Abstract
Adsorption is an efficient method to combat the important issues of water pollution caused by dyes and metal ions. However, due to the surface charge diversity of pollutants, there is a pressing need to develop an all-round, efficient, cheap and environmentally friendly adsorbent. To this end, this work synthesized an amphiprotic adsorbent based on cotton fibers, which were chemically modified with a cationic monomer (3-chloro-2-hydroxypropyl trimethyl ammonium chloride) and anionic monomer (2-acrylamide-2-methyl propane sulfonic acid) respectively. The resultant amphiprotic cotton (AP-cotton) can cope with both of anionic and cationic pollutants. Its adsorption behavior as influenced by the pH value, adsorption time and initial concentration of various adsorbates was investigated. The results demonstrate that the adsorption equilibrium was reached within 4 h for Congo red (CR) and methylene blue (MB), 2 h for Cu2+ and 3 h for Pb2+, respectively. Adsorption kinetics showed that the adsorption rate was well fitted with the pseudo-second-order rate model, and the best adsorption isotherms fitted the Langmuir model. The Langmuir maximum adsorption capacities were 175.1 mg/g for CR, 113.1 mg/g for MB, 88.9 mg/g for Cu2+ and 70.6 mg/g for Pb2+, respectively, and the adsorption capacities could be maintained above 90 % after six regenerations. The all-round adsorption capacity and good regeneration performance of AP-cotton benefited from its hollow, flat-banded structure and amphiprotic characteristic. Therefore, AP-cotton exhibited a much better application potential compared with many other reported adsorbents based on natural materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, a progressive increase in industrialization and urbanization has substantially increased aquatic environmental pollution by the discharge of industrial effluents containing highly toxic organic and inorganic pollutants. Dyes and heavy metals are the most important constituents among the toxic compounds present in the effluent (Gupta et al. 2009; Saad et al. 2010; Sajab et al. 2011; Siddiqi and Pathania 2002). They not only influence the quality of the water body, but also reduce the production and quality of crops and aquatic products, even threatening the health and life of animals and humans through the food chain (Cheng 2003; Duan et al. 2013). The presence of dyes in aquatic bodies increases chemical oxygen demand, color contents, and dissolved and suspended solids; impedes light penetration into water; interferes with photosynthesis of aquatic plants; hinders the growth of microbes; creates microtoxicity for fish and other organisms; etc. Unlike organic pollutants, the heavy metals are persistent in nature and do not undergo physical, chemical or microbial degradation; they have a tendency to accumulate in living organisms, having a severe impact on human health and on the sustainability of ecosystems because of their carcinogenic and mutagenic nature (Bailey et al. 1999; Cay et al. 2004; Roy et al. 2013). Due to the harmful effects of dye and heavy metal ion pollution in water, there is a pressing need to find efficient methods to combat this kind of pollution. Many approaches to the removal of dyes and heavy metal ions from aqueous solution have been carried out, such as adsorption, chemical precipitation, ion exchange, complexation, photodegradation, electrodialysis, reverse osmosis and ultra-filtration membrane separation (Bhattacharyya and Sharma 2005; Gupta et al. 2006; Gupta and Ali 2008; Wang and Zhu 2007). However, most of these techniques have several disadvantages such as inefficiency, high energy and chemical reagent requirements, generation of toxic dreg or other wastes as by-products that need careful treatment and disposal, high capital and operational costs, etc. (Demir et al. 2008). Nevertheless, adsorption is an efficient method that has been used extensively for the removal of dyes and heavy metal ions from industrial wastewater because of its economic viability and technical feasibility (Yin et al. 2008).
Activated carbon (Aber and Sheydaei 2012; Kim et al. 2013), graphene (Zhang et al. 2011; Zhao et al. 2011), sand (Gao et al. 2011), clay (Ozdes et al. 2011), oxides (Shi et al. 2011), composite fiber (Ji et al. 2012, 2013; Ren et al. 2011; Sui et al. 2012; Xia et al. 2013) and polymeric material (Jiang et al. 2012; Neghlani et al. 2011) have been utilized as adsorbents. However, most of these materials are non-renewable or non-biodegradable and may cause secondary pollution. Of course, several natural fiber-based materials have been explored for this purpose, for example, sugar cane bagasse (Gurgel et al. 2008a, b; Júnior et al. 2009; Karnitz Jr et al. 2007), kapok (Duan et al. 2013; Liu et al. 2012; Zheng et al. 2012), luffa cylindrical (Demir et al. 2008; Gupta et al. 2013), lignocellulosic jute fiber (Roy et al. 2012, 2013), wheat straw (Han et al. 2010), peanut shell (Liu et al. 2010), coconut shell (Shrestha et al. 2013), rice husk (Chowdhury et al. 2011), starch (Yin et al. 2008; Zhao et al. 2010b), etc. But most of these adsorbents can only cope with the pollutants with a single surface charge. Nevertheless, usually the constituents of industrial effluents are extremely complex and include both cationic and anionic pollutants. Therefore, the application fields of all the adsorbents mentioned above are seriously restricted.
With this in mind, amphiprotic adsorbents are gaining increasing attention because of their versatile adsorption capability for both cationic and anionic pollutants. Zhang et al. (2012) prepared a novel amphiprotic wheat straw-based adsorbent (AWS) used to adsorb various dyes from aqueous solutions. This AWS was proven effective in eliminating both cationic and anionic dyes [methylene blue (MB) and acid green 25], the maximum adsorption capacity of AWS for MB being around 138 mg/g and for acid green 25 around 360 mg/g. Xu and coworkers (Xu et al. 2005) crosslinked amphiprotic starch (CAS) with quaternary ammonium and carboxymethyl groups and used it to remove Pb2+ from aqueous solutions; the maximum adsorption capacity of CAS was around 53 mg/g for Pb2+.
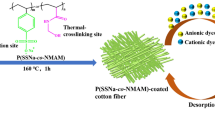
Cellulose is the major component in cotton fibers and the most abundant renewable biopolymer in nature. Due to the characteristic properties of cellulose such as excellent hydrophilicity, biocompatibility and biodegradability, it can easily be chemically modified according to the introduction of various functional groups on hydroxyl (Qu et al. 2009). However, most of the modified cotton fibers were only used as adsorbents for the removal of cationic compounds (metal ions, etc.) instead of anionic pollutants. In this study, we synthesized an amphiprotic adsorbent based on cotton fiber that has a hollow, flat-banded structure. Specifically, cotton fibers were activated by dispersing them into the diluted NaOH aqueous solution; then activated cotton was chemically modified with the cationic monomer (3-chloro-2-hydroxypropyl trimethyl ammonium chloride, CTA) and anionic monomer (2-acrylamide-2-methyl propane sulfonic acid, AMPS) respectively. The resulting amphiprotic cotton fibers (AP-cotton) were used as the adsorbent for the removal of dyes and heavy metal ions from aqueous solutions (Scheme 1). Two typical azo Congo red (CR) and methylene blue (MB) dyes were chosen as the representatives of anionic and cationic pollutants, and Cu2+ and Pb2+ acted as the metal ions for the adsorption study. As shown in Scheme 1, the removal capacity of AP-cotton for dyes and metal ions is remarkable after amphiprotic modification. Furthermore, the influences of pH, adsorption time and initial concentration of dyes and metal ions in the adsorption behavior were investigated. Regeneration tests were carried out to evaluate the practical utility of this modified cotton fiber.
Experimental
Materials
The cotton fibers used were provided by Suzhou Yintong Cotton Co., Ltd. CTA (60 wt%) and AMPS came from Sigma-Aldrich and were used without further purification. Sodium hydroxide (NaOH), hydrochloric acid (HCl), acetone, methanol, ethanol, diethyl ether and ammonium cerium nitrate [Ce(NH4)2(NO3)6], glacial acetic acid, copper chloride dehydrate (CuCl2 2H2O), lead chloride (PbCl2), CR and MB were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and used without any treatment.
Pretreatment of cotton fibers
Cotton fibers (10 g) were boiled in 500 ml NaOH aqueous solution (2 wt%) for 90 min. Then the fibers were filtered and transferred into 300 ml NaOH aqueous solution (18 wt%); the mixture was stirred at room temperature for 2 h. Then the fibers were filtered and washed three times with plenty of deionized water. After drying at 60 °C for 2 h, the activated cotton fiber (AC) was obtained.
Preparation of AP-cotton
ACs were cut into 5–10-mm-long segments by shears. The short fibers (8.0 g) were suspended in 80 ml acetone; under continuous stirring, 6.0 ml NaOH (10 wt%) and 16.0 g CTA respectively were added to the above-mentioned mixture dropwise. The resultant mixture was stirred at 45 °C for 3 h, and its pH value was regulated to be neutral. Then the fibers were filtered and washed with plenty of aqueous methanol solution (85 %) and absolute ethanol, followed by drying at 90 °C for 1 h. The product was named CTA-cotton (CC).
Then 5.0 g CC was added to 200 ml deionized water; the mixture was stirred at room temperature for 10 min under nitrogen atmosphere. Then 10.0 g Ce(NH4)2(NO3)6 was added and stirred for 30 min. Subsequently, 15.0 g AMPS was added to the above mixture and stirred for 1.0 h. The resultant system was stirred continuously at 50 °C for 3.0 h, then the fibers were filtered, and washed with plenty of deionized water, acetone and diethyl ether, followed by drying at 60 °C for 3 h. The product was CTA-AMPS-cotton; because of including both cationic groups and anionic groups, we named it as AP-cotton.
Characterization
The morphology of the fibers was studied using Hitachi S-4800 field emission scanning electron microscopy. The FT-IR spectroscopy measurements were made using a Nicolet 5700. X-ray diffraction studies were performed on an X-ray diffractometer (Brucker AXS, D8 Advance). XPS measurements were conducted with an Axis Ultra HAS system. The concentrations of metal ions were measured using a Thermo Scientific ICAP 6000 DUO inductively coupled plasma atomic emission spectroscopy. The concentration of CR and MB in the supernatant was estimated by UV/visible spectroscopy (Purkinje General, TU-1810) at 498 and 664 nm, respectively. The resultant standard curves of the relationship between the absorbance of dyes solutions and the dye concentration are shown in Figs. S1 and S2.
Adsorption and regeneration experiments
Adsorbents were added to different adsorbate solutions at a dosage of 1.0 g/l for Cu2+ and Pb2+, and 2.0 g/l for CR and MB, respectively. Initial pH values of the solution were calibrated using glacial acetic acid and NaOH solutions. The adsorption experiments were carried out at 30 °C. The adsorption capacity Qe (mg/g) was calculated according to the equation:
where C 0 and C e are the initial and equilibrium adsorbate concentration (mg/l), respectively. V is the volume of adsorbate solution (L), and m is the mass of adsorbent used for adsorption experiment (g). Three sets of experiments were conducted for complete investigation of Cu2+, Pb2+, CR and MB adsorption on AP-cotton. The details are given in Table 1.
For the regeneration study, the AP-cotton used for adsorption of Cu2+ or Pb2+ was placed with 1 M HCl solution at 303 K for 2 h for desorption. After being washed until no metal ions were detected in the filtrate, indicating complete desorption, then it was reemployed in metal ion removal (Duan et al. 2013). The AP-cotton was loaded with CR or MB solution, washed gently with water to remove any unadsorbed dye and dried. Then it was suspended in eluting solvents (0.1 M NaOH used for desorption of CR (Roy et al. 2013), and 0.1 M HCl used for desorption of MB (Liu et al. 2012) was agitated (120–140 rpm) at 303 K for 3 h. The desorbed dye concentration was quantified using a UV/visible spectrophotometer; the desorption was creased until the concentration of desorbed dye had no change. Then the regenerated AP-cotton was reused for the removal of dyes.
Results and discussion
Characterization of AP-cotton
The synthesis route and adsorption efficiency of AP-cotton are shown in Scheme 1. The main component of cotton fiber is cellulose, which accounts for over 94 % in ripe cotton. The other components were wax and ash, which were removed after treatment with NaOH solution. Then the obtained activated cotton (AC) was amphiprotically modified with CTA and AMPS respectively to improve the hydrophilicity and adsorption activity of the fibers. As a result, the AP-cotton product could adsorb both CR (anionic) and MB (cationic) as a versatile adsorbent, and its adsorption capacity for dyes and metal ions was obviously enhanced compared to raw cotton (RC).
SEM study of AP-cotton
Significant changes in the surface morphology of cotton fibers after amphiprotic modification with CTA and AMPS respectively were revealed by SEM (Fig. 1). The surfaces of raw cotton (Fig. 1a) and activated cotton (Fig. 1b) were smooth with few wrinkles. However, after the treatment of CTA, the surface morphology of CTA-cotton was observed to be wrinkled and porous (Fig. 1c, d). The enlarged image (Fig. 1d) shows the average pore size was 10–30 nm. With further treatment by AMPS, the porous nature remained without obvious change in the pore size, but some tablet shapes were observed on the surface of AP-cotton (Fig. 1e, f), which might be coated with AMPS molecules.
FTIR analysis of AP-cotton
As shown in Fig. 2, the bands at 1,649.9 and 1,552.6 cm−1 in the spectrum of AP-cotton were attributed to the amide I (νC=O) and amide II (δN–H), respectively; their appearance confirms that the amide group was introduced onto the AP-cotton after modification with CTA and AMPS. The weakening and even disappearing of the band at 1,430.4 cm−1 with the process of modification from raw cotton to AP-cotton revealed the reduction in inter- or intramolecular hydrogen bonding between the hydroxyl groups of cellulose. This may mean that the crystallinity of the cotton fibers was reduced after amphiprotic modification. In addition, the unchanged characteristic absorption bands of cellulose at about 3,407, 2,903 and 1,060 cm−1 suggest that the main composition of the cotton fibers remained after chemical modification (Saad et al. 2010).
XRD analysis of AP-cotton
The XRD pattern of AP-cotton is shown in Fig. 3 in order to further determine the change in crystallinity of cotton fibers after modification. The characteristic peaks of raw cotton were observed at 22.78 and 14.85 with relative intensities at 8,661 and 3,917 respectively. After cationic modification, the related characteristics peaks of the resultant CTA-cotton decreased obviously. More notably, the characteristic peak intensity of AP-cotton that resulted from the amphiprotic modification was found at 20.46 with relative intensities of 2,015, which decreased more significantly. This suggested that the incorporation of CTA and AMPS led to serious impairment of the fiber crystallinity. Therefore, the resultant AP-cotton developed a random microscopic structure with modification, which is consistent with the result of Fig. 2 and will be useful for the adsorption.
XPS characterization of AP-cotton
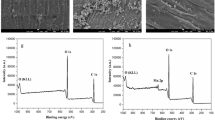
The chemical state of the elements in AP-cotton was further investigated by XPS (Fig. 4). The wide scan XPS spectrum (Fig. 4a) of raw cotton, CTA-cotton and AP-cotton showed photoelectron lines at binding energies of about 168, 285, 400 and 530 eV attributed to S2p, C1s, N1s and O1s, respectively. The high-resolution C1s peaks of raw cotton, CTA-cotton and AP-cotton are displayed in Fig. 4b–d. The C1s peak of raw cotton can be deconvoluted into two components (Fig. 4b): (1) the nonoxygenated C (284.6 eV); (2) the carbon in C–O (286.5 eV) (Wang et al. 2010; Zhao et al. 2010a). The deconvoluted C1s spectra of CTA-cotton contains three components (Fig. 4c): the nonoxygenated ring C (284.6 eV), C–N species (285.7 eV) and C–O species (286.5 eV) (Park et al. 2009), the C–N species coming from the cationic monomer CTA. The deconvoluted C1s spectra of the AP-cotton composite show four peaks at 284.6, 285.7, 286.5 and 287.8 eV, and they correspond to the C–C, C–N, C–O and C=O bonds, respectively (Fig. 4d) (Bai et al. 2009; Park et al. 2008). Apparently, the nitrogen and sulfur element existed in the AP-cotton because of the chemical interaction, which demonstrated that both the cationic monomer CTA and anionic monomer AMPS were introduced into the fibers successfully, and the final product was really AP-cotton.
Adsorption study of AP-cotton for dyes and metal ions
Effect of pH values on adsorption
The initial pH of the dye solution plays a significant role in the sorption process of dyes and metal ions by AP-cotton. The variation of the adsorption capacity for the adsorbent at different pH values can be attributed to the surface chemical character of both the adsorbent and adsorbate, specifically the surface charge of the adsorbent in aqueous solution at a certain pH. The effect of pH on the adsorption efficiency of AP-cotton toward CR, MB, Cu2+ and Pb2+ ions is presented in Fig. 5b. The CR removal increased as the initial pH of the solution increased from 2 to 4, but decreased drastically as the pH further increased from 4 to 13 (Fig. 5b). The adsorption capacity of the adsorbent is affected by the change of solution pH due to the protonation and deprotonation of the active functional groups of the adsorbent and adsorbate (Vieira et al. 2009). CR has a pKa value at 4.5–5.5, which can exist in both anionic form at basic pH and in cationic form at acidic pH (Purkait et al. 2007). Lower adsorption at pH 2 and pH 3 is due to the interionic repulsion between the positively charged CR molecule and adsorbent. At pH 4, the nitrogen atoms and sulfonate groups of the CR molecules become protonated (Aldegs et al. 2008), whereas at pH > 3.8 the surface charge of the adsorbent becomes negative because the pH point of zero net charge of AP-cotton is 3.8 (Fig. 5a). This results in an electrostatic attraction between the positively charged CR molecule and negatively charged adsorbent and leads to the maximum adsorption capacity of CR (around 241 mg/g) by AP-cotton at pH 4.0. On the contrary, as pH increases from 4 to 13, the negative surface charge of AP-cotton hinders the adsorption of CR by electrostatic repulsion between deprotonated CR molecules and negatively charged AP-cotton; consequently, the dye removal capacity decreases to 82.7 mg/g. However, it was clear that the adsorption capacity of AP-cotton for MB remarkably increased from 56 to 134 mg/g as the initial pH of MB solution increased from 2 to 13 (Fig. 5b). The surface of MB is always positively charged at acidic or basic pH. So lower adsorption before pH 3 is also because of the interionic repulsion between the positively charged MB molecules and AP-cotton, and the MB removal increased obviously as the pH increased from 4 to 13, attributed to the electrostatic attraction between positively charged MB molecules and negatively charged AP-cotton (Al-Ghouti et al. 2010). In addition, the effect of pH for the removal of Cu2+ and Pb2+ was similar to the tendency of MB removal (Fig. 5b), which was due to the same adsorption mechanism. The above evidence shows that the versatile AP-cotton can cope with both anionic and cationic molecules in their respective pH environment. Of course, we should note that a considerable amount of adsorption by AP-cotton indicates that the electrostatic mechanism is not the only mechanism for dye and metal ion adsorption; the physical forces are also responsible for adsorption (Chatterjee et al. 2007).
Adsorption kinetics
The adsorption kinetic study is a very important feature of water treatment as it depicts the adsorbate uptake, which in turn controls the residence time of adsorbate uptake at the solid-solution interface (Liu et al. 2012). AP-cotton allows the integrated adsorption process for the dyes and metal ions within 12 h. As shown in Fig. 6a, the adsorption equilibrium was reached within 4 h for CR and MB, 2 h for Cu2+, and 3 h for Pb2+, respectively. However, we should note that more than 50 % of each adsorbate was adsorbed by the AP-cotton within 1 h, whose fast adsorption was due to the electrostatic attraction between the adsorbate and adsorbent, and a considerable amount of adsorption before equilibrium may be attributed to other chemical interactions or physical forces, such as the hydrogen bond, complexation between the adsorbate and the active groups of AP-cotton or its porous, and the needed interaction time may be the result of the slower adsorption process of the dyes and metal ions by AP-cotton.
Adsorption kinetic data were analyzed using pseudo-first-order (2) and pseudo-second-order (3) kinetic equations, which are given as (Cherifi et al. 2013; Roy et al. 2013):
where Q 1e or Q 2e represents the calculated values of the adsorption capacity of the AP-cotton at equilibrium (mg/g), Q t (mg/g) is the adsorption amount at time t (min), and k 1 (min−1) and k 2 [g/(mg min)] are the rate constants of pseudo-first-order and pseudo-second-order kinetics equations, respectively.
The corresponding parameters calculated and experimental adsorption capacities are obtained and tabulated in Table 2. It can be seen that the correlation coefficients from pseudo-second-order kinetics (Fig. 6c) were higher than those from pseudo-first-order kinetics (Fig. 6b), and the theoretical adsorption capacities (Q 2e) calculated from pseudo-second-order kinetic equation were close to the experimental adsorption capacities (Q exp), demonstrating pseudo-second-order kinetics could reasonably describe the adsorption process (Tang et al. 2012; Zhang 2011).
Adsorption isotherm
As shown in Fig. 7a, the adsorption amount at equilibrium for each adsorbate increases dramatically with increasing initial concentration at first and then tends to level off.
Adsorption isotherms are used to describe how the adsorbate interacts with adsorbent, and the constants of the isotherm models are useful to determine the amount of adsorbent needed to adsorb a required amount of adsorbate (Tian et al. 2011). In this study, the Langmuir and Freundlich adsorption isotherms were constructed using the adsorption equilibrium data of CR, MB, Cu2+ and Pb2+ by the AP-cotton at initial adsorbate concentrations of 25–500 mg/l (Table 1). The Langmuir isotherm model presumes that the adsorption is limited to the formation of monolayer coverage of the adsorbate on the homogeneous adsorbent surface. The linearized Langmuir equation is given below (Ahmad 2009):
where C e (mg/l) is the concentration at equilibrium, Q e (mg/g) is the adsorption amount at equilibrium, Q max is the maximum monolayer capacity of adsorbent (mg/g), and b is the Langmuir constant that represents the energy of the adsorption process (l/mg); both were computed from the slopes and intercepts of the straight line (Fig. 7b). In the Langmuir model, a dimensionless parameter called separation factor R L, which can indicate whether the adsorption process is favorable, is defined as follows:
As show in Table 3, the Q max of AP-cotton was 175.1 mg/g for CR, 113.1 mg/g for MB, 88.9 mg/g for Cu2+ and 70.6 mg/g for Pb2+. Having high correlation coefficient (R 2 > 0.99), the Langmuir constant b > 0 and the values of the separation factor R L are between 0 and 1, indicating that the Langmuir isotherm model described the adsorption process of AP-cotton for each dye and ions favorably. In relation to the majority of the adsorbents derived from natural materials in the literature, AP-cotton shows good performance compared with others (Table 4). The good adsorption capacity of AP-cotton first benefits from the hollow, flat-banded structure and excellent hydrophilicity of raw cotton, and the all-round adsorption capacity for various adsorbates is due to the amphiprotic modification of cotton fibers, which is a promising candidate modification method for the fabrication of natural material-based adsorbents for the removal of dyes or metal ions from waste water.
The Freundlich model is based on the assumption that multilayer adsorption occurs on a heterogeneous adsorption surface containing unequally available sites of different adsorption energies and is given by the relation (Hu et al. 2010):
where K F is the Freundlich constant (mg/g), and n is the heterogeneity factor. The graphical presentation of the Freundlich isotherm model is expressed in Fig. 7c. The model parameters reflected that this isotherm model showed poorer fit to the experimental data as compared to the Langmuir isotherm under the studied initial concentration range of each adsorbate (Table 3).
Regeneration of AP-cotton
After six repititions of desorption and re-adsorption loop (Fig. 8), a small decrease was observed in the adsorption capacity for Cu2+ and Pb2+, and a slightly more obvious decrease in the AP-cotton adsorption capacity for CR and MB was observed. Even so, the adsorption capacity retained for each adsorbate was more than 90 % after six regenerations.
Desorption studies of a spent adsorbent can provide important insight into the overall adsorption mechanism. Desorption of the adsorbate by water suggests the involvement of weak physisorption, whereas desorption by organic acids implies that the attachment of the adsorbate to the adsorbent is through chemisorption. If the adsorbate desorption occurs upon use of strong mineral acids or bases, the adsorption is due to an electrostatic interaction (Roy et al. 2013). All desorptions in this article were finished by 0.1–1 M HCl solution or 0.1 M NaOH aqueous solution, and the electrostatic interaction and chemisorption may be the main interactions between the adsorbate and adsorbent. This is consistent with the result from the effect of pH values on adsorption.
Conclusion
Cotton fiber, the most abundant renewable fiber in nature, and having excellent hydrophilicity for its hydroxyl groups, was chemically modified with the cationic monomer CTA and anionic monomer AMPS, respectively. The resultant amphiprotic adsorbent (AP-cotton) can cope with both anionic and cationic pollutants in aqueous solution. It could reach adsorption equilibrium within 4 h for CR and MB, 2 h for Cu2+ and 3 h for Pb2+, respectively. The Langmuir maximum adsorption capacities were 175.1 mg/g for CR, 113.1 mg/g for MB, 88.9 mg/g for Cu2+ and 70.6 mg/g for Pb2+, respectively, and its adsorption capacities retained for each adsorbate were more than 90 % after six regenerations. Due to the hollow, flat-banded structure and amphiprotic characteristic, AP-cotton exhibited an all-round adsorption capacity and regeneration potential for various adsorbates. All the results demonstrate this AP-cotton is a versatile, economical and environmentally friendly adsorbent, which is a promising candidate for practical application in the treatment of wastewater contaminated with dyes or heavy metal ions.
References
Aber S, Sheydaei M (2012) Removal of COD from industrial effluent containing indigo dye using adsorption method by activated carbon cloth: optimization, kinetic, and isotherm studies. CLEAN-Soil Air Water 40:87–94. doi:10.1002/clen.201000434
Ahmad R (2009) Studies on adsorption of crystal violet dye from aqueous solution onto coniferous pinus bark powder (CPBP). J Hazard Mater 171:767–773. doi:10.1016/j.jhazmat.2009.06.060
Aldegs Y, Elbarghouthi M, Elsheikh A, Walker G (2008) Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigment 77:16–23. doi:10.1016/j.dyepig.2007.03.001
Al-Ghouti MA, Li J, Salamh Y, Al-Laqtah N, Walker G, Ahmad MN (2010) Adsorption mechanisms of removing heavy metals and dyes from aqueous solution using date pits solid adsorbent. J Hazard Mater 176:510–520. doi:10.1016/j.jhazmat.2009.11.059
Bai H, Xu Y, Zhao L, Li C, Shi G (2009) Non-covalent functionalization of graphene sheets by sulfonated polyaniline. Chem Commun 1667–1669. doi:10.1039/b821805f
Bailey SE, Olin TJ, Bricka RM, Adrian DD (1999) A review of potentially low-cost sorbents for heavy metals. Water Res 33:2469–2479
Batzias F, Sidiras D, Schroeder E, Weber C (2009) Simulation of dye adsorption on hydrolyzed wheat straw in batch and fixed-bed systems. Chem Eng J 148:459–472. doi:10.1016/j.cej.2008.09.025
Bhattacharyya KG, Sharma A (2005) Kinetics and thermodynamics of methylene blue adsorption on neem (Azadirachta indica) leaf powder. Dyes Pigment 65:51–59
Castro RSD et al (2011) Banana peel applied to the solid phase extraction of copper and lead from river water: preconcentration of metal Ions with a fruit waste. Ind Eng Chem Res 50:3446–3451. doi:10.1021/ie101499e
Cay S, Uyanık A, Özaşık A (2004) Single and binary component adsorption of copper(II) and cadmium(II) from aqueous solutions using tea-industry waste. Sep Purif Technol 38:273–280
Chatterjee S, Chatterjee S, Chatterjee BP, Guha AK (2007) Adsorptive removal of congo red, a carcinogenic textile dye by chitosan hydrobeads: binding mechanism, equilibrium and kinetics. Colloids Surf A 299:146–152. doi:10.1016/j.colsurfa.2006.11.036
Cheng S (2003) Heavy metal pollution in China: origin, pattern and control. Environ Sci Pollut Res 10:192–198
Cherifi H, Fatiha B, Salah H (2013) Kinetic studies on the adsorption of methylene blue onto vegetal fiber activated carbons. Appl Surf Sci 282:52–59. doi:10.1016/j.apsusc.2013.05.031
Chowdhury S, Mishra R, Saha P, Kushwaha P (2011) Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination 265:159–168
Demir H, Top A, Balköse D, Ülkü S (2008) Dye adsorption behavior of Luffa cylindrica fibers. J Hazard Mater 153:389–394
Duan C, Zhao N, Yu X, Zhang X, Xu J (2013) Chemically modified kapok fiber for fast adsorption of Pb2+, Cd2+, Cu2+ from aqueous solution. Cellulose 20:849–860. doi:10.1007/s10570-013-9875-9
Gao W, Majumder M, Alemany LB, Narayanan TN, Ibarra MA, Pradhan BK, Ajayan PM (2011) Engineered graphite oxide materials for application in water purification. ACS Appl Mater Interfaces 3:1821–1826. doi:10.1021/am200300u
Gupta VK, Ali I (2008) Removal of endosulfan and methoxychlor from water on carbon slurry. Environ Sci Technol 42:766–770
Gupta V, Mittal A, Gajbe V, Mittal J (2006) Removal and recovery of the hazardous azo dye acid orange 7 through adsorption over waste materials: bottom ash and de-oiled soya. Ind Eng Chem Res 45:1446–1453
Gupta V, Carrott P, Ribeiro Carrott M, Suhas (2009) Low-cost adsorbents: growing approach to wastewater treatment—a review. Crit Rev Environ Sci Technol 39:783–842
Gupta VK, Agarwal S, Singh P, Pathania D (2013) Acrylic acid grafted cellulosic Luffa cylindrical fiber for the removal of dye and metal ions. Carbohydr Polym 98:1214–1221. doi:10.1016/j.carbpol.2013.07.019
Gurgel LVA, Freitas RP, Gil LF (2008a) Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by sugarcane bagasse and mercerized sugarcane bagasse chemically modified with succinic anhydride. Carbohydr Polym 74:922–929
Gurgel LVA, Júnior OK, Gil RPF, Gil LF (2008b) Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by cellulose and mercerized cellulose chemically modified with succinic anhydride. Bioresour Technol 99:3077–3083
Han R, Zhang L, Song C, Zhang M, Zhu H, Zhang L (2010) Characterization of modified wheat straw, kinetic and equilibrium study about copper ion and methylene blue adsorption in batch mode. Carbohydr Polym 79:1140–1149
Hu Z, Chen H, Ji F, Yuan S (2010) Removal of congo red from aqueous solution by cattail root. J Hazard Mater 173:292–297. doi:10.1016/j.jhazmat.2009.08.082
Ji F, Li C, Tang B, Xu J, Lu G, Liu P (2012) Preparation of cellulose acetate/zeolite composite fiber and its adsorption behavior for heavy metal ions in aqueous solution. Chem Eng J 209:325–333. doi:10.1016/j.cej.2012.08.014
Ji F, Li C, Xu J, Liu P (2013) Dynamic adsorption of Cu(II) from aqueous solution by zeolite/cellulose acetate blend fiber in fixed-bed. Colloids Surf A 434:88–94. doi:10.1016/j.colsurfa.2013.05.045
Jiang N, Xu Y, Dai Y, Luo W, Dai L (2012) Polyaniline nanofibers assembled on alginate microsphere for Cu2+ and Pb2+ uptake. J Hazard Mater 215:17–24
Júnior OK, Gurgel LVA, de Freitas RP, Gil LF (2009) Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by mercerized cellulose and mercerized sugarcane bagasse chemically modified with EDTA dianhydride (EDTAD). Carbohydr Polym 77:643–650
Karnitz O Jr, Gurgel LVA, De Melo JCP, Botaro VR, Melo TMS, Gil RPF, Gil LF (2007) Adsorption of heavy metal ion from aqueous single metal solution by chemically modified sugarcane bagasse. Bioresour Technol 98:1291–1297
Kim DH, Kim DW, Kim BH, Yang KS, Lim YK, Park EN (2013) Study of the adsorbent–adsorbate interactions from Cd(II) and Pb(II) adsorption on activated carbon and activated carbon fiber. J Korean Chem Soc 57:104–108. doi:10.5012/jkcs.2013.57.1.104
Kousha M, Daneshvar E, Sohrabi MS, Jokar M, Bhatnagar A (2012) Adsorption of acid orange II dye by raw and chemically modified brown macroalga Stoechospermum marginatum. Chem Eng J 192:67–76. doi:10.1016/j.cej.2012.03.057
Li Q, Zhai J, Zhang W, Wang M, Zhou J (2007) Kinetic studies of adsorption of Pb(II), Cr(III) and Cu(II) from aqueous solution by sawdust and modified peanut husk. J Hazard Mater 141:163–167. doi:10.1016/j.jhazmat.2006.06.109
Liu Y, Sun X, Li B (2010) Adsorption of Hg2+ and Cd2+ by ethylenediamine modified peanut shells. Carbohydr Polym 81:335–339
Liu Y, Wang J, Zheng Y, Wang A (2012) Adsorption of methylene blue by kapok fiber treated by sodium chlorite optimized with response surface methodology. Chem Eng J 184:248–255
Mall ID, Srivastava VC, Agarwal NK, Mishra IM (2005) Removal of congo red from aqueous solution by bagasse fly ash and activated carbon: kinetic study and equilibrium isotherm analyses. Chemosphere 61:492–501. doi:10.1016/j.chemosphere.2005.03.065
Moussavi G, Khosravi R (2011) The removal of cationic dyes from aqueous solutions by adsorption onto pistachio hull waste. Chem Eng Res Des 89:2182–2189. doi:10.1016/j.cherd.2010.11.024
Neghlani PK, Rafizadeh M, Taromi FA (2011) Preparation of aminated-polyacrylonitrile nanofiber membranes for the adsorption of metal ions: comparison with microfibers. J Hazard Mater 186:182–189
Ozdes D, Duran C, Senturk HB (2011) Adsorptive removal of Cd(II) and Pb(II) ions from aqueous solutions by using Turkish illitic clay. J Environ Manag 92:3082–3090
Park S, An J, Piner RD, Jung I, Yang D, Velamakanni A, Ruoff RS (2008) Aqueous suspension and characterization of chemically modified graphene sheets. Chem Mater 20:6592–6594
Park S, An J, Jung I, Piner RD, An SJ, Li X, Ruoff RS (2009) Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents. Nano Lett 9:1593–1597
Purkait MK, Maiti A, DasGupta S, De S (2007) Removal of congo red using activated carbon and its regeneration. J Hazard Mater 145:287–295. doi:10.1016/j.jhazmat.2006.11.021
Qu R, Sun C, Wang M, Ji C, Xu Q, Zhang Y, Yin P (2009) Adsorption of Au(III) from aqueous solution using cotton fiber/chitosan composite adsorbents. Hydrometallurgy 100:65–71. doi:10.1016/j.hydromet.2009.10.008
Reddad Z, Gerente C, Andres Y, Le Cloirec P (2002) Adsorption of several metal ions onto a low-cost biosorbent: kinetic and equilibrium studies. Environ Sci Technol 36:2067–2073
Ren Y, Yan N, Wen Q, Fan Z, Wei T, Zhang M, Ma J (2011) Graphene/δ-MnO2 composite as adsorbent for the removal of nickel ions from wastewater. Chem Eng J 175:1–7. doi:10.1016/j.cej.2010.08.010
Roy A, Chakraborty S, Kundu SP, Adhikari B, Majumder SB (2012) Adsorption of anionic-azo dye from aqueous solution by lignocellulose-biomass jute fiber: equilibrium, kinetics, and thermodynamics study. Ind Eng Chem Res 51:12095–12106. doi:10.1021/ie301708e
Roy A, Adhikari B, Majumder SB (2013) Equilibrium, kinetic, and thermodynamic studies of azo dye adsorption from aqueous solution by chemically modified lignocellulosic jute fiber. Ind Eng Chem Res 52:6502–6512. doi:10.1021/ie400236s
Saad S, Isa KM, Bahari R (2010) Chemically modified sugarcane bagasse as a potentially low-cost biosorbent for dye removal. Desalination 264:123–128
Sajab MS, Chia CH, Zakaria S, Jani SM, Ayob MK, Chee KL, Chiu WS (2011) Citric acid modified kenaf core fibres for removal of methylene blue from aqueous solution. Bioresour Technol 102:7237–7243
Senthil Kumar P, Ramalingam S, Senthamarai C, Niranjanaa M, Vijayalakshmi P, Sivanesan S (2010) Adsorption of dye from aqueous solution by cashew nut shell: Studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Desalination 261:52–60. doi:10.1016/j.desal.2010.05.032
Shi W, Tao S, Yu Y, Wang Y, Ma W (2011) High performance adsorbents based on hierarchically porous silica for purifying multicomponent wastewater. J Mater Chem 21:15567–15574
Shrestha S, Son G, Lee SH, Lee TG (2013) Isotherm and thermodynamic studies of Zn(II) adsorption on lignite and coconut shell-based activated carbon fiber. Chemosphere 92:1053–1061. doi:10.1016/j.chemosphere.2013.02.068
Siddiqi Z, Pathania D (2002) Studies on heavy metals in surface and ground water of Jalandhar and Ludhiana districts. Indian J Environ Prot 22:201–206
Sui K, Li Y, Liu R, Zhang Y, Zhao X, Liang H, Xia Y (2012) Biocomposite fiber of calcium alginate/multi-walled carbon nanotubes with enhanced adsorption properties for ionic dyes. Carbohydr Polym 90:399–406. doi:10.1016/j.carbpol.2012.05.057
Tang H, Zhou W, Zhang L (2012) Adsorption isotherms and kinetics studies of malachite green on chitin hydrogels. J Hazard Mater 209–210:218–225. doi:10.1016/j.jhazmat.2012.01.010
Tian Y, Wu M, Liu R, Wang D, Lin X, Liu W, Huang Y (2011) Modified native cellulose fibers–a novel efficient adsorbent for both fluoride and arsenic. J Hazard Mater 185:93–100. doi:10.1016/j.jhazmat.2010.09.001
Vieira AP, Santana SA, Bezerra CW, Silva HA, Chaves JA, de Melo JC, Airoldi C (2009) Kinetics and thermodynamics of textile dye adsorption from aqueous solutions using babassu coconut mesocarp. J Hazard Mater 166:1272–1278. doi:10.1016/j.jhazmat.2008.12.043
Wang S, Zhu Z (2007) Effects of acidic treatment of activated carbons on dye adsorption. Dyes Pigment 75:306–314
Wang Y, Shao Y, Matson DW, Li J, Lin Y (2010) Nitrogen-doped graphene and its application in electrochemical biosensing. ACS Nano 4:1790–1798
Waranusantigul P, Pokethitiyook P, Kruatrachue M, Upatham ES (2003) Kinetics of basic dye (methylene blue) biosorption by giant duckweed (Spirodela polyrrhiza). Environ Pollut 125:385–392. doi:10.1016/s0269-7491(03)00107-6
Xia Y, Li T, Chen J, Cai C (2013) Polyaniline (skin)/polyamide 6 (core) composite fiber: preparation, characterization and application as a dye adsorbent. Synth Met 175:163–169. doi:10.1016/j.synthmet.2013.05.012
Xu S-M, Feng S, Peng G, Wang J-D, Yushan A (2005) Removal of Pb(II) by crosslinked amphoteric starch containing the carboxymethyl group. Carbohydr Polym 60:301–305
Yin QF, Ju BZ, Zhang SF, Wang XB, Yang JZ (2008) Preparation and characteristics of novel dialdehyde aminothiazole starch and its adsorption properties for Cu(II) ions from aqueous solution. Carbohydr Polym 72:326–333
Zhang M (2011) Adsorption study of Pb(II), Cu(II) and Zn(II) from simulated acid mine drainage using dairy manure compost. Chem Eng J 172:361–368. doi:10.1016/j.cej.2011.06.017
Zhang W, Zhou C, Zhou W, Lei A, Zhang Q, Wan Q, Zou B (2011) Fast and considerable adsorption of methylene blue dye onto graphene oxide. Bull Environ Contam Toxicol 87:86–90. doi:10.1007/s00128-011-0304-1
Zhang W, Yang H, Dong L, Yan H, Li H, Jiang Z, Cheng R (2012) Efficient removal of both cationic and anionic dyes from aqueous solutions using a novel amphoteric straw-based adsorbent. Carbohydr Polym 90:887–893
Zhao J, Pei S, Ren W, Gao L, Cheng H-M (2010a) Efficient preparation of large-area graphene oxide sheets for transparent conductive films. ACS Nano 4:5245–5252
Zhao P, Jiang J, Zhang F-W, Zhao W-F, Liu J-T, Li R (2010b) Adsorption separation of Ni(II) ions by dialdehyde o-phenylenediamine starch from aqueous solution. Carbohydr Polym 81:751–757
Zhao G, Li J, Ren X, Chen C, Wang X (2011) Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ Sci Technol 45:10454–10462. doi:10.1021/es203439v
Zheng Y, Wang W, Huang D, Wang A (2012) Kapok fiber oriented-polyaniline nanofibers for efficient Cr(VI) removal. Chem Eng J 191:154–161
Acknowledgments
This work was supported by the National High Technology Research and Development Program of China (no. 2012AA030313), Natural Science Foundation of Jiangsu Higher Education Institutions of China (no. 11KJB540002) and Suzhou City Key Technology R&D program (no. ZXS2012008).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xiong, J., Jiao, C., Li, C. et al. A versatile amphiprotic cotton fiber for the removal of dyes and metal ions. Cellulose 21, 3073–3087 (2014). https://doi.org/10.1007/s10570-014-0318-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-014-0318-z