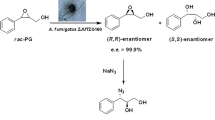
Abstract
l-2-Aminobutyric acid (l-ABA), an unnatural amino acid, is a key intermediate of several important drugs. Although some methods have been developed to prepare pure chiral l-ABA, there are still many drawbacks, including low catalytic efficiency, cumbersome steps and high cost due to the addition of some expensive catalysts or coenzymes. Herein, with chemical and biological approaches together, we discovered a newly isolated Aspergillus tamarii ZJUT ZQ013 strain containing a microbial lipase which could be employed to resolve racemic methyl N-Boc-2-aminobutyrate to produce l-ABA with high enantioselectivity (e.e.s > 99.9%, E = 257). Moreover, the subsequent gram scale experiment confrimed that A. tamarii ZJUT ZQ013 could be an attractive biocatalyst for the efficient preparation of optically pure acid.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
l-2-Aminobutyric acid (l-ABA), an unnatural amino acid, has been used as a precursor for synthesis of many chiral drugs,such as anti-epileptic Levetiracetam, anti-tuberculotic Ethambutol and Brivaracetam [1–4]. For example, (S)-2-amino butanol, a key intermediate of ethambutol, can be synthesized by esterification and hydrogenation reduction starting from l-ABA [5]. Hence, due to the great market demand for l-ABA in both pharmaceutical and chemical industries, the preparation of optically pure l-ABA with high efficiency has attracted much attention.
Up to now, the preparation of l-ABA is mainly achieved by chemical synthesis or enzymatic catalyst. Among the chemical methods, synthesis of l-ABA has been extensively reported including ammonolysis of α-Halogen acid [6], Reduction reaction [7], ammoniation hydrolysis reaction and butanone acid reduction [8]. But these chemical methods had many shortcomings, including poor selectivity, harsh reaction conditions, various byproducts, difficulty in separation and purification.
Compared with chemical synthesis, the biosynthetic methods aroused many interests due to their high selectivity and mild conditions. For example, l-ABA can be biochemically synthesized from the chemical l-threonine catalyzed by l-threonine deaminase and l-leucine dehydrogenase. But it needs formate as a co-substrate for NADH regeneration catalyzed by formate dehydrogenase [9]. Seo developed a fusion protein catalyst (VHb-DAAO) through binding to Vitreoscilla hemoglobin (VHb) on D-amino acid oxidase (DAAO), which can selectively catalyze D-ABA to ketoacid, while l-ABA was kept unchanged [10]. However these processes were complicated and required co-substrate or several enzymes. l-ABA could also be produced in a transamination reaction from l-threonine and l-aspartic acid catalyzed by threonine deaminase and aromatic aminotransferase. 2-Oxobutyric acid and benzylamine using ω-transaminase purified from Vibrio fluvialis JS17 was reported to produce l-ABA, but benzaldehyde, a harmful chemical to the microorganisms was also detected in the reaction [3]. Clearly, these methods have many weaknesses, such as low catalytic efficiency, cumbersome catalytic process, and the demand for expensive catalysts or coenzymes, which could not satisfy mass-production requirements [11].
To overcome the drawbacks of chemical or enzymatic methods, herein, we report a simple method to obtain the optical pure l-ABA by combining both chemical and biological methods. First, methyl rac-Boc-2-aminobutyric ester was synthesized from rac-2-aminobutyric acid. Then, a microbe named Aspergillus tamarii ZJUT ZQ013 was successfully screened out among hundreds strains to resolve methyl rac-Boc-2-aminobutyric ester and (S)-isomer was obtained in a high yield. Its substrate spectrum of rac-N-Boc-2-aminobutyric esters was further determined.
2 Materials and Methods
2.1 Materials
DL-2-aminobutyric acid (purity 99%), Di-tert-butyl dicarbonate (98% purity) and other chemicals were purchased from Aladdin Industrial Corporation.
2.2 Synthesis of Methyl rac-N-Boc-2-aminobutyric Acid
Synthesis of N-Boc-2-aminobutyric acid [12]: 10.3 g (0.1 mol) DL-2-aminobutyric acid was dissolved in the solution of 95mL 1 M NaOH solution and 65mL of methanol, and 27.5mL Di-tert-butyl dicarbonate(0.12 mol) was added in ice bath. Then the solution was warmed to room temperature and stirred for 12 h. Solvent methanol was removed on a rotary evaporator. The pH of residue was adjusted to1–2 with 1 M hydrochloric acid. The product was extracted with ethyl acetate, and then washed with saturated sodium chloride solution (50mL × 3), dried over anhydrous sodium sulfate and concentrated under vacuum to obtain the DL-2-aminobutyric acid (compound a).
DL-N-Boc-2-aminobutyric acid was dissolved in DMF, and NH4HCO3 and CH3I were added at room temperature. The mixture was stirred for 12 h in room temperature. The reaction solution (compound b) was extracted with ethyl acetate, and then washed with saturated sodium chloride solution (50mL × 3), dried over anhydrous sodium sulfate and concentrated under vacuum [13] (Scheme 1).
a White solid (93.4% yield). 1H NMR (500 MHz, CDCl3) δ 4.31 (s, 1H), 1.91 (s, 1H), 1.73 (s, 1H), 1.46 (s, 9H), 0.99 (s, 3H). 13C NMR (126 MHz, CDCl3) ä 177.34, 155.64, 80.14, 54.46,27.81,25.63, 9.48.
b Light yellow liquid (84.3% yield). 1H NMR (500 MHz, CDCl3) δ4.28 (s, 1H), 3.75 (s, 3H), 1.65 (s, 2H), 1.45 (s, 9H), 0.92 (s, 3H). 13C NMR (126 MHz, CDCl3) ä 172.87, 155.09, 79.50, 54.19, 52.07, 27.81, 25.85, 9.54.
2.3 Screening of Strains and Enantioselective Hydrolysis to Methyl rac-N-Boc-2-aminobutyrate
The soil samples were collected from various locations in China, and then the strains were screened by utilizing N-Boc-2-aminobutyrate as a sole carbon source and selective plate with bromocresol purple indicator. Isolated strains were transferred to 150 mL fermentation medium and cultivated at 200 rpm, 30 °C for 48 h. After the cells were harvested, 1 g wet cell were washed and suspended in 5 mL 0.1 M, pH 7.0 potassium phosphate buffer (KPB). Cell suspensions were mixed with 50 μL substrate solution (N-Boc-2-aminobutyrate:acetone = 1:4,V/V) and kept at 200 rpm, 30 °C for 6 h reaction, the enantioselective hydrolysis of the substrate was determined by GC-MS. The screening medium contained (per liter, pH 7) 0.5 g KCl, 0.5 g MgSO4∙7H2O, 1 g K2HPO4∙3H2O, 3 g NaNO3, 0.01 g FeSO4, 0.25 g Bromocresol purple, 20 g agar. Fermentation medium contained (per liter, pH 7) 0.5 g KCl, 0.5 g MgSO4∙7H2O, 1 g K2HPO4∙3H2O, 3 g NaNO3, 0.01 g FeSO4, 20 g sucrose.
2.4 Identification of the Aspergillus tamarii ZJUT ZQ013
The isolated strain was preliminarily identified by morphological and microscopic observation. Furthermore, its 18 S-internal transcribed spacer (ITS) regions of the rDNA were obtained through gene sequencing. The 18 S-ITS region sequence was deposited in the GenBank database. Related sequences were obtained from GenBank database (National Center for Biotechnology Information) using the BLAST system. The 18 S-ITS regions determined were aligned with the reference sequences obtained from GenBank databases using ClustalW ver.1.81 [14]. MEGA ver.5.1 was applied for the calculation of evolutionary distance and finally a phylogenetic tree was constructed using the neighbor-joining method [15, 16].
2.5 Preparation of Derivatives of Methyl rac-N-Boc-2-aminobutyrate
As described in synthesis of methyl rac-N-Boc-2-aminobutyric acid, compounds 1, 6, 7, 8 were synthesized. Compounds 9–12 are purchased from Aladdin. The structure of compounds 1–12 were showed in Table 1.
The synthesis of compounds 2–5 was shown in Scheme 2. 5 mmol (1 eq.) N-Boc-2-amino butyric acid was dissolved in 30 mL dry dichloromethane, adding 0.5 mmol 4-DMAP (0.1 eq.), 6 mmol alcohols (R–OH, 1.2 eq.) and 6 mol DCC (1.2 eq.) at 0 °C. Then the reaction was allowed to warm slowly to room temperature and stirred overnight. The precipitate was removed by filtration, then washed with saturated sodium chloride solution (30mL × 2), dried over anhydrous sodium sulfate and concentrated under vacuum to get the product. Products have been purified by thin layer chromatography.
Compound 1: Light yellow solid (78.7% yield),1 H NMR (500 MHz, CDCl3) δ 5.06 (d, J = 6.9 Hz, 1 H), 4.26 (dd, J = 13.3, 7.2 Hz, 1 H), 3.73 (s, 3 H), 1.83 (q, J = 6.6 Hz, 1 H), 1.67 (dt, J = 14.2, 7.3 Hz, 1 H), 1.44 (s, 9 H), 0.92 (t, J = 7.5 Hz, 3 H).
Compound 2: Light yellow liquid (67.1% yield),1 H NMR (500 MHz, CDCl3) δ 5.06 (d, J = 7.0 Hz, 1 H), 4.27–4.19 (m, 2 H), 1.94–1.79 (m, 1 H), 1.70 (dd, J = 14.2, 7.1 Hz, 1 H), 1.28 (dd, J = 13.8, 6.6 Hz, 10 H), 0.98–0.81 (m, 6 H).
Compound 3: Light yellow liquid (73.4% yield),1 H NMR (500 MHz, CDCl3) δ 5.09 (d, J = 7.6 Hz, 1 H), 4.23 (d, J = 13.1 Hz, 1 H), 4.11–4.01 (m, 3 H), 2.23 (s, 1 H), 1.81 (dd, J = 17.2, 10.1 Hz, 2 H), 1.65 (dt, J = 14.2, 7.0 Hz, 4 H), 1.42 (s, 9 H), 1.22 (s, 2 H).
Compound 4: Light yellow liquid (71.8% yield),1 H NMR (500 MHz, CDCl3) δ5.10 (d, J = 7.7 Hz, 1 H), 4.14–4.09 (m, 2 H), 3.62 (t, J = 6.7 Hz, 2 H), 2.99 (s, 3 H), 2.18 (d, J = 6.0 Hz, 2 H), 1.64 (dq, J = 13.8, 6.8 Hz, 2 H), 1.55 (d, J = 4.3 Hz, 2 H), 1.44 (s, 9 H), 1.34 (d, J = 3.3 Hz, 3 H).
Compound 5: Light yellow liquid (64.2% yield),1 H NMR (500 MHz, CDCl3) δ 4.13–4.08 (m, 1 H), 3.61 (t, J = 6.7 Hz, 3 H), 2.03 (d, J = 6.4 Hz, 2 H), 1.63 (t, J = 11.0 Hz, 1 H), 1.58–1.50 (m, 3 H), 1.43 (s, 4 H), 1.37–1.33 (m, 1 H), 1.28 (d, J = 6.9 Hz, 9 H), 0.98–0.78 (m, 7 H).
Compound 6: Light yellow liquid (76.8% yield),1 H NMR (500 MHz, CDCl3) ä 5.10 (s, 1 H), 4.38–4.25 (m, 1 H), 3.73 (s, 3 H), 1.43 (s, 9 H), 1.37 (d, J = 7.2 Hz, 3 H).
Compound 7: Light yellow liquid (74.2% yield),1 H NMR (500 MHz, CDCl3) ä 5.03 (d, J = 7.5 Hz, 1 H), 4.29 (d, J = 5.5 Hz, 1 H), 4.11 (q, J = 7.1 Hz, 1 H), 3.73 (m, 4 H), 3.70 (s, 3 H), 1.44 (s, 9 H), 0.93 (d, J = 7.3 Hz, 3 H).
Compound 8: Light yellow liquid (79.1% yield),1 H NMR (500 MHz, CDCl3) ä 5.04 (d, J = 8.3 Hz, 1 H), 4.21 (dd, J = 9.0, 4.8 Hz, 1 H), 3.73 (s, 3 H), 2.12 (dd, J = 12.2, 6.6 Hz, 1 H), 1.44 (s, 9 H), 0.94 (s, 3 H), 0.88 (d, J = 6.9 Hz, 3 H).
2.6 Analytical Methods
N-Boc-2-aminobutyric acid and its ester were detected and separated by GC (6890 N of Agilent) with chiral capillary column BGB-175. The initial temperature of oven was 120 °C for 2 min, 4 °C/min to 195 °C for 2 min, and the flow rate was 1mL/min. The value of e.e.s is expressed as enantiomeric excess of the remaining ester, which was calculated using the following equation: e.e.s=([R]−[S])/([R]+[S]). E value was enantiomeric ratios of esters, which was calculated using the following equation: E = ln[(1−c)(1−e.e.s)]/ln[(1−c)(1 + e.e.s)] (c conversion, e.e.s ee of the remaining epoxide) [17].
3 Results and Discussion
3.1 Screening and Identification of a Strain Producing l-ABA
We have successfully isolated more than 60 strains from soil samples using methyl N-Boc-2-aminobutyrate as the sole carbon source. The entioselectivity of the isolated microorgansims towards methyl N-Boc-2-aminobutyratewere checked by chiral GC-MS. More than 30 strains showed (S)-configuration, whereas, 14 of them showed moderate and good enantioselectivity towards R configuration. After the D7 strain was inoculated at 30 °C for 3 days on PDA agar plate, the colonies were flat, velvety, spore dense and violet-black sclerotia in the incubator. In the process, the white colony turned to yellowish green gradually till a deep kelly or brown, with a light brown in the reverse simultaneously. The conidiophores showed a spherical shape, and spore stems are separated under the microscopic observation (Fig. 1).
The ITS sequence analysis of the ZJUT ZQ013 was further carried out [18]. The sequence data had been submitted to GenBank under the accession NO. KJ470706. A phylogenetic tree (Fig. 2) was further constructed, and this strain was closely clustered with Aspergillus tamari (GenBankaccession No. D63701.1), having 99% sequence identity. Based on the results of phylogenetic analysis and phenotypic tests, the isolated strain ZJUT ZQ013 was designated as Aspergillus tamarii ZJUT ZQ013 and deposited in the China Center for Type Culture Collection (CCTCC M 2,014,046).
3.2 Optimization of Biotransformation Conditions
To obtain the optimum reaction conditions for asymmetric hydrolysis of methyl N-Boc-2-aminobutyrate by Aspergillus tamarii ZJUT ZQ013, different factors, such as buffer solution, pH, reaction time, reaction temperature, were investigated [19]. It is well known that buffer solutions and pH values can influence both of structures of enzymes and substrates, resulting in the alternation of the complex between substrates and the active site of enzymes [20–22]. As shown in Fig. 3, the rate of reaction was increased marginally with the increscent of pH when pH was below 5.6, at the cost of the activity and stereo selectivity of enzymes. Interestingly, even at the same pH, the selective catalytic activity in KPB buffer system was significantly better than Tris–HCl. Therefore, 0.1 M KPB (pH 7.2) was selected as the best enzyme catalytic reaction meidum.
The optimization of temperature was analyzed from 15 to 50 °C. As shown in Fig. 4, the e.e.s of methyl N-Boc-2-aminobutyrate reached the maximum 99.999% at 30 °C, and the yield of l- N-Boc −2-amino butyric acid was up to 45.4%.
Under the above optimized condition, the catalytic reaction time was further studied. According to Fig. 5, the e.e.s of methyl N-Boc-2-aminobutyrate gradually increased, it reached higher than 99% at 5.5 h. After 1 h, optical pure N-Boc-L-2- amino butyric acid was detected (e.e.p > 99.9%), and the yield was gradually increased, reaching a maximum of 45.7% at 6 h.
3.3 Substrate Specificity of Chiral Resolution by Aspergillus tamarii ZJUT ZQ013
Enzymes are known to have strict selection towards their substrates [23, 24]. To explore the scopes of substrates and to better understand the interaction between the enzyme and substrate, the substrate spectrum was determined by checking the length of carbon chain on ester and types of amino acids etc. As shown in Table 1, with the extension of the carbon chain of the alcohols (1–5), the entioselectivity of Aspergillus tamarii ZJUT ZQ013 was gradually decreased. The bulky groups on carbon chain of the amino acids also have great impact on the catalytic activity and stereoselectivity of the organism. When a longer and branched carbon chain (7 and 8 respectively) was introduced into the substrate, the selective catalytic ability of Aspergillus tamarii ZJUT ZQ013 was cut down sharply. When the Boc group was replaced with bromide (9), the enantioselectivity of Aspergillus tamari ZJUT ZQ013 decreased significantly (high conversion rate, low E value). The results also show that there is no enantioselectivity for γ-butyrolactone compounds (10–12), indicating that entioselectivity of Aspergillus tamarii ZJUT ZQ013 has strict substrate selectivity.
3.4 Preparative Scale Production of l-2-Aminobutyric Acid
To further evaluate the practical application of Aspergillus tamarii ZJUT ZQ013 in industry, a preparative scale resolution of rac-N-Boc -2-amino butyric acid was carried out in gram scale. Under the optimized resolution conditions, the enantioselective hydrolysis reaction was successfully performed at a concentration of 50 mM of rac-N-Boc -2- amino butyrate. Up to 2.5 g of optically pure N-Boc-l-2- amino butyric acid (99.9% e.e.p) was successfully obtained, and the protecting group Boc was removed in 20% TFA solution affording l-2-aminobutyric acid in 42% total yield. This result indicates that Aspergillus tamarii ZJUT ZQ013 is a powerful biocatalyst which could be employed for the efficient preparation of optically pure l-ABA.
4 Conclusion
l-ABA was an important chiral material and pharmaceutical intermediates. In this work, a newly isolated strain, Aspergillus tamarii ZJUT ZQ013 capable of resolving methyl rac-N-Boc-2-aminobutyrate to l-ABA with high enantioselectivity was reported herein. By single-factor experiment, the conditions of the kinetic resolution, such as pH, reaction time, reaction temperature were optimized. The results showed that Aspergillus tamarii ZJUT ZQ013 could afford an excellent enantio pure l-ABA with e.e.s > 99.9%, conversion rate of 45.7%, and E value of 257, which are, to our best knowledge, the highest values. It was demonstrated that Aspergillus tamarii ZJUT ZQ013 was an efficient biocatalyst and has promising potential for industry application.
References
Taylor PP, Pantaleone DP, Senkpeil RF, Fotheringham IG (1998) Novel biosynthetic approaches to the production of unnatural amino acids unnatural amino acids using transaminases. Trends Biotechnol 16:412–418
Zhu L, Tao RS, Wang Y et al (2011) Removal of L-alanine from the production of l-2-aminobutyric acid by introduction of alanine racemase and d-amino acid oxidase. Appl Microbiol Biotechnol 90(3):903–910
Shin JS, Kim BG (2009) Transaminase-catalyzed asymmetric synthesis of L-2-aminobutyric acid from achiral reactants. Biotechnol Lett 31(10):1595–1599
Futagawa T, Canvat JP, Cavoy E, et al. Process for the preparation of levetiracetam. US Patent 6,107,492, 22 Aug 2000
Ragonese R, Macka M, Hughes J et al (2002) The use of the Box-Behnken experimental design in the optimisation and robustness testing of a capillary electrophoresis method for the analysis of ethambutol hydrochloride in a pharmaceutical formulation. J Pharm Biomed Anal 27(6):995–1007
Jefery E A, Meisters A (1978) Electrochemical synthesis of amino acids by reductive amination of keto acids. I. Reduction at mercury electrodes. Aust J Chem 31(1):79–84
Babievskii KK, Belikov VM, Tikhonova NA (1965) Synthesis of D, L—threonine and D, L-2-aminobutyrie acid based on condensations of nitroacetie ester. Russ Chem Bull, 14(1):76–81
Chiyuki F. Process for production of (+)-2-amino-1-butanol. US Patent 3,979,457, 07 Sept 1976
Tao RS, Jiang Y, Zhu FY, Yang S (2014) A one-pot system for production of L-2-aminobutyric acid from L-threonine by L-threonine deaminase and a NADHregeneration system based on L-leucine dehydrogenase and formate dehydrogenase. Biotechnol Lett 36:835–841
Seo YM, Mathew S, Bea HS, Khang YH, Lee SH, Kim BG, Yun H (2012) Deracemization of unnatural amino acid: homoalanine using D-amino acid oxidase and omega-transaminase. Org Biomol Chem 10(12):2482–2485
Fotheringham IG, Grinter N, Pantaleone DP, Senkpeil RF, Taylor PP (1999) Engineering of a novel biochemical pathway for the biosynthesis of L-2-aminobutyric acid in Escherichia coli K12. Bioorg Med Chem 7:2209–2213
Alvaro G, Decor A, Fontana S, Hamprecht D, Large C, Marasco A. Imidazolidinedione derivatives. WO 2011069951 A1, 16 July 2011
Brenner M, Vecchia LL, Leutert T, Seebach D (2003) (4 S)-4- (1-methylethyl)-5,5- diphenyl- 2-oxazolidinone[(2-oxazolidinone,4-(1-methylethyl)-5,5- diphenyl-, (4 S)-)]. Organ Synth 80:57–65.
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res 22(22):4673–4680
Xu F, Deng G, Cheng S et al (2012) Molecular cloning, characterization and expression of the phenylalanine ammonia-lyase gene from Juglans Regia. Molecules 17(7):7810–7823
Tamura K, Peterson D, Peterson N et al (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739
Chen CH, Sih CJ (1989) General aspects and optimization of enantioselective biocatalysis in organic solvents: the use of lipases. Angew Chem Int Ed 28(6):695–707
Baldwin BG (1992) Phylogenetic utility of the internal transcribed spacers of nuclear ribosomal DNA in plants: an example from the compositae. Mol Phylogenet Evol 1(1):3–16
Dosanjh NS, Kaur J (2002) Immobilization, stability and esterification studies of a lipase from a Bacillus sp. Appl Biochem 36(1):7–12
Dong HP, Wang YJ, Zheng YG (2010) Enantioselective hydrolysis of diethyl 3-hydroxyglutarate to ethyl (S)-3-hydroxyglutarate by immobilized Candida antarctica lipase B. J Mol Catal B Enzym 66(1):90–94
Long WS, Kow PC, Kamaruddin AH (2005) Comparison of kinetic resolution between two racemic ibuprofen esters in an enzymic membrane reactor. Process Biochem 40(7):2417–2425
Guo JL, Mu XQ, Xu Y (2010) Integration of newly isolated biocatalyst and resin-based in situ product removal technique for the asymmetric synthesis of (R)-methyl mandelate. Bioproc Biosyst Eng 33(7):797–804
Zhang Z, Sheng Y, Jiang K (2010) Bio-resolution of glycidyl (o,m,p)-methylphenyl ethers by Bacillus megaterium. Biotechnol Lett 32:513–516
Chang CS, Ho SC (2011) Enantioselective esterification of (R, S)-2-methylalkanoic acid with Carica papaya lipase in organic solvents. Biotechnol Lett 33(11):2247–2253
Acknowledgements
This work was supported by National Science Foundation of China (Nos. 21272212, 21472172) and Zhejiang Natural Science Fund (Nos. LY17B060009, LY12B02018), Project of Science Technology Department of Zhejiang Province (2014C33141), and Project of Department of Science Technology Jinhua City (2013-3-003).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
An, Z., Gu, X., Liu, Y. et al. Bioproduction of l-2-Aminobutyric Acid by a Newly-Isolated Strain of Aspergillus tamarii ZJUT ZQ013. Catal Lett 147, 837–844 (2017). https://doi.org/10.1007/s10562-017-1999-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-017-1999-3