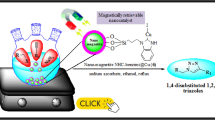
Abstract
Copper(I)–caffeine complex immobilized on silica-coated magnetite nanoparticles was successfully synthesized and fully characterized by analyzing FT-IR, X-ray diffraction, scanning electron microscopy, energy-dispersive X-ray, inductively coupled plasma, thermal gravimetric analysis and vibrating sample magnetometer data. The catalytic activity of these magnetically retrievable nanoparticles was evaluated for green synthesis of 1,2,3-triazoles through the three-component reaction of various terminal alkynes with in situ generated organic azides from organic halides and epoxides in an aqueous medium that was observed to proceed well and products were obtained in good yields. In addition to showing outstanding catalytic activity, the magnetic catalyst is easy to synthesize and can be recycled at least five times with little loss in activity.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The concept of “click chemistry” was originally postulated by Sharpless and colleagues [1]. Since then, a variety of transition metal catalysts (Cu, Ru, Ag, Au, Ir, Ni, Zn, Ln) have been used for “click chemistry”, although the copper(I)-catalyzed 1,3-dipolar azide–alkyne cycloaddition (CuAAC) reaction is still the most popular “click” reaction to produce 1,4-disubstituted 1,2,3-triazoles regioselectively [2,3,4]. 1,2,3-Triazoles are vital structural scaffolds found in a wide variety of biologically active natural compounds and have extensive applications in medicinal chemistry, pharmaceutical industry [5], biochemicals [6] and materials science [7]. Even though most of the previous studies on CuAAC reactions employed pre-isolated organic azides [8, 9], it is difficult to prepare and handling of toxic and potentially explosive azides [10]. To overcome this difficulty, multicomponent one-pot CuAAC reactions using in situ generated organic azides from organic halides and NaN3 have been developed [11, 12]. Recently, alternative one-pot methodologies for the synthesis of 1,2,3-triazoles using aromatic amines [13], diazonium salts [14], and epoxides [15, 16] as precursors of azides have been disclosed.
Various copper-based catalytic systems have been employed for “click chemistry” [11, 17,18,19], among them copper complexes bearing N-heterocyclic carbene (NHC) ligands are prominent as they show appropriate thermal, moisture, and air stability in metal-catalyzed transformations [20,21,22]. Since the first pioneering isolation of stable free NHC by Arduengo et al. in the last two decades [23, 24], NHCs have become ubiquitous ligands with incredible activity in coordination chemistry [25,26,27]. NHC–transition metal complexes with the notable s-electron-donating abilities and the robust metal–carbon bonds are a well-defined family of organometallic catalysts [28,29,30]. The immobilization of such organometallic catalysts and also organocatalysts on magnetic or non-magnetic nanoparticles not only facilitates catalyst separation and recovery but also confers new levels of catalytic activity and selectivity on them [31,32,33,34,35,36]. However, Fe3O4 magnetic nanoparticles are well known to be the most promising nanomaterials for catalytic purposes due to their high catalytic activity and easy separation [37,38,39]. In recent years, a large number of click reactions catalyzed by various copper complex-functionalized magnetic nanoparticles have been reported [40,41,42,43]. An efficient, eco-friendly and recyclable heterogeneous catalyst for “click chemistry” could thus be achieved by a copper–NHC system with high activity that has been immobilized on magnetic nanoparticles. We herein report on the immobilization of caffeine, a natural methylxanthine alkaloid, as NHC ligand on the surface of silica coated magnetite nanoparticles and the formation of a highly efficient, eco-friendly and recoverable Fe3O4@SiO2-caffeine–Cu(I) heterogeneous catalyst for green synthesis of 1,2,3-triazoles through aqueous multicomponent one-pot reactions of terminal alkynes with in situ generated organic azides from organic halides and epoxides in good yields.
2 Experimental
2.1 Materials and Instrumentation
All reagents and solvents were purchased from reputable commercial suppliers and used without further purification. All reactions were carried out in the air. All reported yields are isolated yields. FT-IR spectra were obtained over the region 400–4000 cm− 1 using a Nicolet IR100 FT-IR with spectroscopic grade KBr. The X-ray diffraction pattern was obtained at room temperature using a Philips X-pert 1710 diffractometer with Co Kα (α = 1.78897 Å), 40 kV voltage, 40 mA current and in the range 100–900 (2θ) with a scan speed of 0.020/s. Scanning electron microscopy (SEM; Philips XL 30 and S-4160) was utilized to study the catalyst morphology and size. Magnetic saturation of the catalyst was obtained using a vibrating magnetometer/alternating gradient force magnetometer (VSM/AGFM, MDK Co., Iran). Thermal gravimetric analysis (TGA) was recorded using a thermal analyzer with a heating rate of 20 °C/min over a temperature range of 25–1100 °C under flowing nitrogen. Inductively coupled plasma (ICP) analyse was performed using a Varlan Vista-Pro ICP-OE spectrometer. 1H NMR and 13C NMR spectra were recorded on a Bruker Avance (DRX 250 MHz and DRX 500 MHz) in a pure deuterated CHCl3 solvent with tetramethylsilane as an internal standard.
2.2 Preparation of Silica-Coated Magnetite Nanoparticles of Fe3O4
Magnetite nanoparticles were prepared by co-precipitation method according to a previously reported procedure [44]. Briefly, in 100 mL of deionized water, a mixture of 10 mmol FeCl3_6H2O and 5 mmol FeCl2_4H2O salts were dissolved under vigorous stirring (800 rpm). An aqueous ammonia solution (28% w/w, 30 mL) was then added to the stirring mixture to reach the reaction pH about 11. The resulting black dispersion was stirred vigorously for 1 h at room temperature and then refluxed for 1 h. The resulting black nanoparticles were separated magnetically from the aqueous solution and were washed with water and ethanol several times before being dried in an oven at 60 °C. In order to synthesize silica-coated Fe3O4 nanoparticles, magnetite nanoparticles (1.0 g) were initially dispersed in 80 mL 4:1 ethanol/water solution and the pH of the solution was adjusted to 10 using concentrated aqueous ammonia (1.5 mL, 28 wt%). The resulting dispersion was then sonicated for 20 min. Then, 0.5 mL tetraethylorthosilicate (TEOS) was added subsequently. The mixture was stirred vigorously at 40 °C for 12 h. The resulting nanoparticles were collected magnetically and washed several times with water and ethanol and dried in an oven at 80 °C.
2.3 Immobilization of Caffeine on Silica-Coated Magnetite Nanoparticles (Fe3O4@SiO2-Caffeine) and Complex Preparation [Fe3O4@SiO2-Caffeine–Cu(I)]
The functionalized magnetic nanoparticles (Fe3O4@SiO2-Caffeine) were prepared by treating about 1.0 g of Fe3O4@SiO2 in 50 mL of dry chloroform with (3-chloropropyl) triethoxysilane (3 mL). The resulting suspension was then refluxed. After 18 h, nanoparticles were concentrated by magnetic decantation and washed several times with toluene (2 × 100 mL), methanol (2 × 100 mL), and finally diethyl ether. The resulting nanoparticles were dried under Ar. The resultant nanoparticles were dispersed in 80 mL of dry acetone and then caffeine (0.38 g, 2 mmol) in 20 mL of acetone was added. The resulting suspension was brought to reflux and after 48 h, nanoparticles were magnetically separated and washed with methanol (2 × 100 mL), and acetone (2 × 100 mL). The resulting nanoparticles were dried in an oven. In a round-bottomed flask, Fe3O4@SiO2-caffeine (1.0 g), CuI (0.09 g, 0.5 mmol) and KOt–Bu (0.056 g, 0.5 mmol) were suspended in dry THF (10 mL) and stirred for 12 h at room temperature. The resulting nanoparticles were then magnetically concentrated and washed with THF (2 × 100 mL), and DCM (2 × 100 mL) before drying the particles overnight in an oven.
2.4 General Procedure for Synthesis of 1,2,3-Triazoles
In a round-bottomed flask, an appropriate alkyl halide (1.0 mmol) or epoxide (1.0 mmol), terminal alkyne (1.0 mmol), and sodium azide (1.1 mmol) were added in water (5 mL). Then the suspension was magnetically stirred at 70 °C in the presence of 25 mg (0.30 mol%) nano-Fe3O4@SiO2-caffeine–Cu(I) magnetic catalyst. The progress of the reaction was monitored by TLC. After completion of the reaction, the resulting mixture was decanted as much as possible followed by dilution of the residue with hot ethanol and separation of catalyst from the mixture by an external magnet. The solution was evaporated to afford the desired product. The pure crystalline products were obtained by re-crystallization from EtOH:H2O (3:1 v/v). In some cases products had to be isolated using chromatography on silica gel. After separation of the catalyst, it was washed with ethanol (2 × 10 mL) and dried in an oven for reuse in subsequent reactions under the same conditions.
3 Results and Discussion
The method for the synthesis of this catalyst system is shown in Scheme 1. Silica-coated magnetite nanoparticles (Fe3O4@SiO2) were synthesized based on literature procedures. Fe3O4 nanoparticles were prepared according to conventional co-precipitation method of ferrous and ferric ions in alkali solution. To improve the chemical stability of Fe3O4 nanoparticles, TEOS was employed to modify the surface of magnetic Fe3O4 with a thin layer of silica. The abundant surface hydroxyl groups of Fe3O4@SiO2 provide this possibility for grafting of 3-chloropropyltrimethoxysilane to produce chloropropyl-functionalized magnetic nanoparticles. Subsequently, the reaction of these magnetic nanoparticles with caffeine led to the desired magnetic nanoparticle-supported organocatalyst. Finally, treatment of caffeine functionalized magnetic nanoparticles with CuI in the presence of KOtBu in THF for 12 h provided Fe3O4@SiO2-caffeine–Cu(I).
The catalyst was fully characterized using various techniques such as FT-IR, SEM, energy-dispersive X-ray (EDX), TGA, X-ray diffraction (XRD), ICP and vibrating sample magnetometer (VSM). The FT-IR spectra of the magnetic nanoparticles show the peaks that confirm the successful synthesis of the catalyst (Fig. 1) [45]. The peaks appearing at 590 and 1097 cm− 1 for the Fe3O4@SiO2 sample could be associated with the presence of stretching vibrations of Fe–O and Si–O–Si bonds, respectively (Fig. 1a). In the spectrum for chloropropyl-functionalized magnetic nanoparticles (Fig. 1b), the additional bands around 2927 cm− 1 are related to C–H stretching vibrations that confirm grafting of 3-chloropropyltriethoxysilane on the surface of Fe3O4@SiO2 [46]. In the spectrum for Fe3O4@SiO2-caffeine (Fig. 1c), the peaks appearing at 1697, 1633 and 1377 cm− 1 are ascribed to the C=O, C=N, and C–N stretching vibrations, respectively. Comparing the FT-IR spectra of Fe3O4@SiO2-caffeine and Fe3O4@SiO2-caffeine–Cu(I) proved formation of the complex.
The surface morphology of the catalyst was evaluated by SEM. The SEM image of the catalyst shows that the magnetic particles obtained in the presence of caffeine–Cu(I) have a nearly spherical shape (Fig. 2a). Also, particles of the catalyst were observed in nano scale. EDX spectrum of the obtained nanomaterials (Fig. 2b) confirmed the presence of the expected elements in the structure of Fe3O4@SiO2-caffeine–Cu(I), namely iron, oxygen, silicon, and copper with wt% of 6.18, 59.99, 18.87 and 3.69, respectively.
TGA was performed for quantitative determination of inorganic and organic components in the catalyst. Curves a, b and c at Fig. 3 represent the TGA results of Fe3O4@SiO2–Cl, Fe3O4@SiO2-caffeine, and Fe3O4@SiO2-caffeine–Cu(I), respectively. In the three cases, the weight loss at temperatures below 200 °C can be mainly attributed to the water desorption from the magnetite surface. As shown as Fig. 3a, the magnetic Fe3O4@SiO2–Cl nanoparticles showed a slight weight loss of about 4.2% in the range of 185–550 °C, and it could be attributed to the thermal decomposition of the organic groups. In the TGA curve of the Fe3O4@SiO2-caffeine (Fig. 3b), a weight loss of about 6.1% in the range of 185–600 °C should be attributed to the evaporation and subsequent decomposition of organic moieties grafted on the surface of the magnetic nanoparticles. The TGA curve for Fe3O4@SiO2-caffeine–Cu(I) (Fig. 3c), represents a weight loss of 7% in the range of 160–600 °C corresponding to the main decomposition of the complex. The third weight loss could be assigned to the sublimation of iodine (melting point of CuI: 602 °C) [47]. According to TGA analysis, the caffeine content of Fe3O4@SiO2-caffeine–Cu(I) magnetic nanoparticles was evaluated to be 0.33 mmol/g. By Comparing the TGA curves of Fe3O4@SiO2-caffeine–Cu(I) and Fe3O4@SiO2-caffeine, the mass fraction of copper iodine on the surface of Fe3O4@SiO2 could be deduced to be 2.39% and the amount of adsorbed copper iodide was evaluated to be 0.12 mmol/g. ICP analysis was also used to indicate the 0.12 mmol/Cu g loading for the catalyst.
To determine the crystalline structure of the magnetic nanoparticles, XRD pattern was studied in a domain of 10°–90°. As shown at Fig. 4a, diffraction peaks at around 35.17°, 41.53°, 50.53°, 63.61°, 67.77°, and 74.61° corresponding to (220), (311), (400), (422), (511), and (440) are quite identical to characteristic peaks of the cubic magnetite (JCPDS card no. 19-0629). The broad peaks at 2θ from 21° to 30° of the XRD pattern are assigned to the silica phase, representing the core–shell structure of the catalyst (Fig. 4b). The appearance of the same peaks in XRD pattern after each modification with organic groups followed by CuI shows that the crystalline structure of magnetic nanoparticles is maintained (Fig. 4c). Any other characteristic peaks due to the impurities of other oxides of iron were not detected.
Magnetic hysteresis measurements of the nanoparticles were explored in an applied magnetic field at room temperature, with the field sweeping from − 10,000 to + 10,000 Oe using a VSM. As shown in Fig. 5, the saturation magnetization (Ms( values of nanoparticles are 52.63, 29.68 and 21.83 for Fe3O4, Fe3O4@SiO2, and Fe3O4@SiO2-caffeine–Cu(I), respectively, demonstrating that the catalyst is paramagnetic. Some decreasing of the value of Ms in compare to pure Fe3O4 is attributed to the silica and organic layer on the surface of Fe3O4 [48].
To verify the practicability of the projected route, we selected a model reaction between phenyl acetylene (1 mmol), benzyl bromide (1 mmol), and sodium azide (1.2 mmol) (Scheme 2). The results are shown in Table 1. In an initial experiment, when the mixture was heated to 70 °C for a long time in the absence of catalyst in water, no reaction was observed (Table 1, entry 1). In the next step, when the reaction was performed in the presence of pure Fe3O4 in water at 70 °C, we observed the formation of a trace amount of the desired product (Table 1, entry 2). To examine the effect of different solvents, the model reaction was performed in the presence of various solvents and the results showed that experiments proceeded in acetone, acetonitrile, methanol and water to afford the desired product in good to excellent yields (Table 1, entries 3–6). Among them, water was found to be the best solvent (Table 1, entry 6) in terms of the time and yield of desired product. Subsequently, we optimized the catalyst amount and according to the obtained results (Table 1, entries 6–9) 25 mg (0.3 mol%) of the catalyst was chosen as the best catalyst amount. As shown in Table 1, the best result was obtained by carrying out the reaction using 0.3 mol% Fe3O4@SiO2-caffeine–Cu(I) at 70 °C in water (Table 1, entry 6).
To demonstrate the generality of this method, the scope of the reaction was investigated under the optimized conditions (Scheme 3), and the results are summarized in Tables 2 and 3. We found that the provided conditions are useful for a wide range of organic halides, epoxides, and alkynes. Different benzyl bromides have reacted readily with a variety of alkynes and desired triazoles have been prepared in high yields (Table 2, entries 2–9). When benzyl chloride was employed in the reaction, the corresponding product was generated in 94% yield (Table 2, entry 1). Allyl bromide reacted with sodium azide and phenyl acetylene leading to the desired triazole in 87% yield (Table 2, entry 10). When various phenacyl bromides were employed in the reaction, the desired products were obtained in good to excellent yields (Table 2, entries 11–13). Following the same procedure as described above, when various epoxides were used in place of organic halides, the corresponding triazoles were obtained in excellent yields. The results are presented in Table 3. Aliphatic epoxides, as well as aromatic epoxides, reacted with sodium azide and phenyl acetylene by this procedure.
Based on the above observations we proposed a plausible mechanistic pathway for this reaction (Scheme 4). In the first step, Cu(I)–acetylidine complex (a) was generated from the reaction of Cu(Ӏ) and aryl acetylene. Then, azide group adds to the complex (a) and a π-complex is formed as an intermediate product. In the next step, the distal nitrogen of the azide attacks to the carbon (C-2) of the Cu–acetylidine to give a six-membered metallacycle (b). Finally, ring contraction to a Cu(I)–triazolide complex (c) is followed by protonolysis that delivers the target product along with regeneration of Cu(I) catalyst.
In order to examine the recyclability of the catalyst in three-component click reaction for synthesis of 1,2,3-triazoles, the model reaction was repeated under optimized conditions. In each cycle, after completion of the reaction, the catalyst was magnetically concentrated and washed with ethanol several times, dried and was used in the next cycle. We found that the catalyst was recovered for five runs without considerable loss of its activity, as shown in Fig. 6.
In this research, we also report amounts of copper leaching in three-component click reaction for synthesis of 1,2,3-triazoles by checking the copper loading amount before and after recycling of the catalyst by ICP analysis. It can be seen that the amount of copper in the fresh catalyst and the recycled catalyst after five times recycling is 0.120 and 0.098 mmol/g, respectively, which showed that the copper content of this catalyst did not decrease appreciably after the reaction.
Efficiency of the prepared nanocatalyst was compared with previously reported catalysts in the literature for click chemistry. It can be seen in Table 4 that the present catalyst showed a good catalytic activity. Noticeably, this new catalyst is comparable in terms of price, non-toxicity, recyclability, commercially available materials, and easy separation.
4 Conclusions
In conclusion, we have developed a highly efficient, recyclable and eco-friendly catalytic system, magnetic nanoparticles-supported CuI–caffeine, for green synthesis of 1,2,3-triazoles through the three-component reaction of various terminal alkynes with in situ generated organic azides from organic halides and NaN3 in an aqueous medium. The corresponding triazoles were prepared with various epoxides and terminal alkynes in good yields. In addition, recovery and reusability of the catalyst have been investigated in three-component click reaction and the catalyst was reused in at least five cycles without a significant loss of activity.
References
Kolb HC, Finn MG, Sharpless KB (2001) Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed 40(11):2004–2021
Meldal M, Tornøe CW (2008) Cu-catalyzed azide–alkyne cycloaddition. Chem Rev 108(8):2952–3015
Miao T, Wang L (2008) Regioselective synthesis of 1,2,3-triazoles by use of a silica-supported copper(I) catalyst. Synthesis 2008(03):363–368
Wang C, Ikhlef D, Kahlal S, Saillard J-Y, Astruc D (2016) Metal-catalyzed azide–alkyne “click” reactions: mechanistic overview and recent trends. Coord Chem Rev 316:1–20
Thirumurugan P, Matosiuk D, Jozwiak K (2013) Click chemistry for drug development and diverse chemical–biology applications. Chem Rev 113(7):4905–4979
Such GK, Quinn JF, Quinn A, Tjipto E, Caruso F (2006) Assembly of ultrathin polymer multilayer films by click chemistry. J Am Chem Soc 128(29):9318–9319
Lutz J-F (2007) 1,3-Dipolar cycloadditions of azides and alkynes: a universal ligation tool in polymer and materials science. Angew Chem Int Ed 46(7):1018–1025
Baig RBN, Varma RS (2013) Copper on chitosan: a recyclable heterogeneous catalyst for azide–alkyne cycloaddition reactions in water. Green Chem 15(7):1839–1843
Jang S, Sa YJ, Joo SH, Park KH (2016) Ordered mesoporous copper oxide nanostructures as highly active and stable catalysts for aqueous click reactions. Catal Commun 81:24–28
Campbell-Verduyn LS, Mirfeizi L, Dierckx RA, Elsinga PH, Feringa BL (2009). Phosphoramidite accelerated copper(I)-catalyzed [3 + 2] cycloadditions of azides and alkynes. Chem Commun. https://doi.org/10.1039/B822994E
Tavassoli M, Landarani-Isfahani A, Moghadam M, Tangestaninejad S, Mirkhani V, Mohammadpoor-Baltork I (2016) Copper dithiol complex supported on silica nanoparticles: a sustainable, efficient, and eco-friendly catalyst for multicomponent click reaction. ACS Sustain Chem Eng 4(3):1454–1462
Zhang C, Huang B, Chen Y, Cui D-M (2013) Porous copper catalyzed click reaction in water. N J Chem 37(9):2606–2609
Roy S, Chatterjee T, Islam SM (2013) Polymer anchored Cu(II) complex: an efficient and recyclable catalytic system for the one-pot synthesis of 1,4-disubstituted 1,2,3-triazoles starting from anilines in water. Green Chem 15(9):2532–2539
Alonso F, Moglie Y, Radivoy G, Yus M (2011) Click chemistry from organic halides, diazonium salts and anilines in water catalysed by copper nanoparticles on activated carbon. Org Biomol Chem 9(18):6385–6395
Alonso F, Moglie Y, Radivoy G, Yus M (2011) Multicomponent click synthesis of 1,2,3-triazoles from epoxides in water catalyzed by copper nanoparticles on activated carbon. J Org Chem 76(20):8394–8405
Noshiranzadeh N, Emami M, Bikas R, Kozakiewicz A (2017) Green click synthesis of β-hydroxy-1,2,3-triazoles in water in the presence of a Cu(II)–azide catalyst: a new function for Cu(II)–azide complexes. N J Chem 41(7):2658–2667
Tavassoli M, Landarani-Isfahani A, Moghadam M, Tangestaninejad S, Mirkhani V, Mohammadpoor-Baltork I (2015) Polystyrene-supported ionic liquid copper complex: a reusable catalyst for one-pot three-component click reaction. Appl Catal A 503:186–195
Sarkar SM, Rahman ML (2017) Cellulose supported poly(amidoxime) copper complex for click reaction. J Clean Prod 141:683–692
Liu X, Novoa N, Manzur C, Carrillo D, Hamon J-R (2016) New organometallic Schiff-base copper complexes as efficient “click” reaction precatalysts. N J Chem 40(4):3308–3313
Tasca E, La Sorella G, Sperni L, Strukul G, Scarso A (2015) Micellar promoted multi-component synthesis of 1,2,3-triazoles in water at room temperature. Green Chem 17(3):1414–1422
Bidal YD, Leiseur M, Melaimi M, Nahra F, Cordes DB, Arachchige AKS et al (2015) Copper(I) complexes bearing carbenes beyond classical N-heterocyclic carbenes: synthesis and catalytic activity in “Click Chemistry”. Adv Synth Catal 357(14-15):3155–3161
Li P, Wang L, Zhang Y (2008) SiO2–NHC–Cu(I): an efficient and reusable catalyst for [3 + 2] cycloaddition of organic azides and terminal alkynes under solvent-free reaction conditions at room temperature. Tetrahedron 64(48):10825–10830
Arduengo AJ, Harlow RL, Kline M (1991) A stable crystalline carbene. J Am Chem Soc 113(1):361–363
Hopkinson MN, Richter C, Schedler M, Glorius F (2014) An overview of N-heterocyclic carbenes. Nature 510:485
Hahn FE, Jahnke MC (2008) Heterocyclic carbenes: synthesis and coordination chemistry. Angew Chem Int Ed 47(17):3122–3172
Nolan SP (2014) N-heterocyclic carbenes: effective tools for organometallic synthesis. Wiley, Mannheim
Nelson DJ, Nolan SP (2013) Quantifying and understanding the electronic properties of N-heterocyclic carbenes. Chem Soc Rev 42(16):6723–6753
Schaper L-A, Hock SJ, Herrmann WA, Kühn FE (2013) Synthesis and application of water-soluble NHC–transition metal complexes. Angew Chem Int Ed 52(1):270–289
César V, Zhang Y, Kośnik W, Zieliński A, Rajkiewicz AA, Ruamps M, Bastin S et al (2017) Ruthenium catalysts supported by amino-substituted N-heterocyclic carbene ligands for olefin metathesis of challenging substrates. Chem Eur J 23(8):1950–1955
Xu S, Song K, Li T, Tan B (2015) Palladium catalyst coordinated in knitting N-heterocyclic carbene porous polymers for efficient Suzuki–Miyaura coupling reactions. J Mater Chem A 3(3):1272–1278
Kumari S, Shekhar A, Pathak DD (2016) Synthesis and characterization of a Cu(II) Schiff base complex immobilized on graphene oxide and its catalytic application in the green synthesis of propargylamines. RSC Adv 6(19):15340–15344
Javad SG, Safura Z (2015) l-Proline-functionalized Fe3O4 nanoparticles as a novel magnetic chiral catalyst for the direct asymmetric Mannich reaction. Appl Organomet Chem 29(8):566–571
Khalafi-Nezhad A, Nourisefat M, Panahi F (2015) l-Cysteine functionalized magnetic nanoparticles (LCMNP): a novel magnetically separable organocatalyst for one-pot synthesis of 2-amino-4H-chromene-3-carbonitriles in water. Org Biomol Chem 13(28):7772–7779
Nikoorazm M, Ghorbani-Choghamarani A, Mahdavi H, Esmaeili SM (2015) Efficient oxidative coupling of thiols and oxidation of sulfides using UHP in the presence of Ni or Cd salen complexes immobilized on MCM-41 mesoporous as novel and recoverable nanocatalysts. Microporous Mesoporous Mater 211:174–181
Christina K, Thomas W (2015) Recyclable bifunctional polystyrene and silica gel-supported organocatalyst for the coupling of CO2 with epoxides. ChemSusChem 8(12):2031–2034
Price GA, Hassan A, Chandrasoma N, Bogdan AR, Djuric SW, Organ MG (2017) Pd-PEPPSI-IPent-SiO2: a supported catalyst for challenging Negishi coupling reactions in flow. Angew Chem Int Ed 56(43):13347–13350
Zhang L, Li P, Li H, Wang L (2012) A recyclable magnetic nanoparticles supported palladium catalyst for the Hiyama reaction of aryltrialkoxysilanes with aryl halides. Catal Sci Technol 2(9):1859–1864
Yu L, Wang M, Li P, Wang L (2012) Fe3O4 nanoparticle-supported copper(I): magnetically recoverable and reusable catalyst for the synthesis of quinazolinones and bicyclic pyrimidinones. Appl Organomet Chem 26(11):576–582
Li P, Wang L, Zhang L, Wang GW (2012) Magnetic nanoparticles-supported palladium: a highly efficient and reusable catalyst for the Suzuki, Sonogashira, and Heck reactions. Adv Synth Catal 354(7):1307–1318
Collinson J-M, Wilton-Ely JDET, Diez-Gonzalez S (2013) Reusable and highly active supported copper(I)–NHC catalysts for Click chemistry. Chem Commun 49(97):11358–11360
Wang D, Etienne L, Echeverria M, Moya S, Astruc D (2014) A highly active and magnetically recoverable tris(triazolyl)–CuI catalyst for alkyne–azide cycloaddition reactions. Chem Eur J 20(14):4047–4054
Bahrami K, Sheikh Arabi M (2016) Copper immobilized ferromagnetic nanoparticle triazine dendrimer (FMNP@TD-Cu(II))-catalyzed regioselective synthesis of 1,4-disubstituted 1,2,3-triazoles. N J Chem 40(4):3447–3455
Mohammadi L, Zolfigol MA, Khazaei A, Yarie M, Ansari S, Azizian S et al (2018) Synthesis of nanomagnetic supported thiourea–copper(I) catalyst and its application in the synthesis of triazoles and benzamides. Appl Organomet Chem 32(1):e3933
Arefi M, Saberi D, Karimi M, Heydari A (2015) Superparamagnetic Fe(OH)3@Fe3O4 nanoparticles: an efficient and recoverable catalyst for tandem oxidative amidation of alcohols with amine hydrochloride salts. ACS Comb Sci 17(6):341–347
Togashi T, Naka T, Asahina S, Sato K, Takami S, Adschiri T (2011) Surfactant-assisted one-pot synthesis of superparamagnetic magnetite nanoparticle clusters with tunable cluster size and magnetic field sensitivity. Dalton Trans 40(5):1073–1078
Zhang Q, Su H, Luo J, Wei Y (2012) A magnetic nanoparticle supported dual acidic ionic liquid: a “quasi-homogeneous” catalyst for the one-pot synthesis of benzoxanthenes. Green Chem 14(1):201–208
Yuan S, Wang H, Wang D-X, Lu H-F, Feng S-Y, Sun D (2013) Reactant ratio-modulated six new copper(I)–iodide coordination complexes based on diverse [CumIm] aggregates and biimidazole linkers: syntheses, structures and temperature-dependent luminescence properties. CrystEngComm 15(38):7792–7802
Garkoti C, Shabir J, Mozumdar S (2017) An imidazolium based ionic liquid supported on Fe3O4@SiO2 nanoparticles as an efficient heterogeneous catalyst for N-formylation of amines. N J Chem 41(17):9291–9298
Huang L, Liu W, Wu J, Fu Y, Wang K, Huo C et al (2014) Nano-copper catalyzed three-component reaction to construct 1,4-substituted 1,2,3-triazoles. Tetrahedron Lett 55(14):2312–2316
Jia Z, Wang K, Li T, Tan B, Gu Y (2016) Functionalized hypercrosslinked polymers with knitted N-heterocyclic carbene–copper complexes as efficient and recyclable catalysts for organic transformations. Catal Sci Technol 6(12):4345–4355
Gupta M, Gupta M, Paul S, Kant R, Gupta VK (2015) One-pot synthesis of 1,4-disubstituted 1,2,3-triazoles via Huisgen 1,3-dipolar cycloaddition catalysed by SiO2–Cu(I) oxide and single crystal X-ray analysis of 1-benzyl-4-phenyl-1H-1,2,3-triazole. Chem Mon 146(1):143–148
Mouradzadegun A, Alsadat Mostafavi M (2016) Copper-loaded hypercrosslinked polymer decorated with pendant amine groups: a green and retrievable catalytic system for quick [3 + 2] Huisgen cycloaddition in water. RSC Adv 6(48):42522–42531
Pourjavadi A, Motamedi A, Hosseini SH, Nazari M (2016) Magnetic starch nanocomposite as a green heterogeneous support for immobilization of large amounts of copper ions: heterogeneous catalyst for click synthesis of 1,2,3-triazoles. RSC Adv 6(23):19128–19135
Jahanshahi R, Akhlaghinia B (2016) CuII immobilized on guanidinated epibromohydrin functionalized γ-Fe2O3@TiO2 (γ-Fe2O3@TiO2-EG-CuII): a novel magnetically recyclable heterogeneous nanocatalyst for the green one-pot synthesis of 1,4-disubstituted 1,2,3-triazoles through alkyne-azide cycloaddition in water. RSC Adv 6(35):29210–29219
Tajbakhsh M, Farhang M, Baghbanian SM, Hosseinzadeh R, Tajbakhsh M (2015) Nano magnetite supported metal ions as robust, efficient and recyclable catalysts for green synthesis of propargylamines and 1,4-disubstituted 1,2,3-triazoles in water. N J Chem 39(3):1827–1839
Shao C, Zhu R, Luo S, Zhang Q, Wang X, Hu Y (2011) Copper(I) oxide and benzoic acid ‘on water’: a highly practical and efficient catalytic system for copper(I)-catalyzed azide–alkyne cycloaddition. Tetrahedron Lett 52(29):3782–3785
Rapakousiou A, Deraedt C, Gu H, Salmon L, Belin C, Ruiz J et al (2014) Mixed-valent click intertwined polymer units containing biferrocenium chloride side chains form nanosnakes that encapsulate gold nanoparticles. J Am Chem Soc 136(40):13995–13998
Acknowledgements
We acknowledge Tarbiat Modares University for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that no conflict of interest exists.
Additional information
This article is dedicated to memory of Yadollah Shojaee.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salamatmanesh, A., Kazemi Miraki, M., Yazdani, E. et al. Copper(I)–Caffeine Complex Immobilized on Silica-Coated Magnetite Nanoparticles: A Recyclable and Eco-friendly Catalyst for Click Chemistry from Organic Halides and Epoxides. Catal Lett 148, 3257–3268 (2018). https://doi.org/10.1007/s10562-018-2523-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-018-2523-0