. Continuing to increase the DS made no increase in film tensile strength. It added evidence to the hypothesis that modification of hemicelluloses at a very low degree might be enough and necessary to make biodegradable films with strong tensile strength facilitating their possible substitution to petroleum-based films.
Within our studied DS ranges, the GA crosslinking removed the non-Newtonian behavior of the unmodified AX suspensions at high concentration (10 % w/w) creating a more Newtonian fluid, which was likely due to the intra-molecular crosslinking being prevalent. Compared to unmodified AX coated paper, GAX coated paper showed significant increase in dry TI at low DS and in wet TI at high DS. This can possibly be explained by GAX coatings forming covalent bonds with paper fibers, whose quantities might increase with increased DS. Additionally, GAX coating at very low DS (0.03) imparted paper with better hydrophobicity. When mixing with calcium carbonate, GAX coating with DS = 0.03 showed higher hydrophobicity, and similar mechanical and optical performances compared to PVA coating, suggesting its potential substitution to commercial petroleum-based paper coating binder.
Abbreviations
- AX:
-
Arabinoxylan
- AXU:
-
Arabinoxylose unit
- DG:
-
Distillers’ grains
- DS:
-
Degree of substitution
- GA:
-
Glutaraldehyde
- GAX:
-
Glutaraldehyde crosslinked arabinoxylan
- MOE:
-
Modulus of elasticity
References
Aharoni SM (1977) Intramolecular crosslinking. Angew Makromol Chem 62:115–133
Alekhina M, Mikkonen KS, Alen R, Tenkanen M, Sixta H (2014) Carboxymethylation of alkali extracted xylan for preparation of bio-based packaging films. Carbohydr Polym 100:89–96
Arguelles-Monal W, Goycoolea FM, Peniche C, Higuera-Ciapara I (1998) Rheological study of the chitosan glutaraldehyde chemical gel system. Polym Gels Netw 6:429–440
Aulin C, Lindström T (2011) Biopolymer coatings for paper and paperboard. In: Plackett D (ed) Biopolymers—new materials for sustainable films and coatings. Wiley, Chichester
Aulin C, Gallstedt M, Lindstrom T (2010) Oxygen and oil barrier properties of microfibrillated cellulose films and coatings. Cellulose 17:559–574
Aydt TP, Wller C, Testin RF (1991) Mechanical and barrier properties of edible corn and wheat protein films. Trans ASAE 34:207–211
Balkenende AR, van de Boogaard H, Scholten M, Willard NP (1998) Evaluation of different approaches to assess the surface tension of low-energy solids by means of contact angle measurements. Langmuir 14:5907–5912
Barhoum A, Rahier H, Abou-Zaied RE, Rehan M, Dufour T, Hill G, Dufresne A (2014) Effect of cationic and anionic surfactants on the application of calcium carbonate nanoparticles in paper coating. ACS Appl Mater Interfaces 6:2734–2744
Bigi A, Cojazzi G, Panzavolta S, Rubini K, Roveri N (2001) Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 22:763–768
Brown EE, Laborie MG, Zhang J (2012) Glutaraldehyde treatment of bacterial cellulose/fibrin composites: impact on morphology, tensile and viscoelastic properties. Cellulose 19:127–137
Ebringerová A, Heinze T (2000) Xylan and xylan derivatives—biopolymers with valuable properties, 1—Naturally occurring xylans structures, procedures and properties. Macromol Rapid Commun 21:542–556
Ebringerová A, Hromádková Z, Heinze T (2005) Hemicellulose. In: Heinze T (ed) Polysaccharides 1: structure, characterization and use. Springer, New York, pp 1–55
El-Tahlawy K, Venditti RA, Pawlak JJ (2007) Aspects of the preparation of starch microcellular foam particles crosslinked with glutaraldehyde using a solvent exchange technique. Carbohydr Polym 67:319–331
Fardim P (2002) Paper and surface chemistry-part 2-coating and printability. Tappi J 1:1–13
Gáspár M, Kalman G, Reczey K (2007) Corn fiber as a raw material for hemicellulose and ethanol production. Process Biochem 42:1135–1139
Gebben B, Vandenberg HWA, Bargeman D, Smolders CA (1985) Intramolecular crosslinking of polyvinyl-alcohol. Polymer 26:1737–1740
Ghassemzadeh J, Hashemi M, Sartor L, Sahimi M (2001) Pore network simulation of imbibition into paper during coating: I. Model development. AIChE J 47:519–535
Gliko-Kabir I, Penhasi A, Rubinstein A (1999) Characterization of crosslinked guar by thermal analysis. Carbohydr Res 316:6–13
Guilbert S, Cuq B, Gontard N (1997) Recent innovations in edible and/or biodegradable packaging materials. Food Addit Contam Part A 14:741–751
Hansen NML, Plackett D (2008) Sustainable films and coatings from hemicelluloses: a review. Biomacromolecules 9:1493–1505
Hartman J, Albertsson AC, Lindblad MS, Sjoberg J (2006a) Oxygen barrier materials from renewable sources: material properties of softwood hemicellulose-based films. J Appl Polym Sci 100:2985–2991
Hartman J, Albertsson AC, Sjoberg J (2006b) Surface- and bulk-modified galactoglucomannan hemicellulose films and film laminates for versatile oxygen barriers. Biomacromolecules 7:1983–1989
Höije A, Gröndahl M, Tømmeraas K, Gatenholm P (2005) Isolation and characterization of physicochemical and material properties of arabinoxylans from barley husks. Carbohydr Polym 61:266–275
Jain RK, Sjostedt M, Glasser WG (2000) Thermoplastic xylan derivatives with propylene oxide. Cellulose 7:319–336
Laine C, Harlin A, Hartman J, Hyvarinen S, Kammiovirta K, Krogerus B, Pajari H, Rautkoski H, Setala H, Sievanen J, Uotila J, Vaha-Nissi M (2013) Hydroxyalkylated xylans—their synthesis and application in coatings for packaging and paper. Ind Crops Prod 44:692–704
Laudone GM, Matthews GP, Gane PAC (2006) Modelling the shrinkage in pigmented coatings during drying: a stick-slip mechanism. J Colloid Interface Sci 304:180–190
Leinster P, Baum JM, Baxter PJ (1993) An assessment of exposure to glutaraldehyde in hospitals: typical exposure levels and recommended control measures. Br J Ind Med 50:107–111
Lindblad MS, Albertsson AC (2005) Chemical modification of hemicelluloses and gums. In: Dumitriu S (ed) Polysaccharides: structural diversity and functional versatility. Marcel Dekker, New York
Loudon M (2009) Organic chemistry, 5th edn. Roberts and Company Publishers, Greenwood Village
Lv S, Gong R, Hu J (2012) Study on modified starch by glutaraldehyde and its properties and application. Adv Mater Res 455–456:575–581
Matsuda S, Iwata H, Se N, Ikada Y (1999) Bioadhesion of gelatin films crosslinked with glutaraldehyde. J Biomed Mater Res 45:20–27
Maurer HW (2009) Starch in the paper industry. In: BeMiller J, Whistler R (eds) Starch: chemistry and technology, 3rd edn. Elsevier, New York, pp 658–706
Mikkonen KS, Tenkanen M (2012) Sustainable foodpackaging materials based on future biorefinery products: xylans and mannans. Trends Food Sci Technol 28:90–102
Monteiro OAC, Airoldi C (1999) Some studies of crosslinking chitosan-glutaraldehyde interaction in a homogeneous system. Int J Biol Macromol 26:119–128
Nishiwaki-Akine Y, Watanabe T (2014) Dissolution of wood in alpha-keto acid and aldehydic carboxylic acids and fractionation at room temperature. Green Chem 16:3569–3579
Petzold K, Schwikal K, Heinze T (2006) Carboxymethyl xylan—synthesis and detailed structure characterization. Carbohydr Polym 64:292–298
Saghir S, Iqbal MS, Hussain MA, Koschella A, Heinze T (2008) Structure characterization and carboxymethylation of arabinoxylan isolated from Ispaghula (Plantago ovata) seed husk. Carbohydr Polym 74:309–317
Saghir S, Iqbal MS, Koschella A, Heinze T (2009) Ethylation of arabinoxylan from Ispaghula (Plantago ovata) seed husk. Carbohydr Polym 77:125–130
Soppirnath KS, Aminabhavi TM (2002) Water transport and drug release study from cross-linked polyacrylamide grafted guar gum hydrogel microspheres for the controlled release application. Eur J Pharm Biopharm 53:87–98
Stevanic JS, Joly C, Mikkonen KS, Pirkkalainen K, Serimaa R, Rémond C, Toriz G, Gatenholm P, Tenkanen M, Salmén L (2011) Bacterial nanocellulose-reinforced arabinoxylan films. J Appl Polym Sci 122:1030–1039
Sun JX, Sun XF, Sun RC, Su YQ (2004) Fractional extraction and structural characterization of sugarcane bagasse hemicelluloses. Carbohydr Polym 56:195–204
Tipson RS, Isbell HS, Stewart JE (1959) Infrared absorption spectra of some cyclic acetals of sugars. J Res Natl Bur Stand 62:257–282
Walt DR, Agayn VI (1994) The chemistry of enzyme and protein immobilization with glutaraldehyde. TrAC Trends Anal Chem 13:425–430
Wang Q, Zhu JY, Considine JM (2013) Strong and optically transparent films prepared using cellulosic solid residue recovered from cellulose nanocrystals production waste stream. ACS Appl Mater Interfaces 5:2527–2534
Xiang Z, Runge T (2014) Co-production of feed and furfural from dried distillers’ grains to improve corn ethanol profitability. Ind Crops Prod 55:207–216
Xiang Z, Watson J, Tobimatsu Y, Runge T (2014a) Film-forming polymers from distillers’ grains: structural and material properties. Ind Crops Prod 59:282–289
Xiang Z, Anthony R, Tobimatsu Y, Runge T (2014b) Emulsifying properties of an arabinoxylan-protein gum from distillers’ grains and the co-production of animal feed. Cellulose 21:3623–3635
Xiang Z, Sen SK, Roy A, Min D, Savithri D, Jameel H, Chiang V, Chang HM (2015) Wood characteristics and enzymatic saccharification efficiency of field-grown transgenic black cottonwood with altered lignin content and structure. Cellulose 22:683–693
Xing R, Qi W, Huber G (2011) Production of furfural and carboxylic acids from waste aqueous hemicellulose solutions from the pulp and paper and cellulosic ethanol industries. Energy Environ Sci 4:2193–2205
Yu J, Chang PR, Ma X (2010) The preparation and properties of dialdehyde starch and thermoplastic dialdehyde starch. Carbohydr Polym 79:296–300
Zhang P, Whistler RL (2004) Mechanical properties and water vapor permeability of thin film from corn hull arabinoxylan. J Appl Polym Sci 93:2896–2902
Zhu JY, Sabo R, Luo X (2011) Integrated production of nano-fibrillated cellulose and cellulosic biofuel (ethanol) by enzymatic fractionation of wood fibers. Green Chem 13:1339–1344
Acknowledgments
This work was supported by U.S. Department of Agriculture, under contract USDA Critical Agricultural Material Grant (2013-38202-20400). NMR experiments were carried out at the Great Lakes Bioenergy Research Center funded by U.S. Department of Energy, the Office of Science (BER DE-FC02-07ER64494) and the Wisconsin Energy Institute (WEI). Contact angle measurements were carried out at the University of Wisconsin Materials Research Science and Engineering Center funded by NSF (DMR-1121288). The authors gratefully acknowledge Dr. John Ralph and Dr. Dharshana Padmakshan for NMR analysis, Dr. Sundaram Gunasekaran for FTIR analysis, USDA Forest Products Laboratory (FPL) for paper optical property measurements, and Didion Milling Inc. for materials and valuable discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Xiang, Z., Anthony, R., Lan, W. et al. Glutaraldehyde crosslinking of arabinoxylan produced from corn ethanol residuals. Cellulose 23, 307–321 (2016). https://doi.org/10.1007/s10570-015-0828-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-015-0828-3