Abstract
In this current work, a series of cellulose-based flocculants, carboxymethyl cellulose-graft-poly[(2-methacryloyloxyethyl) trimethyl ammonium chloride] (CMC-g-PDMC) with different grafting ratios were successfully synthesized. CMC-g-PDMC bears high flocculation performance in removal of an anionic dye, Acid Green 25 (AG25), at various pH conditions, which is due to improvement of both positive charge and molecular weight after modification. Moreover, the dye removal efficiency is mostly improved with the increase of grafting ratio. Among the four tested salt additives, CMC-g-PDMC exhibits good salt resistance except for sodium chloride in the measured salt concentration range. Furthermore, the image analysis in combination with fractal theory has been employed to investigate the flocs properties including floc size and fractal structure for studying the flocculation mechanism in detail. It is confirmed that charge neutralization and bridging flocculation effects both play important roles in removal of AG25 from water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dyes are widely used in textile and leather industries. (Tian et al. 2014; Verma et al. 2012; Zhou et al. 2014) In addition to increase of the chemical oxygen demand (COD) of the water body, the dye effluents are highly visible and interfere with light penetration into water thereby retard photosynthesis and inhibit the growth of aquatic organisms seriously. (Khraisheh et al. 2005; Xiong et al. 2014; Zahrim et al. 2011) Hence, it is of vital importance to efficiently remove dye pollutants from wastewater.
Various technologies have been employed for treatment of the dye-containing wastewater including flocculation, adsorption, filtration, oxidation, electrolysis, and chemical/photochemical/microbial degradation (Batmaz et al. 2014; Liang et al. 2014; Rafatullah et al. 2010; Verma et al. 2012; Wang et al. 2013a; Yang et al. 2013a). Among them, flocculation is one of the most effective pre-treatment processes in terms of low-cost, easy-operation and energy-saving (Aboulhassan et al. 2006; dos Santos et al. 2007; Riera-Torres et al. 2010; Wang et al. 2009b; Zahrim et al. 2011). The flocculation performance highly depends on the choice of flocculants. Up to now, inorganic metal-based flocculants and synthetic organic polymeric ones are two kinds of the most popular flocculants which are widely applied. However, both of them bear the risk of secondary pollution for residual metal ions and noxious monomers of some synthetic polymeric flocculants (Roussy et al. 2005; Song et al. 2010; Zhao et al. 2011). Therefore, development of safer, cheaper and more effective flocculants is still one of the hottest topics in flocculation.
Recently, natural polymer-based flocculants have become attractive because of their nontoxic, widely-sourced, environment-friendly and cost-effective aspects (Renault et al. 2009; Shi et al. 2012; Wang et al. 2009a; Zahrim et al. 2011). Among them, cellulose, the most abundant natural polymers in the world, is an excellent candidate (Cai et al. 2013; Klemm et al. 2005). However, the disadvantages of cellulose itself always limit its practical applications in wastewater treatment such as the poor water solubility and low reactivity. Accordingly, the chemical modification has been employed (Liu et al. 2008; Song et al. 2010). Carboxymethylation (Bhandari et al. 2012; Qi et al. 2009) is a simple and efficient way to overcome those drawbacks of cellulose. The carboxymethyl rectified product, carboxymethyl cellulose (CMC), shows significantly increased water solubility for destruction of its original ordered structure. Besides, grafting is also a valid modification approach (Li et al. 2007). The flexible long chain grafted onto the rigid cellulose backbone can increase the conformational freedom of the polymeric flocculants in solution, resulting in improved adsorption and bridging effects (Yang et al. 2013b). Moreover, as most of the contaminants in water bodies carry some charges, the attraction and approachability to these contaminants can be greatly enhanced when the flocculants have opposite charges (Cai et al. 2013; Ghimici et al. 2010). As for dyes, many acidic and reactive ones with negative charges, containing sulfonic or carboxylic groups, are widely applied in the industrial fields (He 2004; Yagub et al. 2014). However, they are difficult to be completely removed due to high water solubility (Zahrim et al. 2011). Therefore, increasing the positive charge density of flocculants is an effective way to improve flocculation efficiency for removal of those anionic dyes from water.
In this present work, a series of graft cellulose-based flocculants with strong positive charges, carboxymethyl cellulose-graft-poly[(2-methacryloyloxyethyl) trimethyl ammonium chloride] (CMC-g-PDMC) have been designed and synthesized to flocculate Acid Green 25 (AG25, a typical anionic dye) from water. The effects of both internal (grafting ratio) and external factors (pH, flocculant dosage, and salt additives) on flocculation of AG25 have been studied systematically. Moreover, the floc properties (i.e., floc size and compactness) are also crucial during flocculation process (Fabrizi et al. 2010; Jarvis et al. 2005) since larger and denser flocs are usually preferred in order to yield lower sludge volume and reduce the disposal and handling cost. The floc size and two-dimensional fractal dimension (D 2) (Chakraborti et al. 2000) which describes the compactness of flocs, have been determined by image analysis (IA) (Yang et al. 2013b) in combination with fractal theory (Mandelbrot 1983). The flocculation mechanisms of the cellulose-based flocculants have been discussed in detail on the basis of the flocculation performance and floc properties.
Materials and methods
Materials
Cellulose was purchased from Shanghai Jinsui Bio-Technology Co., Ltd. Monochloroacetic acid (Zibo Lushuo Economic Trade Co., Ltd.), ammonium persulfate (APS) (Shanghai Lingfeng Chemical Reagent Co., Ltd.), (2-methacryloyloxyethyl) trimethyl ammonium chloride (DMC) (Shanghai Bangcheng Bilogical Technol. Co., Ltd.), and AG25 (Shanghai Jingchun Reagent Co., Ltd.) were used without further purification. All other chemicals were purchased from Nanjing Chemical reagent Co. Ltd. All the reagents are of analytical grade, and distilled water was used in all experiments.
Preparation of CMC-g-PDMC
CMC-g-PDMC has been obtained through two steps. The former step was to prepare CMC by carboxymethylation of cellulose to improve the water solubility, and the latter one was to graft PDMC chains onto CMC backbone in a homogeneous solution. The detailed synthesis process is described in Scheme 1.
Firstly, the desired amount of cellulose was added into an ethanol/NaOH blending aqueous solution to swell and alkalize at 80 °C in a water bath for 1 h. Then monochloroacetic acid was added into the reaction mixture dropwise and stirred for 1.5 h at 80 °C. The product was filtered and rinsed in 75 % ethanol and finally dried under vacuum at 60 °C. The substitution degree of carboxymethyl groups is 17.4 % calculated from the mass fraction of sodium, which was obtained by atomic absorption spectrum (AAS).
Then, a CMC aqueous solution with known concentration was freshly prepared and stirred under nitrogen for 30 min. After that, a certain amount of APS as the initiator was added. To suppress the formation of the PDMC homopolymer, the CMC solution was kept for 5 min before the DMC monomer aqueous solution was added (Sonmez et al. 2002). The reaction was carried out for 3 h under nitrogen and was then stopped by precipitating the mixture in acetone. The solid product was filtered and washed with ethanol for three times, then extracted using acetone as the solvent in a Soxhlet apparatus for 48 h to remove impurities (da Silva et al. 2007; Wang et al. 2013b). Finally CMC-g-PDMC was dried under vacuum at 60 °C. Five different cellulose-based samples with various mass feed ratios of CMC and DMC (1:2, 1:3, 1:4, 1:5 and 1:8) were synthesized and named CMC-g-PDMC12, CMC-g-PDMC13, CMC-g-PDMC14, CMC-g-PDMC15 and CMC-g-PDMC18, respectively. The grafting ratio (G) of various CMC-g-PDMC samples was calculated by weighting method according to Eq. (1) (Wang et al. 2009a) as shown below.
where w g and w 0 are the weight of CMC-g-PDMC and CMC, respectively.
The calculated grafting ratios of various CMC-g-PDMC samples including other details are all listed in Table 1. The grafting ratio increases with increasing the feed mass of DMC monomers from Table 1.
Characterization
Fourier transform infrared (FTIR) spectra of various samples were recorded on a Bruker Model IFS 66/S FTIR spectrometer. The interval of measured wave numbers was 600–3800 cm−1.
1H nuclear magnetic resonance (1H NMR) spectra of different samples were carried out using a Bruker AVANCE Model DRX-500 spectrometer, operating at 500 MHz with D2O as solvent.
Zeta potential (ZP) of various cellulose sample solutions was measured using a Malvern Model Nano-Z Zetasizer.
Hydrodynamic radius (R h), a parameter commonly used to characterize the size of the polymer in solution, was determined by a Brookhaven Model BI200SM dynamic light scattering apparatus.
Flocculation experiment
Jar tests
The AG25 aqueous solution with a concentration of 100 mg/L was employed as synthetic wastewater. The pH levels were adjusted by diluted HCl or NaOH aqueous solutions. Jar tests were conducted using 250 mL jars at room temperature. After adding a desired amount of flocculants as solid powder into 100 mL AG25 solution, the mixture was fast stirred at 200 rpm for 3 min followed by a slowly stirring at 50 rpm for 10 min, and finally left to settle for 2 h. The supernatants were collected after reaching flocculation equilibrium using a syringe and filtrated through the membrane with a pore diameter of 0.45 μm. A UV–visible spectrophotometry (Model UV-2401, Shimadzu Co.) was used to determine the concentration of residual dye in water. A calibration curve of AG25 was prepared in advance. The absorbance was measured at the wavelength of 642 nm. The colour removal efficiency was calculated as follows:
where C 0 and C are the dye concentrations in solution before and after flocculation, respectively (Yang et al. 2011).
The effect of salt additives on the flocculation performance of CMC-g-PDMC was studied at pH 7.0. CMC-g-PDMC15 was selected as a representative. Four different salts, NaCl, NaNO3, Na2CO3 and Na3PO4, were tested respectively. The flocculant dosage in each experiment was 90 mg/L, and the initial dye concentration was 100 mg/L. A similar analysis method using UV–visible spectrophotometry, as mentioned above, was employed to detect the final dye concentration once flocculation equilibrium was reached.
Floc properties measurement
Floc properties were measured by image analysis (IA). After sedimentation, the flocs were carefully withdrawn from the jars and then transferred into a glass dish with water. A Pentax Model K-m digital camera with a 200-mm lens was used to take photos. The characteristic length (l) and the projected area (A) were derived from the photos using image analysis software, Imagepro® Plus 6.0. In this work, l was defined as the largest projection length and used to evaluate the floc size. The coefficient of two-dimensional fractal dimension (D 2) was then obtained from the slope of the log–log plot of A and l (Chakraborti et al. 2000; Lin et al. 2008). Larger D 2 indicates denser flocs.
Result and discussion
Characterization of CMC-g-PDMC
CMC-g-PDMC has been prepared through a two-step route on the basis of Scheme 1 described in detail in the experimental part. The FTIR and 1H NMR spectra of CMC, monomer DMC and various CMC-g-PDMC samples have been measured and illustrated in Figs. 1 and 2, respectively. The bands of 1025 cm−1 (C–OH) and 3320 cm−1 (O–H) in Fig. 1a are the characteristic FTIR peaks of cellulose. The peak at 1595 cm−1 in CMC is ascribed to carboxymethyl groups. Besides the aforementioned peaks, peaks around 1716 cm−1 are attributed to carbonyl (C=O) on the PDMC chains and peaks at 1474 and 944 cm−1 are vibrations of methyl groups (C–H) on quaternary ammonium salt in PDMC branches (Wang et al. 2009a) from Fig. 1c–g, which are well consistent with those of DMC as shown in Fig. 1b. As the PDMC homopolymer has been removed during the preparation process after the products were washed and extracted by acetone in the Soxhlet apparatus, the detected PDMC chain should be chemically bonded onto the cellulose backbone. Furthermore, the characteristic FTIR peaks of PDMC in Fig. 1c–g all increase with the increase of grafting ratio as listed in Table 1.
As for the 1H NMR spectra, the overlapped signals between 3.10 and 4.30 ppm in Fig. 2a are assigned to the protons H1–H6 on the glycosidic ring of carboxymethyl cellulose. For various CMC-g-PDMC samples, the new significantly strong signals at around 3.25 ppm in Fig. 2 b–f are the resonances of methyl protons on the PDMC chains. The FTIR and 1H NMR spectra both confirm that CMC-g-PDMC has been successfully synthesized.
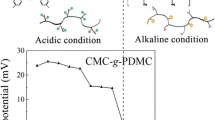
Moreover, the zeta potentials of various cellulose-based sample solutions have been measured before flocculation measurement, since charge properties of ionic flocculants would highly affect the flocculation efficiency (Wang et al. 2009a; Yang et al. 2012). The ZP-pH curves were demonstrated and shown in Fig. 3.
It is found that CMC shows negative ZP in the whole measured pH range (from 2.0 to 12.0) due to the presence of carboxymethyl groups. However, after PDMC branches were grafted onto CMC backbones, all the five CMC-g-PDMC samples bear positive charges and exhibit the characteristics of strong cationic polyelectrolytes. Furthermore, the ZPs of all CMC-g-PDMC samples decrease with increasing pH due to the fact that the concentrations of counterions increase. In addition, the ZPs of different CMC-g-PDMC samples increase with increasing grafting ratio resulting from more cationic quaternary ammonium groups contained. Since AG25 is a typical anionic dye and bears negative charge in aqueous solution from Fig. 3, the strong cationic characteristics of CMC-g-PDMC samples are expected to wield excellent flocculation ability in the following jar tests.
Flocculation performance of CMC-g-PDMC
The flocculation performance of different CMC-g-PDMC samples at various dosages for removal of AG25 was systematically investigated under acidic (pH 3.0), neutral (pH 7.0) and alkaline conditions (pH 11.0), respectively, since flocculant dosage and pH are two very important factors that affect the flocculation efficiency of flocculants, especially for ionic ones (Oladoja and Aliu 2009; Shi et al. 2012). The results are all depicted in Fig. 4.
Effect of dosage
To start with, the optimal dosages of the cellulose-based flocculants at various pH conditions have been observed from Fig. 4 which are corresponding to the maximal dye removal efficiencies. It is found that the maximal dye removal efficiencies of all CMC-g-PDMC samples are quite high in each measured pH level. This improvement can be mainly ascribed to the strong cationic characteristics of the flocculants in the whole tested pH range for the grafted PDMC chains with high positive charge density. The strong electrostatic attractions between the flocculants and the anionic AG25 are beneficial for dye removal.
Moreover, all the colour removal efficiency–dosage profiles in Fig. 4 give demonstrations of up-climax-down trend, suggesting that charge neutralization effect (Pal et al. 2011) plays an important role in the flocculation process. In order to investigate the flocculation mechanism further, the ZPs of the final supernatants after reaching flocculation equilibrium as a function of dosage at various pH levels have been measured, as showed in Fig. 5 along with the colour removal efficiencies.
CMC-g-PDMC13 and CMC-g-PDMC15 were selected as representatives with relatively low and high grafting ratio respectively. Under each condition, ZPs always display an upward trend with increasing the dosage of flocculants. Moreover, most of the ZPs of the supernatants are very close to zero at their corresponding optimal dosages from Fig. 5, suggesting that simple charge neutralization is the dominant flocculation mechanism in these cases. At the optimal points, the cationic flocculants fully neutralize the opposite charges of dyes and then insoluble complexes are formed which further aggregate together to be larger flocs and settle down. However, insufficient flocculants are not enough for complete neutralization whereas excessive ones lead to restabilization effect due to the electrostatic repulsion among the dye flocs bounded with oppositely charged flocculants.
As for CMC-g-PDMC13 under alkaline condition in Fig. 5c, the ZP is slightly negative at optimal dosage, which indicates that patch mechanism may also have some contributions to dye removal besides simple charge neutralization. It can be mainly ascribed to more collapsed conformation and lower ZP of CMC-g-PDMC13 at higher pH condition. In consequence, its velocity of migration to and adsorption on dye turns slower in this situation and there is enough time for collision among some of the primary dye flocs which are not completely neutralized yet.
In addition to charge neutralization effect, the polymeric flocculants with highly charged branches would present an extended macromolecular conformation in aqueous solutions due to intra-chain electrostatic repulsion (Flory 1953), which also engenders improved approachability of the flocculants to those dye molecules. Therefore, both charge neutralization and bridging effects result in high dye removal efficiency of CMC-g-PDMC.
Effect of pH
From Fig. 4, it is roughly found that there are quite different pH influences on the flocculation performance among those various graft cellulose-based flocculants. The dye removal efficiency of CMC-g-PDMC with relatively low grafting ratio (CMC-g-PDMC12, CMC-g-PDMC13 and CMC-g-PDMC14) shows pH dependent, but that of CMC-g-PDMC with high grafting ratio (CMC-g-PDMC15 and CMC-g-PDMC18) is pH independent. In order to give a clearer comparison and discussion, CMC-g-PDMC13 and CMC-g-PDMC15 were selected as representatives to test their flocculation performance at five different pH levels, which is shown in Fig. 6.
From Fig. 6a, CMC-g-PDMC13 exhibits higher dye removal efficiency with lower optimal dosage at acidic conditions, but more dosage is required at high pHs. It is due to the fact that the positive charge of CMC-g-PDMC would be reduced with increasing pH from Fig. 3 as the flocculants chains are surrounded with a growing number of OH− counterions which would compress the electrical double layer of flocculants much more. Accordingly, on the one hand, the charge neutralization effects would be reduced obviously. On the other hand, the conformation of the polymeric flocculants in water would be collapsed for reduction of the net charge on the macromolecular chain (Flory 1953; Jiang et al. 2011) resulting in the bridging flocculation effects weakened. Therefore, the flocculation performance of CMC-g-PDMC13 decreases with the increase of pH.
It is notable that both the optimal dosage and colour removal efficiency of CMC-g-PDMC15 with relatively high grafting ratio have almost no change at various pH levels on the basis of Fig. 6b. Although the positive charge of CMC-g-PDMC15 also decreases with increasing pH, its ZP at pH 11.0 is still close to that of CMC-g-PDMC13 at pH 4.0 according to Fig. 3. Hence, the positive charge density of CMC-g-PDMC15 is still high enough to keep high flocculation performance at alkaline conditions. Moreover, CMC-g-PDMC with higher grafting ratio also has higher molecular weight resulting in enhanced bridging effect. Therefore, the flocculation performance of CMC-g-PDMC15 shows pH independent and always remains at a high level.
Effect of grafting ratio (G)
Besides the influence of external factors such as dosage and pH as mentioned above, the grafting ratio, an important structural parameter, also plays a key role in the flocculation properties of graft flocculants. In fact, structural factor is the final determining one for the materials’ performance.
On the basis of Fig. 4, the optimal dosage of the cellulose-based flocculants would decrease from CMC-g-PDMC12 to CMC-g-PDMC18 with increasing grafting ratio at each pH level but the corresponding maximal colour removal efficiency roughly increases. Meanwhile, the slopes of colour removal efficiency–dosage curves at the beginning turn much larger and sharper for CMC-g-PDMC with higher grafting ratio. All of the aforementioned results indicate that CMC-g-PDMC with higher grafting ratio has more PDMC branch chains and higher positive charge density resulting in higher flocculation efficiency and much more sensitive to dosage. Therefore, the colour removal efficiency increases more quickly at the beginning and the restabilization effect is also more serious at overdose conditions for CMC-g-PDMC with higher grafting ratio on the basis of Fig. 4.
Moreover, the flocculation performance of various flocculants at pH 7.0 is selected as the representative, and the detailed optimal dosages as well as their corresponding colour removal efficiencies based on Fig. 4 are all listed in Table 1 for better comparison. Besides, the flocs properties including floc size and D 2, measured through IA under each optimal condition, are also illuminated in Table 1. In addition to better colour removal efficiency and lower dosage requirement, the larger values of average l and D 2 reveal that larger flocs with denser structure are generated by the cellulose-based flocculants with higher grafting ratio. The comb-like copolymer with higher amount of PDMC branch chains would enhance the approachability to dye molecules, and larger flocs could connect with other primary flocs and seize residual dye molecules together through improved bridging (Yang et al. 2010) and sweep (Canizares et al. 2006) effects, respectively. Meanwhile, larger D 2 indicates the more compact structure of flocs, which is close to 2.0, the largest value in theory. It can be ascribed to the stronger charge attraction between cationic flocculants and anionic dye molecules.
However, it doesn’t mean unrestrained PDMC amount to the flocculants’ performance. As listed in Table 1, although CMC-g-PDMC18 with the highest grafting ratio in this work bears the smallest optimal dosage among the five cellulose-based flocculants, it is unfavorable for the highest colour removal efficiency and best flocs properties. It may be due to the fact that the graft copolymer is facile to chemical crosslink with excessive monomers hurting its application performance (Ceresa 1973). Accordingly, the grafting ratio of flocculant should be controlled in a suitable range (around 180 % in this current work) to ensure better cost performance for not only lower optimal dosage and higher dye removal efficiency but also larger and denser flocs.
Effect of salt additives
It is well known that the real water is normally very complicated where many solutes coexist such as inorganic salts. The effects of four common salt additives (NaCl, NaNO3, Na2CO3 and Na3PO4) on the flocculation performance of CMC-g-PDMC for dye removal were studied also. CMC-g-PDMC15 was selected as the representative. Figure 7 shows the concentration effects of various salts on the colour removal efficiency of CMC-g-PDMC15. It is found that the dye removal efficiencies all decrease with increasing salt concentration. It may be ascribed to two facts. One is that the positive charges of the flocculants have been partially screened with the increase of the ionic strength, thus the electrostatic interactions between flocculants and dyes are weakened. The other is that the inorganic anions might compete with and replace AG25 on the activated flocculation sites of flocculants. However, except NaCl, the extent of decrease in colour removal efficiency is quite slight in the presence of the other three salts. CMC-g-PDMC15 still shows good salt resistance in the measured salt concentration range, and the dye removal efficiency still remains around 90 % at the maximal tested salt concentration approximately 0.2 mol/L.
Additionally, the flocs properties (including floc size and D 2) and hydrodynamic radius (R h) of the flocculants in the presence of each salt with concentration of 0.1 mol/L are all listed in Table 2. The molar concentration of inorganic anions is kept constant, since CMC-g-PDMC flocculants bear the characteristics of cationic polyelectrolytes. It is found that the final floc size and D 2 both decrease as well as the colour removal efficiency in the presence of each salt. On the basis of the shrunk hydrodynamic size, the polymeric flocculants have more curled morphology, (Flory 1953; Jiang et al. 2011) and the bridging flocculation effect has been also reduced besides charge interactions. Interestingly, the degrees of decrease in floc size, D 2 and R h, by NaCl are the most remarkable which are full consistent with the results of salt concentration effects as shown in Fig. 7. The stronger reduction effect of NaCl additive may result from certain efficient interactions between quaternary ammonium groups of flocculants and chloride ions for smaller hydrated ion radius of Cl−.
Conclusion
The tailored graft cellulose-based flocculants, CMC-g-PDMC, were successfully prepared which can efficiently flocculate an anionic dye, AG25, from aqueous solutions at different pH levels. It is due to the enhanced charge neutralization and bridging effects for improvement of both positive charge and molecular weight of the polymeric flocculants after PDMC branches grafted. Furthermore, the grafting ratio plays an important role in both flocculation performance and flocs properties. With increasing grafting ratio, the colour removal efficiency as well as floc size and compactness (D 2) mostly increases but the required dosage decreases. The flocculation performance of the cellulose-based flocculants with various grafting ratio shows quite different pH dependent. The dye removal efficiency of CMC-g-PDMC with high grafting ratio is pH independent for stronger positive charge and higher molecular weight, while that of CMC-g-PDMC with relatively low grafting ratio would be reduced in alkaline conditions. The cellulose-based flocculants bear good salt resistance except for NaCl. It may be ascribed to certain more efficient attraction between flocculants and chloride ions.
References
Aboulhassan MA, Souabi S, Yaacoubi A et al (2006) Improvement of paint effluents coagulation using natural and synthetic coagulant aids. J Hazard Mater 138:40–45
Batmaz R, Mohammed N, Zaman M (2014) Cellulose nanocrystals as promising adsorbents for the removal of cationic dyes. Cellulose 21:1655–1665
Bhandari PN, Jones DD, Hanna MA (2012) Carboxymethylation of cellulose using reactive extrusion. Carbohydr Polym 87:2246–2254
Cai T, Yang Z, Li HJ et al (2013) Effect of hydrolysis degree of hydrolyzed polyacrylamide grafted carboxymethyl cellulose on dye removal efficiency. Cellulose 20:2605–2614
Canizares P, Martinez F, Jimenez C et al (2006) Coagulation and electrocoagulation of wastes polluted with dyes. Environ Sci Technol 40:6418–6424
Ceresa RJ (1973) Block and graft copolymerization, vol 1. Wiley Interscience, New York
Chakraborti RK, Atkinson JF, Van Benschoten JE (2000) Characterization of alum floc by image analysis. Environ Sci Technol 34:3969–3976
da Silva DA, de Paula RCM, Feitosa JPA (2007) Graft copolymerisation of acrylamide onto cashew gum. Eur Polym J 43:2620–2629
dos Santos AB, Cervantes FJ, van Lier JB (2007) Review paper on current technologies for decolourisation of textile wastewaters: perspectives for anaerobic biotechnology. Bioresour Technol 98:2369–2385
Fabrizi L, Jefferson B, Parsons SA et al (2010) The role of polymer in improving floc strength for filtration. Environ Sci Technol 44(16):6443–6449
Flory PJ (1953) Principles of polymer chemistry. Cornell University Press, Ithaca
Ghimici L, Constantin M, Fundueanu G (2010) Novel biodegradable flocculanting agents based on pullulan. J Hazard Mater 181(1–3):351–358
He HL (2004) Dye. Chemical Industry Press, Beijing
Jarvis P, Jefferson B, Parsons SA (2005) Breakage, regrowth, and fractal mature of natural organic matter flocs. Environ Sci Technol 39(7):2307–2314
Jiang X, Cai K, Zhang J et al (2011) Synthesis of a novel water-soluble chitosan derivative for flocculated decolorization. J Hazard Mater 185:1482–1488
Khraisheh MAM, Al-Ghouti MA, Allen SJ et al (2005) Effect of OH and silanol groups in the removal of dyes from aqueous solution using diatomite. Water Res 39:922–932
Klemm D, Heublein B, Fink HP et al (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed 44:3358–3393
Li A, Zhang JP, Wang AQ (2007) Utilization of starch and clay for the preparation of superabsorbent composite. Bioresour Technol 98(2):327–332
Liang CZ, Sun SP, Li FY et al (2014) Treatment of highly concentrated wastewater containing multiple synthetic dyes by a combined process of coagulation/flocculation and nanofiltration. J Membr Sci 469:306–315
Lin JL, Huang CP, Chin CJM et al (2008) Coagulation dynamics of fractal flocs induced by enmeshment and electrostatic patch mechanisms. Water Res 42:4457–4466
Liu C, Sun R, Qin M et al (2008) Succinoylation of sugarcane bagasse under ultrasound irradiation. Bioresour Technol 99:1465–1473
Mandelbrot BB (1983) The fractal geometry of nature. Freeman, New York
Oladoja NA, Aliu YD (2009) Snail shell as coagulant aid in the alum precipitation of malachite green from aqua system. J Hazard Mater 164:1496–1502
Pal S, Ghorai S, Dash MK et al (2011) Flocculation properties of polyacrylamide grafted carboxymethyl guar gum (CMG-g-PAM) synthesised by conventional and microwave assisted method. J Hazard Mater 192:1580–1588
Qi HS, Liebert T, Meister F et al (2009) Homogenous carboxymethylation of cellulose in the NaOH/urea aqueous solution. React Funct Polym 69:779–784
Rafatullah M, Sulaiman O, Hashim R et al (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177:70–80
Renault F, Sancey B, Badot PM et al (2009) Chitosan for coagulation/flocculation processes-an eco-friendly approach. Eur Polym J 45(5):1337–1348
Riera-Torres M, Gutierrez-Bouzan C, Crespi M (2010) Combination of coagulation-flocculation and nanofiltration techniques for dye removal and water reuse in textile effluents. Desalination 252:53–59
Roussy J, Van Vooren M, Dempsey BA et al (2005) Influence of chitosan characteristics on the coagulation and the flocculation of bentonite suspensions. Water Res 39:3247–3258
Shi YL, Ju BZ, Zhang SF (2012) Flocculation behavior of a new recyclable flocculant based on pH responsive tertiary amine starch ether. Carbohydr Polym 88:132–138
Song YB, Zhang J, Gan WP et al (2010) Flocculation properties and antimicrobial activities of quaternized celluloses synthesized in NaOH/Urea aqueous solution. Ind Eng Chem Res 49:1242–1246
Sonmez HB, Senkal BF, Bicak N (2002) Poly(acrylamide) grafts on spherical bead polymers for extremely selective removal of mercuric ions from aqueous solutions. J Polym Sci A Polym Chem 40:3068–3078
Tian D, Zhang XX, Lu CH et al (2014) Solvent-free synthesis of carboxylate-functionalized cellulose from waste cotton fabrics for the removal of cationic dyes from aqueous solutions. Cellulose 21:473–484
Verma AK, Dash RR, Bhunia P (2012) A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J Environ Manag 93:154–168
Wang JP, Chen YZ, Yuan SJ et al (2009a) Synthesis and characterization of a novel cationic chitosan-based flocculant with a high water-solubility for pulp mill wastewater treatment. Water Res 43:5267–5275
Wang Y, Gao B, Yue Q et al (2009b) Flocculation performance of epichlorohydrin-dimethylamine polyamine in treating dyeing wastewater. J Environ Manag 91:423–431
Wang CP, Zhang YW, Yu L et al (2013a) Oxidative degradation of azo dyes using tourmaline. J Hazard Mater 260:851–859
Wang JP, Yuan SJ, Wang Y et al (2013b) Synthesis, characterization and application of a novel starch-based flocculant with high flocculation and dewatering properties. Water Res 47:2643–2648
Xiong JQ, Jiao CL, Li CM et al (2014) A versatile amphiprotic cotton fiber for the removal of dyes and metal ions. Cellulose 21:3073–3087
Yagub MT, Sen TK, Afroze S et al (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interface Sci 209:172–184
Yang Z, Gao BY, Li CX et al (2010) Synthesis and characterization of hydrophobically associating cationic polyacrylamide. Chem Eng J 161:27–33
Yang Z, Shang YB, Lu YB et al (2011) The flocculation properties of biodegradable amphoteric chitosan-based flocculants. Chem Eng J 172:287–295
Yang Z, Yuan B, Huang X et al (2012) Evaluation of the flocculation performance of carboxymethyl chitosan-graft-polyacrylamide, a novel amphoteric chemically bonded composite flocculant. Water Res 46:107–114
Yang Y, Murthy BN, Shapter JG et al (2013a) Benzene carboxylic acid derivatized graphene oxide nanosheets on natural zeolites as effective adsorbents for cationic dye removal. J Hazard Mater 260:330–338
Yang Z, Yang H, Jiang ZW et al (2013b) Flocculation of both anionic and cationic dyes in aqueous solutions by the amphoteric grafting flocculant carboxymethyl chitosan-graft-polyacrylamide. J Hazard Mater 254:36–45
Zahrim AY, Tizaoui C, Hilal N (2011) Coagulation with polymers for nanofiltration pre-treatment of highly concentrated dyes: a review. Desalination 266:1–16
Zhao YX, Gao BY, Shon HK et al (2011) The effect of second coagulant dose on the regrowth of flocs formed by charge neutralization and sweep coagulation using titanium tetrachloride (TiCl(4)). J Hazard Mater 198:70–77
Zhou KQ, Zhang QJ, Wang B et al (2014) The integrated utilization of typical clays in removal of organic dyes and polymer nanocomposites. J Clean Prod 81:281–289
Acknowledgments
This work was supported by the Natural Science Foundation of China (Grant No. 51438008 and 51378250), Major Science and Technology Program for Water Pollution Control and Treatment (Grant No. 2015ZX07204-002), and the Fundamental Research Funds for the Central Universities (Grant No. 20620140494).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, T., Li, H., Yang, R. et al. Efficient flocculation of an anionic dye from aqueous solutions using a cellulose-based flocculant. Cellulose 22, 1439–1449 (2015). https://doi.org/10.1007/s10570-015-0571-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-015-0571-9