Abstract
In this present work, a series of hydrolyzed polyacrylamide grafted carboxymethyl cellulose (CMC-g-HPAM) was prepared. The structure and solution properties of CMC-g-HPAM were characterized by FTIR, 1H-NMR, elemental analysis and zeta potential measurements. The graft copolymers were applied as flocculants to remove methylene blue (MB), a cationic dye, from aqueous solutions. In comparison with its precursors, carboxymethyl cellulose (CMC) and polyacrylamide CMC-g-PAM, CMC-g-HPAM exhibited higher removal efficiencies. Furthermore, the flocculation performance of the copolymers was significantly improved with the increase of the hydrolysis degree, and the MB removal efficiency was more than 90 % when the hydrolysis degree of CMC-g-HPAM was higher than 80 %. More importantly, image analysis in combination with fractal theory demonstrated that the graft copolymers could produce notably denser and larger flocs, which was of great significance in practical water treatment. The improved flocculation performance was ascribed to both charge neutralization and bridging effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapid development of modern industrial sections, such as dyeing, printing, textile, leather, and coating industries, the environmental pollution is becoming more and more serious, which has been drawn much attention. Dyes are the main pollutants in the sewage from the aforementioned industries (Crini 2006; Zahrim et al. 2011). It is estimated that more than 70,000 tons of dyes are discharged in the world every year. Dyes exert serious influences on the environment in two main aspects. On the one hand, they are highly visible and interfere with light penetration in the receiving water bodies thereby disturbing the biological processes. Many dyes degrade into compounds that are toxic and hazardous to mammals and aquatic organisms. On the other hand, most dyes are organic compounds of which the excess would increase the chemical oxygen demand (COD) of the water body (Beltran-Heredia et al. 2009; Zahrim et al. 2011). Among all these dyes, methylene blue (MB) is one of the most commonly used substances for dyeing cotton, wood and silk. MB can cause eye burn which is very harmful to human and animals. Even worse, MB can also increase heart rate, vomiting, shock, Heinz body formation, cyanosis, jaundice, and tissue necrosis in humans (Tan et al. 2008; Vadivelan and Kumar 2005). Thus, removal of dyes from wastewater becomes a significant issue, and environmental regulations in many countries have made it mandatory before discharging wastewaters (Crini 2006; Mishra et al. 2006).
In recent years, various combined techniques, including flocculation, adsorption, filtration, oxidation, and electrolysis, have been developed to remove dyes from wastewaters (Beltran-Heredia et al. 2011; Dukkanci et al. 2010; Fang et al. 2010; Rafatullah et al. 2010; Sabah and Cengiz 2004). Therein, flocculation is one of the most widely applied methods in primary purification due to its low cost and easy operation (Bratby 2006; Hogg 2000). The choice of flocculants during a flocculation process is significant to the final effect. Until now, inorganic metal-based flocculants and synthetic polymeric ones are two of the most extensively applied flocculants. However, the increased concentration of metal ions of inorganic flocculants or residual noxious monomers in synthetic ones when these chemicals are used may have potential disadvantages to both ecosystem and human health (Bratby 2006; Hogg 2000; Li et al. 2005).
Nowadays, great attention has been paid to natural polymer based flocculants, since they are considered as wide-resourced, nontoxic, environment-friendly and high-efficient materials. They have been even acclaimed as “Green Flocculants of twenty-first Century” (Xiao and Zhou 2005). Among them, cellulose is one of the most important natural polymers, and the first most abundant one in the world (Gao and Tang 1996). In term of its novel characteristics, cellulose and its derivatives have a very wide application in many fields such as pharmaceutical, food, paper, and building material industries (Gao and Tang 1996; Klemm et al. 2005). In addition, cellulose also has good water purification effects because it has abundant free –OH groups on the chain, enabling efficient removal of metal ions and organic matters from water for excellent chelating effect. However, owning to poor water solubility and relatively low chemical reactivity, application of cellulose as a flocculant is always limited. To overcome those shortcomings, modified cellulose materials have been manufactured, and carboxymethylation is a conventional and useful method for chemical modification. Among them, carboxymethyl cellulose (CMC) is the simplest one. After introducing the carboxyl group, not only the solubility of cellulose has been greatly improved for destroying the original ordered structure, but also the negative charges on CMC can enhance the electrostatic attraction with some cationic matters such as MB. Additionally, grafting copolymerization is also an efficient way for chemical modification. It is well known that polyacrylamide (PAM) is widely used in flocculation (Lee et al. 2011) and show high flocculation efficiency. PAM can produce dense flocs for bridging flocculation effect, which are usually favored in water treatment. Thus, grafting PAM onto CMC is more promising, since the tailored graft copolymers have combined the advantages of both (Sanghi et al. 2006; Yang et al. 2012).
Moreover, it has been widely accepted that floc properties also count much in the flocculation process in addition to impurity removal efficiency. Dense flocs are preferred in order to produce lower sludge volumes and reduce the disposal and handling cost. Previous work (Mandelbrot 1983; Yang et al. 2012) has proven that the irregular flocs can be treated as geometrically fractal objects. Consequently, fractal dimension, a powerful parameter in fractal theory, can be used to describe the compactness of flocs. Larger fractal dimension indicates denser flocs, and is favored in flocculation. According to the space dimension, fractal dimensions can be divided into several forms. One commonly applied fractal dimension is two-dimensional one (D 2) (Chakraborti et al. 2000; Yang et al. 2012), defined by the power law relationship between the projected area (A) and the characteristic length (l), which can be measured by image analysis (IA) (Chakraborti et al. 2000; Mandelbrot 1983; Yang et al. 2012).
In the current work, PAM was grafted onto CMC with ammonium persulfate as the initiator. In order to further improve the flocculation performance for removal of MB, a cationic dye, the graft copolymers were hydrolyzed under an alkaline condition (Song et al. 2011). By controlling the hydrolysis time, a series of hydrolyzed polyacrylamide grafted carboxymethyl cellulose (CMC-g-HPAM) with different degree of hydrolysis (DH) was obtained. The structure and solution properties of the final copolymers were characterized by Fourier transform infrared (FTIR), 1H nuclear magnetic resonance (1H NMR) spectra, zeta potential (ZP) and elemental analysis measurements. The effect of hydrolysis degree on the MB removal efficiency was systematically evaluated. Besides, the properties of flocs were especially investigated in detail combined with the fractal theory (Liao et al. 2006; Moghaddam et al. 2010).
Experimental
Materials
Carboxymethyl cellulose (CMC) was purchased from Sinopharm Chemical Reagent Co., Ltd. Its viscosity with the concentration of 20 g L−1 is 800–1,200 MPa·s at 25 °C and its weight-average molecular weight is 150,000 g mol−1. The substitution degree of carboxyl group is approximately 68.69 %. Acrylamide (AM) (Nanjing Chemical Reagent Co., Ltd.), ammonium persulfate (Shanghai Lingfeng Chemical Reagent Co., Ltd.), MB (Tianjin Chemical Reagent research institute) were used without further purification. All other chemicals were purchased from Nanjing Chemical reagent Co. Ltd. Distilled water was used in all experiments.
Preparation of CMC-g-HPAM
A desired amount of solid CMC was dissolved in 100 mL distilled water. After 30 min of stirring under N2, a certain amount of ammonium persulfate was added as the initiator. Before the dropwise addition of the acrylamide monomer aqueous solution, the CMC solution was kept for 5 min for pretreatment by the initiator to suppress the formation of the PAM homopolymer. The mass ratio between CMC and AM is 1:5. The reaction was carried out for 3 h under N2. Then, the mixture was precipitated in acetone. The solid product was filtered and washed by ethanol, then extracted using acetone as the solvent in a Soxhlet apparatus for 48 h for removal of impurities (Ali and Singh 2009; da Silva et al. 2007) and finally vacuum dried at 60 °C. The obtained product was CMC-g-PAM, which had a PAM grafting ratio of 323 %, calculated by weighting method as shown below.
where w o and w g are the weight of raw CMC and CMC-g-PAM, respectively.Then, a specific amount of CMC-g-PAM was added into 100 mL mixed solution containing ethanol and 3 mol L−1 sodium hydroxide aqueous solution (Vethanol: VNaOH = 85:15). The hydrolysis reaction was carried out at 55 °C. After finishing the reaction, the mixture was filtered. Then, the solid product was washed by a mixed solution of ethanol and water (Vethanol: Vwater = 85:15) and finally vacuum dried at 60 °C. The detailed synthesis process of CMC-g-HPAM has been described in Scheme 1.
For preparation of various CMC-g-HPAM samples with different hydrolysis degree, various hydrolysis time for each reaction has been carried out, which was 10, 30 min, 1, 1.5, 3, 4, and 6 h, respectively. The final corresponding products were named CMC-g-HPAM1, CMC-g-HPAM2, CMC-g-HPAM3, CMC-g-HPAM4, CMC-g-HPAM5, CMC-g-HPAM6, and CMC-g-HPAM7, respectively. The hydrolysis degree of various CMC-g-HPAM samples was calculated according to Eq. (2) as shown below based on elemental analysis method.
where ωCMC-g-HPAM and ωCMC-g-PAM are the N/C ratio in CMC-g-HPAM and CMC-g-PAM, respectively, which were obtained from an elemental analyzer model of Elementar vario ELIII. The calculated DH of various samples including other details were all listed in Table 1.
Characterization
Fourier transform infrared (FTIR) spectra of various samples were recorded on a Bruker Model IFS 66/S FTIR spectrometer. The interval of measured wave numbers was 600–3,800 cm−1.
1H NMR spectra of different samples were carried out using a Bruker AVANCE Model DRX-500 spectrometer, operating at 500 MHz with D2O as solvent.
ZP of various sample solutions was measured using a Malvern Model Nano-Z Zetasizer. Here, dilute hydrochloric acid and sodium hydroxide aqueous solutions were employed to adjust the pH of various solutions.
Flocculation experiment
A MB aqueous solution with concentration of 100 mg L−1 was employed as synthetic wastewater. After addition of a desired amount of flocculants as solid powder into 100 mL synthetic wastewater, the mixture was fast stirred at 200 rpm for 3 min, followed by a slowly stirring at 50 rpm for 10 min, and finally left to settle for 2 h. The supernatants were collected using a syringe and filtrated through a membrane with 0.45 μm pore diameter. An UV–visible spectrophotometry (Model UV-2401, Shimadzu Co.) was used to measure the concentration of residual dye in water. A calibration curve of MB was prepared in advance. The absorbance was measured at the wavelength of 662 nm. The colour removal efficiency was calculated as follows:
where C 0 and C were the dye concentrations in the solution before and after flocculation, respectively (Yang et al. 2011).
Floc properties were measured by IA according to our previous work (Yang et al. 2012). After flocculation, the flocs were carefully withdrawn from the beakers and transferred into a glass dish. A Leica Model DMLP microscope equipped with a digital camera (Victor Company of Japan, Ltd.) was used to take photos. Then, A and l were derived from the photos using Image-pro® Plus 6.0. In this work, l was defined as the largest projection length and employed to evaluate the floc size. D 2 was obtained from the slope of the log–log plot of A and l (Chakraborti et al. 2000; Liao et al. 2006; Lin et al. 2008; Mandelbrot 1983).
where A is the projected area of flocs. For guaranteeing the reliability of the calculated D 2, more than 50 data points have been collected randomly in each plot.
Result and discussion
Characterization of the CMC-g-HPAM
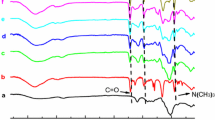
The detailed preparation process of CMC-g-HPAM was described in experimental part and summarized in Scheme 1. The FTIR spectra of CMC, homopolymer PAM, CMC-g-PAM and various CMC-g-HPAM samples were illustrated in Fig. 1. From Fig. 1a, the FTIR spectrum of CMC showed characteristic peaks of C = O around 1,590 cm−1 and hydroxyl groups on the glycosidic ring appeared around 3,300 cm−1. As for the FTIR spectrum of CMC-g-PAM as shown in Fig. 1c, the overlapped peaks around 1,660 and 1,610 cm−1 were assigned to the amide I and II bands, respectively, and a shoulder peak appeared at 3,190 cm−1 was ascribed to N–H stretching vibration, which were both well consistent with those of PAM as shown in Fig. 1b. In addition, since the prepared samples were washed and extracted by acetone in the Soxhlet apparatus thoroughly, and the homopolymer of PAM, initiator, monomer and other unwanted residues have been successfully removed (Ali and Singh 2009; da Silva et al. 2007) the detected PAM chain should be chemically grafted on the backbone of cellulose chain. Furthermore, in comparison with the FTIR spectrum of CMC-g-PAM, those of various hydrolyzed CMC-g-PAM, CMC-g-HPAM, with different hydrolysis time in Fig. 1d–f, it was found that the intensity of the characteristic peak at 1,660 cm−1 due to C = O of amide group decreased whereas that at 1,560 cm−1 corresponding to C = O on carboxyl group increased with the increase of hydrolysis time. It was due to the fact that amide groups in graft PAM were consumed gradually and more carboxyl groups produced, which confirmed that the hydrolysis reaction occurred. Furthermore, the hydrolysis degree of various CMC-g-HPAM samples was calculated according to Eq. (2) based on elemental analysis method and listed in Table 1. DH of hydrolyzed samples increased with increasing hydrolysis time and reached a plateau at approximately 3 h.
In addition, the 1H NMR spectra of various samples as shown in Fig. 2 provided further evidences of the structure changes. The overlapped signals between 3.10 to 4.30 ppm in Fig. 2a were assigned to the protons H1–H6 on the glycosidic ring of carboxymethyl cellulose. The new intense peaks at around 1.58 and 2.14 ppm in Fig. 2b–f were corresponding to the characteristic signals of methylene (Hb) and methine (Ha) protons in the PAM chains (Yang et al. 2012) respectively. Moreover, the signals of CMC-g-HPAM as shown in Fig. 2d–f turned broader than those of CMC-g-PAM in Fig. 2c. It may be due to the fast spin–spin relaxation time (T 2) resulting from the fact that hydrolysis reaction has destroyed the regular structure of samples and chain segments are more mobile.
As for the detailed grafted point of hydrolyzed polyacrylamide in the final products, based on previous reported works (Guo et al. 2013; Wang et al. 2013), the grafted point of hydrolyzed polyacrylamide may be C2 or C3 on glucopiranose ring. Furthermore, since ammonium persulfate was applied as initiator here, the glucopiranose ring would not be broken during the grafting reaction. In addition, the C2–OH was on the axial bond while the C3–OH was on the equatorial bond, therefore C2 was more active than C3. So it could be inferred roughly that the grafting reaction has more probability to take place on C2. However, further experiments are very necessary for confirming the fine structure of the cellulose based flocculants.
In addition, it was well known that charge properties of ionic flocculants would affect the flocculation efficiency greatly (Wang et al. 2009). Hence, the pH dependences of ZP of various samples solutions were measured at the beginning and demonstrated in Fig. 3.
Based on Fig. 3, it was found that ZP of CMC was less negative than that of each CMC-g-HPAM but more than that of CMC-g-PAM. It was ascribed to the fact that more carboxyl groups were produced in the final products with the hydrolysis process, resulting in the decease of ZP. Furthermore, the ZPs of different CMC-g-HPAM samples decreased further when increasing the reaction time and hydrolysis degree. As for CMC-g-PAM, the non-ionic PAM branch chains would screen the charges on the CMC backbone and reduce the surface charge (Song et al. 2009). It was interesting to observe from Fig. 3 that, in the pH range from 8.0 to 13.0, ZP values of CMC-g-HPAM increased with the increase of pH. The reason for this trend could be explained as follows: Before pH at 8.0, more –COO− groups were formed and thus the zeta potential decreased as the pH increased; then, at pH around 8.0 or 9.0, nearly all the carboxyl groups were deprotonized; after that, with pH increase further which need more NaOH, the ionic strength in solution increased greatly. Since the number of –COO− has already reached saturation, the increased ionic strength would compress electrical double layer of flocculants much more and the flocculants chains were surrounded with a growing number of sodium ions. As a result, zeta potential of flocculants increased and became close to zero.
Flocculation performance of CMC-g-HPAM
pH Effects on the optimal dosage and colour removal efficiency
Then, CMC-g-HPAM was employed as flocculants for removal of a cationic dye, MB, from aqueous solutions. Among numbers of external parameters which can affect the flocculation efficiency of ionic flocculants, pH was usually one of the most important ones (Sand et al. 2010). Thus, pH effects on the optimal dosage and colour removal efficiency of CMC-g-HPAM for flocculation of MB were studied and the results were summarized in Fig. 4. Here, dilute hydrochloric acid and sodium hydroxide aqueous solutions were employed to adjust the pH of various dye solutions.
As revealed in Fig. 4, under acidic condition, the required optimal dosages were relatively high but the corresponding colour removal efficiencies were relatively low. However, under alkaline condition, the situations were fully reversed and the flocculation performance of CMC-g-HPAM turned better with lower dosage required yet higher removal efficiencies. As was known, MB is a typical cationic dye and bear positive charge in aqueous solution. As for CMC-g-HPAM, the negative charges of flocculants were relatively weak at pH lower than 7.0 based on their ZP profiles as shown in Fig. 3, since most of carboxyl groups on the CMC-g-HPAM were protonized. As a consequence, the electrostatic attractions between flocculants and positively charged dye were not strong. Nevertheless, under alkaline condition, the carboxyl groups of flocculants were deprotonized and formed –COO− groups, which is in favor of flocculation of MB by neutralizing effect.
Besides, it was found from Fig. 4 that the optimal pH for the removal of MB was pH at 9.0 instead of the highest one (pH = 11.0) in tested pH range. This phenomenon could be ascribed to two factors. On the one hand, the increased zeta potential of the flocculants as illustrated in Fig. 3 engendered weaker electrostatic attraction between the flocculants and positively charged dye. On the other hand, large numbers of hydroxyl groups (OH−) at high pH surrounded the MB molecules tightly through charge attraction, which also prevented the attraction between CMC-g-HPAM and dye molecules and reduced the colour removal efficiency (Yang et al. 2013). Since the flocculant had the highest colour removal efficiency and the lowest dosage at pH 9.0, this optimal pH level was selected in further flocculation experiments.
Effect of the DH of the flocculants
Then, the flocculation performance of CMC, CMC-g-PAM and various CMC-g-HPAM samples for removal of MB was investigated at pH 9.0, and the results were all presented in Fig. 5. The detailed optimal dosages of various flocculants and their corresponding colour removal efficiency based on Fig. 5 were listed in Table 1.
From Fig. 5 and Table 1, the colour removal efficiency of CMC-g-PAM has been improved in a minor way but the optimal dosage was slightly increased in comparison with those of CMC. They were resultant from two opposite effects. On the one hand, the flexible PAM chains on the comb-like copolymer of CMC-g-PAM enhanced the probability of netting and seizing more MB molecules around from aqueous solutions, which engendered better flocculation performance. On the other hand, the surface charge on the flocculants has been partially screened after PAM chains grafting onto CMC backbone according to its ZP profile as shown in Fig. 3, which reduced the charge neutralization flocculation effects. Therefore, more flocculants were required.
Then, it was also found from Fig. 5 and Table 1 that various CMC-g-HPAM samples all showed better flocculation performance for removal of dye from aqueous solutions than their two precursors, CMC and CMC-g-PAM. Most of CMC-g-HPAM had higher colour removal efficiency but lower optimal dosage. It was ascribed to more carboxyl groups and active flocculation sites on the flocculants, which also further confirmed that charge neutralization effect was the main flocculation mechanism in this case. However, it was interesting that the colour removal efficiency of each CMC-g-HPAM as shown in Fig. 5 all increased with increasing dosage and then reached a plateau at its optimal dosage, but did not decreased with the increase of dosage further. As was known, the colour removal efficiency-dosage profiles always showed an up-climax-down trend, when the flocculation process was controlled only by charge neutralization mechanism (Ghimici et al. 2010; Ghimici and Nichifor 2010; Yang et al. 2011). The up-plateau trend and wider flocculation window of CMC-g-HPAM in current system as shown in Fig. 5 revealed that bridging flocculation effect had also much contribution to the dye removal besides charge neutralization effect, since numerous grafted HPAM chains were on the CMC backbone.
Furthermore, the flocculation performance of CMC-g-HPAM has been improved significantly with increasing hydrolysis time and hydrolysis degree based on Fig. 5 and Table 1. According to Fig. 5, the dependence of the hydrolysis degree on the colour removal efficiency after reaching flocculation equilibrium has been summarized in Fig. 6. It was obvious that the colour removal efficiency increased linearly with hydrolysis degree increased. When the hydrolysis degree of CMC-g-HPAM was higher than 80 %, the colour removal efficiency was larger than 90 %. The enhanced flocculation performance was ascribed to the hydrolysis effect and production of more carboxyl groups and active flocculation sites on the graft chain. On the one hand, the carboxyl groups were mainly deprotonized and existed as carboxyl anions at pH 9.0, and the increased negative charges on the flocculants made the electrostatic attraction forces between CMC-g-HPAM and MB stronger. As a result, charge neutralization flocculation effect was distinctly improved. On the other hand, the side HPAM chains with increased negative charge would result in a more extended morphology due to the intra-molecular electrostatic repulsion, which further enlarged the probability of getting close to free MB in wastewater. Therefore, the bridging flocculation effect has been also improved. In terms of the cooperative effects of the aforementioned two flocculation mechanisms (Rasteiro et al. 2008), better flocculation performance of CMC-g-HPAM was obtained.
The effect of hydrolysis degree on the colour removal efficiency after reaching flocculation equilibrium based on Fig. 5
In addition, the flocculation performance of CMC-g-HPAM would be beneficial to higher hydrolysis degree. It was also found that the hydrolysis degree would reach a plateau at hydrolysis time around 3 h according to Table 1. Therefore, the hydrolysis process should be controlled within 3 h for better flocculation performance and saving energy.
Floc properties
Besides flocculation efficiency, floc properties are also very important in real water treatment, and larger and denser flocs are usually preferred for reducing the cost of disposal of and handling the sludge, as mentioned above. Hence, the flocs properties, including floc size (l) and D 2, under each optimal condition were also measured through IA method, which were listed in Table 1 too. In this work, l was defined as the largest floc length and employed to evaluate the floc size. D 2 was obtained from the slope of the log–log plot of A and l (Chakraborti et al. 2000; Liao et al. 2006; Lin et al. 2008; Mandelbrot 1983). Although the values of the determination coefficient R 2 were not quite close to 1.0 based on Table 1, D 2 were still very reliable. Because more than 50 data points have been collected randomly in every plot.
From Table 1, the flocs size turned much larger after PAM chains grafted, and the average l of flocs by CMC-g-PAM was near three times of that by CMC. Furthermore, the hydrolyzed graft copolymer flocculants would produce even larger flocs than CMC-g-PAM, and the size of flocs increased with increasing hydrolysis degree. The larger size of flocs produced by CMC-g-HPAM was due to the enhanced bridging flocculation effect resulting from more extended morphology of flocculants for the intra-molecular electrostatic repulsion of the charged hydrolyzed PAM graft chain.
In addition to flocs size, the flocs by CMC-g-HPAM with higher DH also had denser structure with larger D 2. When the hydrolysis degree was higher than 80 %, D 2 of the flocs by CMC-g-HPAM was more than 1.9 and very near to 2.0, the largest value in theory, which indicated higher strength of flocs and stronger charge attraction between flocculants and dyes. However, CMC could also produce flocs with higher D 2, which may be due to higher charge density on the backbone of CMC or certain efficient attraction way with MB.
Anyway, the flocs with larger size and compacted structure produced by CMC-g-HPAM would be beneficial for rapid separation and low handling cost, which further indicated that CMC-g-HPAM was a kind of efficient flocculants.
Conclusion
In this work, a kind of novel flocculants, CMC-g-HPAM, has been prepared successfully by hydrolysis of PAM grafted carboxymethyl cellulose under strong alkaline condition. In comparison with its precursors, CMC and CMC-g-PAM, CMC-g-HPAM exhibited much better flocculation performance for removal of MB, a cationic dye, from aqueous solutions, i.e., remarkably higher colour removal efficiency and considerably larger and denser flocs. Investigation of pH effect indicated that higher dye removal efficiency would take place at alkaline conditions. Furthermore, the flocculation performance of CMC-g-HPAM has been improved significantly with increasing hydrolysis time and hydrolysis degree. Based on experimental results, hydrolysis time was better controlled in 3 h. The final improved flocculation performance of CMC-g-HPAM was ascribed to the cooperative effects of the charge neutralization and bridging flocculation mechanisms. Above all, CMC-g-HPAM could be regarded as a kind of cost-efficient flocculants in future water treatment work.
References
Ali SKA, Singh RP (2009) An investigation of the flocculation characteristics of polyacrylamide-grafted chitosan. J Appl Polym Sci 114:2410–2414
Beltran-Heredia J, Sanchez-Martin J, Delgado-Regalado A et al (2009) Removal of Alizarin Violet 3R (anthraquinonic dye) from aqueous solutions by natural coagulants. J Hazard Mater 170:43–50
Beltran-Heredia J, Sanchez-Martin J, Davila-Acedo MA (2011) Optimization of the synthesis of a new coagulant from a tannin extract. J Hazard Mater 186:1704–1712
Bratby J (2006) Coagulation and flocculation in water and wastewater treatment, 2nd edn. IWA Publishing, London
Chakraborti RK, Atkinson JF, Van Benschoten JE (2000) Characterization of alum floc by image analysis. Environ Sci Technol 34:3969–3976
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. Bioresour Technol 97:1061–1085
da Silva DA, de Paula RCM, Feitosa JPA (2007) Graft copolymerisation of acrylamide onto cashew gum. Eur Polym J 43:2620–2629
Dukkanci M, Gunduz G, Yilmaz S et al (2010) Heterogeneous fenton-like degradation of Rhodamine 6G in water using CuFeZSM-5 zeolite catalyst prepared by hydrothermal synthesis. J Hazard Mater 181:343–350
Fang R, Cheng XS, Xu XR (2010) Synthesis of lignin-base cationic flocculant and its application in removing anionic azo-dyes from simulated wastewater. Bioresour Technol 101:7323–7329
Gao J, Tang L (1996) Cellulose science. Science Press, Beijing
Ghimici L, Nichifor M (2010) Novel biodegradable flocculanting agents based on cationic amphiphilic polysaccharides. Bioresour Technol 101:8549–8554
Ghimici L, Constantin M, Fundueanu G (2010) Novel biodegradable flocculanting agents based on pullulan. J Hazard Mater 181:351–358
Guo YZ, Liu QL, Chen H et al (2013) Direct grafting modification of pulp in ionic liquids and self-assembly behavior of the graft copolymers. Cellulose 20:873–884
Hogg R (2000) Flocculation and dewatering. Int J Miner Process 58:223–236
Klemm D, Heublein B, Fink HP et al (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed 44:3358–3393
Lee KE, Teng TT, Morad N et al (2011) Flocculation activity of novel ferric chloride-polyacrylamide (FeCl(3)-PAM) hybrid polymer. Desalination 266:108–113
Li FT, Zhang SF, Zhao Y (2005) Coagulants and flocculants. Chemical Industry Press, Beijing
Liao JYH, Selomulya C, Bushell G et al (2006) On different approaches to estimate the mass fractal dimension of coal aggregates. Part Part Syst Charact 22:299–309
Lin JL, Huang CP, Chin CJM et al (2008) Coagulation dynamics of fractal flocs induced by enmeshment and electrostatic patch mechanisms. Water Res 42:4457–4466
Mandelbrot BB (1983) The fractal geometry of nature. W.H.Freeman, New York
Mishra A, Bajpai M, Pandey S (2006) Removal of dyes by biodegradable flocculants: a lab scale investigation. Sep Sci Technol 41:583–593
Moghaddam SS, Moghaddam MRA, Arami M (2010) Coagulation/flocculation process for dye removal using sludge from water treatment plant: optimization through response surface methodology. J Hazard Mater 175:651–657
Rafatullah M, Sulaiman O, Hashim R et al (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177:70–80
Rasteiro MG, Garcia FAP, Ferreira P et al (2008) The use of LDS as a tool to evaluate flocculation mechanisms. Chem Eng Process 47:1329–1338
Sabah E, Cengiz I (2004) An evaluation procedure for flocculation of coal preparation plant tailings. Water Res 38:1542–1549
Sand A, Yadav M, Behari K (2010) Preparation and characterization of modified sodium carboxymethyl cellulose via free radical graft copolymerization of vinyl sulfonic acid in aqueous media. Carbohydr Polym 81:97–103
Sanghi R, Bhattacharya B, Singh V (2006) Use of Cassia javahikai seed gum and gum-g-polyacrylamide as coagulant aid for the decolorization of textile dye solutions. Bioresour Technol 97:1259–1264
Song H, Wu D, Zhang RQ et al (2009) Synthesis and application of amphoteric starch graft polymer. Carbohydr Polym 78:253–257
Song Y, Gan W, Li Q et al (2011) Alkaline hydrolysis and flocculation properties of acrylamide-modified cellulose polyelectrolytes. Carbohydr Polym 86:171–176
Tan IAW, Ahmad AL, Hameed BH (2008) Adsorption of basic dye on high-surface area activated carbon prepared from coconut husk: equilibrium, kinetic and thermodynamic studies. J Hazard Mater 154:337–346
Vadivelan V, Kumar KV (2005) Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J Colloid Interface Sci 286:90–100
Wang JP, Chen YZ, Yuan SJ et al (2009) Synthesis and characterization of a novel cationic chitosan-based flocculant with a high water-solubility for pulp mill wastewater treatment. Water Res 43:5267–5275
Wang JP, Yuan SJ, Wang Y et al (2013) Synthesis, characterization and application of a novel starch based flocculant with high flocculation and dewatering properties. Water Res 47:2643–2648
Xiao J, Zhou Q (2005) Natural polymer flocculants. Chemical Industry Press, Beijing
Yang Z, Shang Y, Lu Y et al (2011) The flocculation properties of biodegradable amphoteric chitosan based flocculants. Chem Eng J 172:287–295
Yang Z, Yuan B, Huang X et al (2012) Evaluation of the flocculation performance of carboxymethyl chitosan-graft-polyacrylamide, a novel amphoteric chemically bonded composite flocculant. Water Res 46:107–114
Yang Z, Yang H, Jiang Z et al (2013) Flocculation of both anionic and cationic dyes in aqueous solutions by the amphoteric grafting flocculant carboxymethyl chitosan-graft-polyacrylamide. J Hazard Mater 254–255:36–45
Zahrim AY, Tizaoui C, Hilal N (2011) Coagulation with polymers for nanofiltration pre-treatment of highly concentrated dyes: a review. Desalination 266:1–16
Acknowledgments
This work was supported by the Natural Science Foundation of China (Grant No. 51073077), and the Open Fund from State Key Laboratory of Pollution Control and Resource Reuse of Nanjing University (Grant No. PCRRF11004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, T., Yang, Z., Li, H. et al. Effect of hydrolysis degree of hydrolyzed polyacrylamide grafted carboxymethyl cellulose on dye removal efficiency. Cellulose 20, 2605–2614 (2013). https://doi.org/10.1007/s10570-013-9987-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-013-9987-2