Abstract
Neighborhoods encompass complex environments comprised of unique economic, physical, and social characteristics that have a profound impact on the residing individual’s health and, collectively, on the community’s wellbeing. Neighborhood disadvantage (ND) is one of several factors that prominently contributes to racial breast cancer (BC) health disparities in American women. African American (AA) women develop more aggressive breast cancer features, such as triple-negative receptor status and more advanced histologic grade and tumor stage, and suffer worse clinical outcomes than European American (EA) women. While the adverse effects of neighborhood disadvantage on health, including increased risk of cancer and decreased longevity, have recently come into focus, the specific molecular mechanisms by which neighborhood disadvantage increases BC risk and worsens BC outcomes (survivorship, recurrence, mortality) are not fully elucidated. This review illuminates the probable biological links between neighborhood disadvantage and predominantly BC risk, with an emphasis on stress reactivity and inflammation, epigenetics and telomere length in response to adverse neighborhood conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the US, cancer health disparities have persisted despite considerable progress made with regards to cancer treatment, screening, diagnosis, and prevention. BC recurrence and mortality are higher in African American (AA) than white/non-Hispanic white or European American (EA) and Hispanic women. AA women tend to be diagnosed at a younger age, have more aggressive BC, and poorer survival outcomes compared with EA women. While 28% of EA women are diagnosed with regional BC, the percentage is higher in AA women (34%) [1,2,3,4,5,6]. Triple-negative BC, a particularly aggressive type of cancer that accounts for 10–20% of all diagnosed BCs, occurs more often in AA women (22%) than EA (12%) women [4, 7, 8]. AA women are also more likely to have larger tumors, higher grade disease, and more advanced stage disease [9,10,11]. Thus, while the lifetime probability of developing BC is slightly higher in white women (1 in 8 vs. 1 in 9 for AA women), AA women disproportionally experience greater mortality [6, 11]. The racial inequalities can be explained in part by biological disparities that include anomalies seen at the molecular, cellular, organellar, and genetic level. For example, racial disparity has been observed in the tumor micro-immune environment between AA and EA BC patients [12]. Differences not only in gene expression but also alterations in gene copy number has been recorded among AA and EA breast tumors [12, 13], a phenotype which correlates with tumor aggressiveness, large tumor size, and spread to lymph nodes [14, 15].
Nevertheless, a substantial fraction of the observed imparities stem from non-biological reasons broadly covered under cultural/spiritual, environmental, socioeconomic, and lifestyle influences. It is well documented that socioeconomic status, lack of medical coverage/health insurance, barriers to cancer screening, and unequal access to improvements in cancer treatment are significant contributory factors [16,17,18,19,20]. Neighborhood characteristics also shape an individual’s health and have an important bearing on BC risk and outcomes [21, 22]. Attributes that define neighborhoods as advantaged or disadvantaged in light of health risk take into account residential pollution, neighborhood socioeconomic status (e.g., median household income, health insurance coverage, education), racial residential segregation, lack of medical coverage/health insurance, spatial access to mammography and health resources, barriers to physical/outdoor activity (e.g., lack of green space or facilities, neighborhood violence, cancer-related factors like pain and other comorbidities), food availability, neighborhood esthetics, and level of social cohesion in communities [16, 23,24,25,26,27,28]. Unfavorable neighborhood conditions are associated with an increased risk of mortality, mental illnesses, and a host of chronic diseases (e.g., diabetes, hypertension, obesity and cancers) [29, 30].
Neighborhood socioeconomic status (SES) and access to healthcare coverage, among other factors, have consistently been cited to explain racial disparities in cancer outcomes [31,32,33]. A study evaluating interactions between race/ethnicity and SES yielded statistically significant interactions for BC. In general, improved survival was associated with higher SES but disparity in BC survival between non-Hispanic black and non-Hispanic whites persisted in both low-SES and high-SES areas [28]. The general trend in studies examining health insurance status and cancer outcomes, shows higher rates of mortality for uninsured or Medicaid (and other public insurance) patients than those privately insured [34,35,36]. Surprisingly, a study found that insured urban women in certain Washington DC neighborhoods presented with high rates of advanced BC despite healthcare access [37], suggesting that the urban setting itself may be implicated in BC progression. The study identified fear and personal factors (fear of cancer and its effects, financial impact) as impediments to BC screening. An educational intervention by community health workers improved respondents’ perception of safety and efficacy of mammography [37].
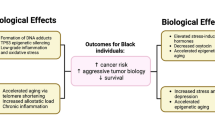
Neighborhood concentrated disadvantage, a term that embodies clusters of poverty characteristics, impacts late-stage BC diagnosis (census tract poverty being associated with late stage at diagnosis) [21, 35, 38, 39]. Furthermore, areas of high ethnic density (i.e., areas that are highly segregated) are associated with poor health outcomes owing at least in part to their weak socioeconomic structure [40]. Relying on a novel metric to measure segregation, Pruitt et al., reported that residential racial segregation considered as a standalone factor contributed to racial disparity and was adversely associated with BC mortality in AAs [41]. However, the literature is mixed with respect to the impact of residential segregation on BC risk and outcomes, with some studies attributing seeming disparities to differences in methodologies for determining segregation [41, 42]. To tease apart the complex web of evidence regarding the influence of neighborhood disadvantage on individual BC risk, it is necessary to devise a model that incorporates biological links between adverse neighborhood characteristics and perturbations at the cellular and molecular levels, which can identify inroads for therapeutic interventions and may compel changes in public policy (Fig. 1).
Molecular mechanisms delineating how disadvantaged neighborhoods increase the likelihood of cancer development. As discussed expansively in the text, neighborhood disadvantage generates chronic stress that activates the sympathetic nervous system (SNS) and hypothalamic pituitary adrenal axis (HPA) which release a host of chemical mediators in response. This in turn leads to chronic inflammation that exerts its effects via epigenetic modifications and telomere shortening, resulting in enhanced BC risk
Towards the goal of identifying biological links, ample research has identified that disadvantaged neighborhoods elicit chronic stress resulting in weathering of endocrine and inflammatory response systems in the body [43]. In this review, the impact of neighborhood disadvantage on biological markers indicative of stress like elevated cortisol and C-reactive protein levels, altered patterns of DNA methylation and histone modification, and telomere length attenuation are described. Moreover, the connections of these biological phenomena to increased BC risk, mortality, and racial BC disparities are explored. Establishing a biological background for BC disparities stemming from ND may help to narrow the disparity gap by shining a light on these issues at all operating levels from the most proximal (tumor biology) to the most distal (health policy initiatives), which can illuminate potentially actionable tumor biology and neighborhood conditions, both of which may need to be targeted.
ND-generated chronic stress and links to inflammation
Psychosocial stress in response to environmental demands has adverse biological effects and has been connected to the development of several diseases including cancer risk and progression. Stress in women also triggers unhealthy behavioral responses such as increased smoking, alcohol consumption, lack of sleep and exercise, as well as poor dietary lifestyle, which in turn puts them at an increased risk of cancer. Chronic stress engendered by long-term exposure to environmental, physiological and psychological stressors such as those encountered in an impoverished residential environment may upset the body’s homeostasis. Distress activates the sympathetic nervous system and the hypothalamic–pituitary–adrenal axis (the classic stress systems), which release chemical mediators to combat the perceived threat [44]. This restorative body reaction is termed ‘allostasis’.
However, when challenged with prolonged or exaggerated stress stimuli, chronically increased allostasis results in disease. These are referred to as stress-related diseases and include cardiovascular and metabolic diseases, depression, and cancer [45]. Catecholamines and glucocorticoids are two such hormones released by these stress systems that suppress cellular immune responses, which also guard against malignant cells, thus preparing ground for tumor initiation and development [46]. Norepinephrine (a catecholamine-family hormone) is known to increase levels of C-reactive protein and the cytokine interleukin 6, both of which function as proinflammatory molecules. C-reactive protein is a prognostic marker in some cancers, and interleukin 6 induces angiogenesis, a critical step in tumor progression [47].
One of the ways by which elevated levels of corticosteroids (during stress) induces immune suppression is via the proinflammatory nuclear factor (NF-κB) mediated signaling [47]. NF-κB is involved in the initiation and progression of BC, and crosstalk with glucocorticoid receptors plays an important role in determining the survival or apoptosis of BC cells [48]. Stress can also act via subduing the normal cortisol pattern, which is considered a risk factor for tumor initiation and progression [49, 50]. Stress induces inflammation by unsettling the immune system balance and appears to be a common pathway for various diseases including cancer. A link between inflammation and BC was first proposed, back in 1863 by German scientist Rudolf Virchow, who suggested that cancers are “born” at sites of chronic inflammation [51]. Chronic inflammation is considered key in BC development and progression. Survivors with chronic inflammation are at a greater risk of recurrence as inflammatory processes have adverse effects on cell growth [52]. Proinflammatory cytokines interleukin 6 and TNF-alpha induces BC cell aggregation and adhesion causing it to metastasize [53]. C-reactive protein and serum amyloid A proteins, secreted in response to cytokines (including interleukin 6 and TNF-alpha), are established biomarkers of low-grade chronic inflammation, are associated with BC risk, and are predictors of BC survival [54].
Social environment impresses upon the epigenome
A suspected biological mechanism by which stress-induced inflammation, which can arise from unfavorable neighborhood conditions, mediates BC risk is epigenetic modification, such as aberrant DNA methylation, histone modification, and alterations in non-coding RNAs expression. These modifications can be long-lasting with even transgenerational effects. Since each factor leaves a specific imprint on the epigenome, it can be inferred that disadvantaged neighborhoods where members are disproportionately exposed to multiple environmental assaults would imprint the genome differently than advantaged neighborhoods, resulting in different epigenomic signatures [55]. Results from a very recent population-based longitudinal study (multi-ethnic study of atherosclerosis) by Smith et al., demonstrated that neighborhood characteristics influence DNA methylation levels of genes involved in the stress response and inflammation pathways, subsequently impacting expression of these genes [56].
Exposure to different environmental and social factors leaves an imprint on the epigenome [57, 58]. Epigenetic changes, in turn, can alter gene expression, resulting in greater susceptibility to diseases. Epigenetic profiles in tissues can help to distinguish diseased individuals from healthy controls [59,60,61]. In the case of cancer and tumor-suppressor genes, the influence of epigenetic changes on gene expression is well understood [62, 63]. In a quest to study how genome-wide aberrant DNA methylation patterns affect the transcriptome and to identify potentially actionable biology, Fleischer et al. conducted genome-wide expression–methylation quantitative trait loci (emQTL) analysis between DNA methylation and gene expression in three BC cohorts [64]. They discovered that two gene regulatory networks were affected by aberrant DNA methylation. One related to estrogen receptor signaling with DNA methylation at enhancers with transcription factor-binding regions for ERα, FOXA1 and GATA3 (transcription factors that regulate genes linked to estrogen dependent tumor growth). They found that levels of methylation at these regulatory regions is BC-subtype specific. The second network was related to tumor-infiltrating immune cells. It has previously been suggested that epigenetic deregulation brought on by aberrant DNA methylation can occur in cells exposed to inflammation, which increases risk of developing various diseases [65,66,67], and different tumor-infiltrating immune cells may drive specific epigenetic modifications [64].
Alterations in DNA methylation patterns occur early during tumor development and are a hallmark of different cancers including BC. They are associated with several clinical and histopathological features of BC and clinical outcomes (tumor stage, hormone receptor status, survival time, molecular subtypes, somatic mutations) [68,69,70,71,72,73,74,75]. Interestingly, the DNA methylation landscape displays far greater changes in estrogen receptor (ER)-positive breast tumors than ER-negative tumors [68,69,70,71]. which may reflect variation in etiology and impact treatment efficacy as well as long-term prognosis.
Another route through which ND manifests as BC-related molecular changes is via poor dietary behavior. Neighborhoods with a large percentage of minorities may not have supermarkets in the vicinity with fresh fruits and vegetables. One important nutrient that is found in fruits and green leafy vegetables is folate that is responsible for maintaining proper DNA methylation patterns [76]. Low intake of folate by women living in neighborhoods with poor resources puts them at greater risk of being diagnosed with ER-negative tumors [77]. One study found that the risk of hormone receptor-negative BC in AA women was inversely related to their total vegetable intake [78]. Research by Harris et al. suggested that women who during adolescence or early adulthood followed a diet that promotes chronic inflammation are at an increased risk of pre-menopausal BC [79]. Maternal diet during pregnancy impacts the in utero environment, mediated partly by DNA methylation [80,81,82,83]. Although these studies are compelling, there is still a need for further research to fully elucidate the influence of different dietary patterns and specific nutrients on the epigenome. Once established, epidemiological studies in the future will benefit by incorporating access to healthy food and dietary lifestyle as one of the contextual factors in their modeling systems.
Obesity has been linked to BC and particularly postmenopausal BC [84, 85]. McCullough et al. revealed a positive correlation between hormone receptor-positive BC and postmenopausal obesity and little to no recreational physical activity with DNA methylation as one of the underlying mechanisms [86]. Obesity disproportionately affects AAs, the prevalence being 1.4 times more than in EA women [87]. This may be attributed in part to living in poorer neighborhoods that lack green spaces and public parks, potentially compounded by a lack of interest/motivation in physical activity, which may be linked to SES, culture, beliefs, and education level, all of which are inextricably intertwined. Obesity is associated with elevated levels of estrogen, hyperinsulinemia, and chronic inflammation, which conspire to generate a cancer-conducive environment [52, 88,89,90,91,92]. Increased physical activity may be a promising approach to lower risk of several cancers [93]. In tune with this strategy, a multi-ethnic study showed that maternal physical activity resulted in reduced birth weight and favorable methylation differences at PLAG1 (a candidate tumor-suppressor gene) [94].
Air pollutants, an integral component of ND, have endocrine-disrupting properties and epigenome-modifying effects. A joint report by the advocacy group Clean Air Task Force and the National Association for the Advancement of Colored People, released in November 2017, found that more than 1 million AAs live within half a mile of an oil and gas operation, while 6.7 million (roughly 14% of the national population) live in a county with a refinery. The proximity to oil and gas refineries means AAs are disproportionately hit by air pollution-related health issues. A finding to similar effect was also reported by the Environmental Protection Agency earlier in 2018. Several studies have been conducted to evaluate the risk of BC associated with vehicular and industrial air pollution [95, 96]. Epidemiologic evidence suggests a link between nitrogen dioxide, nitrogen oxide, and Polycyclic Aromatic Hydrocarbons (PAHs) levels and BC risk [96, 97]. Polycyclic Aromatic Hydrocarbons as well as PM2.5 have shown to impact global methylation of among others, promoter sequences of genes involved in cancer [98, 99]. A study by White et al. revealed that women residing in areas with high levels of airborne toxic metals (lead, cobalt) tended to have dense breasts, a marker of BC risk [100].
A majority of the studies conducting epigenome-wide analysis have concentrated on DNA methylation even though global histone modification profiling can also be revealing about the epigenome dynamics at play [101]. There is little research on the effects of individual components of disadvantaged neighborhoods on histone modifications, and larger studies need to be carried out to bridge this information chasm. Histone proteins can undergo a range of modifications including acetylation, methylation and phosphorylation (usually on lysine and arginine residues) that are maintained during cell division and, when disturbed, can lead to the development of cancer [62]. Zhao et al. profiled histone modifications in an in vitro BC transformation model employing biochemical and epigenomic approaches. They demonstrated a decrease in levels of the histones H3K9me2 and H3K9me3 during BC transformation. In addition, the research identified an increase in KDM3A/JMJD1A, a H3K9me2 demethylase that was responsible for the observed reduction of H3K9me2 [102].
Telomere length: a promising ND and BC risk biomarker
Telomeres are repetitive nucleotide sequences at the termini of eukaryotic chromosomes that maintain chromosomal integrity [103]. Telomere length (TL) is influenced by genetics and non-genetic factors i.e., lifestyle and environment and typically shortens with age [104, 105]. The amount of shortening depends upon the stress experienced by an individual, most notably by oxidative stress, as it interacts with its environment [106]. Environmental stressors can act through increased oxidative stress and inflammatory events and thus accelerate telomere attrition, which has been proposed as an evolutionary tactic to block growth of cells exposed to high risk of mutation [106, 107]. Chronic exposure to stress affects health and longevity via effects on telomere dynamics [108]. The rate of attrition has been linked to lifespan in several species since critically short telomeres bring upon cell senescence and death [109,110,111,112]. There is growing evidence of an association between poor health outcomes and telomere length [113]. Shorter telomeres have been linked to aging, cancer and diabetes among other health outcomes [114,115,116,117,118]. Blood leukocyte telomere length has been implicated as a cancer biomarker. Several studies concerning both adults and children have suggested an inverse relationship between quality of the neighborhood environment and telomere length [119,120,121,122,123,124,125]. The neighborhood characteristics covered in these studies included unstable family structure, low income, low maternal education, violence, noise, social cohesion, crime, esthetics and poverty.
A telling study among these is the one by Mitchell et al., using data from the Fragile Families and Child Wellbeing Study, which compared telomere lengths of AA boys living in disadvantaged environments versus those living in advantaged home environments. They found that the former had significantly shorter telomeres by age nine [121]. Further, applying the same data for white and AA mothers, they found that ND impacted mothers of both races with shorter telomere length. Critical telomere shortening results in genomic instability through chromosomal rearrangements, gains and losses of segments of the chromosome, and is proposed as a driving force for carcinogenesis [126]. Telomeres have thus long been considered as a potential biomarker especially in early stages of cancer development [115, 127].
There is emerging evidence of a strong association between cancers, and specifically BC, and TL. A very recent systematic review that took into account thirty-six studies, evaluated blood and/or tumor TL in relation to BC survival or prognostic factors, finding a tendency of longer telomeres in tumor being associated with better outcomes and suggested that TL could be an effective/a useful BC prognostic marker [128]. Kammori and colleagues’ study investigated a total of 44 BCs and observed significantly shorter telomeres in cancer cells than those in normal epithelial cells, in all histological types. TL corresponded with degree of cancer progression. Patients with advanced TNM stage, large tumors and node positive displayed considerably shorter mean TL [129]. An interesting observation was made by Ennour-Idrissi et al. while studying the correlation between TL and BC prognostic factors. They found that peripheral blood cell telomeres were lengthier in more active BC patients and recommended regular low-intensity physical activity (even that related to transportation or occupation) to BC patients [130]. The typical method to determine telomere length in peripheral blood and tumor tissue (blood leukocytes, tumor tissue and peripheral white blood cells) is by quantitative real-time PCR technique/method and by tissue-quantitative fluorescence in situ hybridization (Q-FISH). Telomere shortening has been suggested as a potential mechanism for BC risk in female night-shift workers as a disruption in circadian rhythm may affect TL. This link came out of a nested BC case–control study of Norwegian nurses published in 2017 [131].
These studies make for a strong case for the impact an individual’s residential environment can have, via telomere attrition, on the risk of cancer and death. Most of the studies have, however, used single time measurement of TL and larger, prospective, longitudinal studies need to be conducted in the future to cement the above association.
Future perspectives
The National Cancer Institute defines cancer health disparities as differences observed in cancer measures such as incidence, prevalence, mortality, morbidity, survivorship, burden of cancer or related health conditions, screening rates and stage at diagnosis, in specific population groups. For example, while in the past BC incidence rate of AA women was lower than EA women, it is similar today, and BC mortality rate of blacks is considerably higher compared with whites, indicating a widening of the mortality gap. Disparities in cancer outcomes are the result of multifaceted interactions between sociodemographic, biological, behavioral, and environmental factors. However, most studies on cancer outcomes in the United States have focused on individual-level factors, with far fewer addressing community-level and geographical contextual factors [132,133,134]. Researchers and government agencies increasingly recognize the importance of geographical contextual factors and the urgency of conducting multilevel modeling in cancer epidemiologic research [135]. A host of contextual factors are implicated in BC and other diseases such as quality of the built and natural environments, healthcare access, SES, residential segregation, rural/urban status, insurance status and healthcare quality, lifestyle/habits, biologic differences, and cultural practices [136]. Representatives from the American Association for Cancer Research, the American Cancer Society, the American Society of Clinical Oncology, and the National Cancer Institute came together in 2015 to try to map the future of cancer health disparities research. Their recommendations to improve research geared towards diminishing disparities in cancer incidence and outcomes included: selecting the highest quality tools to define sociodemographic and economic characteristics of individuals and groups (including neighborhood social and built environmental factors) to measure the most granular data; developing a health disparities research network to gather relevant data, design studies and recruit participants; designing strategies to inform underserved patients, their providers and institutions regarding participation in clinical trials and research studies; and engaging with communities to develop research [136].
Contexts also change over time, for example, people living in one neighborhood may work in another or for other reasons spend considerable time in other neighborhoods or altogether migrate to a new one; therefore, to take into account changing environmental conditions, it is imperative that future studies incorporate rapidly advancing geospatial methods and spatiotemporal data [135, 137]. Similarly, it is important to examine behavioral, environmental, and biologic characteristics prospectively, in population-based cohorts, with ongoing examination of risk factors and ascertainment of bio-specimens. Given that potential drivers are time-varying, and the temporal relationship between exposure and outcome often distorted, it is no longer sufficient to examine these factors using cross-sectional data, particularly as we begin to infer causal pathways between neighborhood deprivation and disparate breast cancer outcomes. Methods in causal inference (i.e., interaction, mediation, and decomposition) almost always require longitudinal study designs, and there are existing methodologies (e.g., marginal structural models) that can be used to assess the joint effects of time-varying exposures and account for interaction between the exposure and outcome over time [138, 139].
Disadvantaged neighborhoods give rise to multiple stressors, each of which may mark the epigenome creating a specific pattern. Epigenome can function as a biosensor of individual as well as combined exposure to different social and environmental stressors over a length of time. Epigenetic changes often precede disease pathology meaning that it has potential in BC diagnosis. Epigenome-wide analysis including looking at the amount of methylation across the whole genome for different stressors is becoming routine in research now. Further matching such patterns with known BC-linked epigenome changes will help effectively translate this knowledge into a BC-diagnostic or -prognostic blood test for regular use in the clinic. Making such a test cost-effective will entail not just looking for a pattern (where DNA methylation occurs) but focusing upon changes in a few key areas/pathways. Large-scale collaborative studies need to be conducted to identify robust DNA methylation signatures of BC risk. Combining it with other –omics can further our understanding of the metabolic pathways affected by ND and its contribution to health disparities.
ND has a marked effect on telomere length which can simply be a biomarker for ongoing disease but can well prime the cell for development of disease pathology. It is believed that telomeres can be lengthened or in the least their shortening delayed by making lifestyle and dietary changes, exercising and managing chronic stress. Epigenetic changes are also modifiable and reversible and can be used for long-term monitoring of an individual’s risk changes i.e., whether creating an advantaged environment by introducing risk-reducing initiatives helps ameliorate BC risk.
Armed with the knowledge of how ND effects molecular changes in women, accelerating their risk of developing BC, and that these changes in the epigenome are reversible makes for a strong case for implementing tailored, community-based, culturally sensitive interventions in a bid to reduce health disparities. An active community political engagement can act as a powerful advocate for making a residential environment health centric. The community leaders, local government, private enterprises, and health providers need to collaborate in this regard. Creating safer neighborhoods, increasing amount of green spaces/public parks, opening supermarkets in the locality, encouraging a more active and healthier lifestyle, ensuring equitable access to diagnostic and health facilities, discouraging conditions that lead to highly segregated housing are a few measures that may help to alleviate racial BC disparities.
References
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66(1):7–30
Cronin KA et al (2018) Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer 124(13):2785–2800
Gupta V et al (2018) Racial disparity in breast cancer: can it be mattered for prognosis and therapy. J Cell Commun Signal 12(1):119–132
Howlader N et al (2014) US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. https://doi.org/10.1093/jnci/dju055
Huo D et al (2017) Comparison of breast cancer molecular features and survival by african and european ancestry in the cancer genome atlas. JAMA Oncol 3(12):1654–1662
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68(1):7–30
Bauer KR et al (2007) Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer 109(9):1721–1728
Brewster AM, Chavez-MacGregor M, Brown P (2014) Epidemiology, biology, and treatment of triple-negative breast cancer in women of African ancestry. Lancet Oncol 15(13):e625–e634
Cadoo KA, Fornier MN, Morris PG (2013) Biological subtypes of breast cancer: current concepts and implications for recurrence patterns. Q J Nucl Med Mol Imaging 57(4):312–321
Elston CW, Ellis IO (2002) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. C. W. Elston & I. O. Ellis. Histopathology 1991;19:403–410. Histopathology 41(3A):151–152, (discussion 152-153)
Kohler BA, et al (2015) Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst 107(6):djv048
Martin DN et al (2009) Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS ONE 4(2):e4531
Ozdemir BC, Dotto GP (2017) Racial differences in cancer susceptibility and survival: more than the color of the skin? Trends Cancer 3(3):181–197
Chlebowski RT et al (2005) Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst 97(6):439–448
Elledge RM et al (1994) Tumor biologic factors and breast cancer prognosis among white, hispanic, and black women in the United States. J Natl Cancer Inst 86(9):705–712
Akinyemiju TF et al (2015) Residential environment and breast cancer incidence and mortality: a systematic review and meta-analysis. BMC Cancer 15:191
Akinyemiju TF et al (2013) Individual and neighborhood socioeconomic status and healthcare resources in relation to black-white breast cancer survival disparities. J Cancer Epidemiol 2013:490472
Coughlin SS et al (2008) Contextual analysis of breast and cervical cancer screening and factors associated with health care access among United States women, 2002. Soc Sci Med 66(2):260–275
Du XL, Fang S, Meyer TE (2008) Impact of treatment and socioeconomic status on racial disparities in survival among older women with breast cancer. Am J Clin Oncol 31(2):125–132
Elkin EB et al (2010) Geographic access and the use of screening mammography. Med Care 48(4):349–356
DeGuzman PB et al (2017) Impact of urban neighborhood disadvantage on late stage breast cancer diagnosis in virginia. J Urban Health 94(2):199–210
Smith BP, Madak-Erdogan Z (2018) Urban neighborhood and residential factors associated with breast cancer in african american women: a systematic review. Horm Cancer 9(2):71–81
Ellis L et al (2018) Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol 36(1):25–33
Haji-Jama S et al (2016) Disparities report: disparities among minority women with breast cancer living in impoverished areas of california. Cancer Control 23(2):157–162
Jones A, Paxton RJ (2015) Neighborhood disadvantage, physical activity barriers, and physical activity among african american breast cancer survivors. Prev Med Rep 2:622–627
Kim S, Chukwudozie B, Calhoun E (2013) Sociodemographic characteristics, distance to the clinic, and breast cancer screening results. J Health Dispar Res Pract 6(1):70
Kim S et al (2015) The effects of navigation and types of neighborhoods on timely follow-up of abnormal mammogram among black women. Med Res Arch 1(3):1–10. https://doi.org/10.18103/mra.v0i3.111
Kish JK et al (2014) Racial and ethnic disparities in cancer survival by neighborhood socioeconomic status in Surveillance, Epidemiology, and End Results (SEER) Registries. J Natl Cancer Inst Monogr 2014(49):236–243
Barber S et al (2016) Neighborhood disadvantage, poor social conditions, and cardiovascular disease incidence among african American adults in the jackson heart study. Am J Public Health 106(12):2219–2226
Ross CE, Mirowsky J (2001) Neighborhood disadvantage, disorder, and health. J Health Soc Behav 42(3):258–276
Farmer MM, Ferraro KF (2005) Are racial disparities in health conditional on socioeconomic status? Soc Sci Med 60(1):191–204
Kawachi I, Daniels N, Robinson DE (2005) Health disparities by race and class: why both matter. Health Aff (Millwood) 24(2):343–352
Woods LM, Rachet B, Coleman MP (2006) Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol 17(1):5–19
Ellis L et al (2018) Trends in cancer survival by health insurance status in california from 1997 to 2014. JAMA Oncol 4(3):317–323
Henry KA et al (2013) The joint effects of census tract poverty and geographic access on late-stage breast cancer diagnosis in 10 US States. Health Place 21:110–121
Niu X et al (2013) Cancer survival disparities by health insurance status. Cancer Med 2(3):403–411
Huerta EE et al (2018) Take care of your neighborhood. Breast Cancer Res Treat 167(1):225–234
Henry KA et al (2011) Breast cancer stage at diagnosis: is travel time important? J Commun Health 36(6):933–942
Sampson RJ, Raudenbush SW, Earls F (1997) Neighborhoods and violent crime: a multilevel study of collective efficacy. Science 277(5328):918–924
Fang CY, Tseng M (2018) Ethnic density and cancer: a review of the evidence. Cancer 124(9):1877–1903
Pruitt SL et al (2015) Residential racial segregation and mortality among black, white, and Hispanic urban breast cancer patients in Texas, 1995 to 2009. Cancer 121(11):1845–1855
Bemanian A, Beyer KM (2017) Measures matter: the local exposure/isolation (LEx/Is) metrics and relationships between local-level segregation and Breast cancer survival. Cancer Epidemiol Biomarkers Prev 26(4):516–524
Hill TD, Ross CE, Angel RJ (2005) Neighborhood disorder, psychophysiological distress, and health. J Health Soc Behav 46(2):170–186
Liu YZ, Wang YX, Jiang CL (2017) Inflammation: the common pathway of stress-related diseases. Front Hum Neurosci 11:316
Cohen S, Janicki-Deverts D, Miller GE (2007) Psychological stress and disease. JAMA 298(14):1685–1687
Costanzo ES, Sood AK, Lutgendorf SK (2011) Biobehavioral influences on cancer progression. Immunol Allergy Clin North Am 31(1):109–132
Nagaraja AS et al (2016) SnapShot: stress and disease. Cell Metab 23(2):388–388.e1
Ling J, Kumar R (2012) Crosstalk between NFkB and glucocorticoid signaling: a potential target of breast cancer therapy. Cancer Lett 322(2):119–126
Cash E et al (2015) Circadian disruption and biomarkers of tumor progression in breast cancer patients awaiting surgery. Brain Behav Immun 48:102–114
Fu L, Lee CC (2003) The circadian clock: pacemaker and tumour suppressor. Nat Rev Cancer 3(5):350–361
Agnoli C et al (2017) Biomarkers of inflammation and breast cancer risk: a case–control study nested in the EPIC-Varese cohort. Sci Rep 7(1):12708
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917):860–867
Geng Y et al (2013) Phenotypic switch in blood: effects of pro-inflammatory cytokines on breast cancer cell aggregation and adhesion. PLoS ONE 8(1):e54959
Pierce BL et al (2009) Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol 27(21):3437–3444
Olden K, Olden HA, Lin YS (2015) The role of the epigenome in translating neighborhood disadvantage into health disparities. Curr Environ Health Rep 2(2):163–170
Smith JA et al (2017) Neighborhood characteristics influence DNA methylation of genes involved in stress response and inflammation: the Multi-Ethnic Study of Atherosclerosis. Epigenetics 12(8):662–673
Notterman DA, Mitchell C (2015) Epigenetics and understanding the impact of social determinants of health. Pediatr Clin North Am 62(5):1227–1240
Szyf M (2011) The early life social environment and DNA methylation: DNA methylation mediating the long-term impact of social environments early in life. Epigenetics 6(8):971–978
Chen E et al (2011) Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Mol Psychiatry 16(7):729–737
Kyrtopoulos SA (2013) Making sense of OMICS data in population-based environmental health studies. Environ Mol Mutagen 54(7):468–479
Marshall KW et al (2010) A blood-based biomarker panel for stratifying current risk for colorectal cancer. Int J Cancer 126(5):1177–1186
Esteller M (2008) Epigenetics in cancer. N Engl J Med 358(11):1148–1159
Jaenisch R, Bird A (2003) Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33(Suppl):245–254
Fleischer T et al (2017) DNA methylation at enhancers identifies distinct breast cancer lineages. Nat Commun 8(1):1379
Kaise M et al (2008) CpG island hypermethylation of tumor-suppressor genes in H. pylori-infected non-neoplastic gastric mucosa is linked with gastric cancer risk. Helicobacter 13(1):35–41
Nakajima T et al (2006) Higher methylation levels in gastric mucosae significantly correlate with higher risk of gastric cancers. Cancer Epidemiol Biomarkers Prev 15(11):2317–2321
Ushijima T (2007) Epigenetic field for cancerization. J Biochem Mol Biol 40(2):142–150
Bediaga NG et al (2010) DNA methylation epigenotypes in breast cancer molecular subtypes. Breast Cancer Res 12(5):R77
Fackler MJ et al (2011) Genome-wide methylation analysis identifies genes specific to breast cancer hormone receptor status and risk of recurrence. Cancer Res 71(19):6195–6207
Fleischer T et al (2014) Genome-wide DNA methylation profiles in progression to in situ and invasive carcinoma of the breast with impact on gene transcription and prognosis. Genome Biol 15(8):435
Holm K et al (2010) Molecular subtypes of breast cancer are associated with characteristic DNA methylation patterns. Breast Cancer Res 12(3):R36
Jovanovic J et al (2010) The epigenetics of breast cancer. Mol Oncol 4(3):242–254
Kamalakaran S et al (2011) DNA methylation patterns in luminal breast cancers differ from non-luminal subtypes and can identify relapse risk independent of other clinical variables. Mol Oncol 5(1):77–92
Ronneberg JA et al (2011) Methylation profiling with a panel of cancer related genes: association with estrogen receptor, TP53 mutation status and expression subtypes in sporadic breast cancer. Mol Oncol 5(1):61–76
van Hoesel AQ et al (2013) Assessment of DNA methylation status in early stages of breast cancer development. Br J Cancer 108(10):2033–2038
Cacioppo JT, Hawkley LC (2003) Social isolation and health, with an emphasis on underlying mechanisms. Perspect Biol Med 46(3 Suppl):S39–S52
Zhang SM et al (2005) Folate intake and risk of breast cancer characterized by hormone receptor status. Cancer Epidemiol Biomarkers Prev 14(8):2004–2008
Boggs DA et al (2010) Fruit and vegetable intake in relation to risk of breast cancer in the Black Women’s Health Study. Am J Epidemiol 172(11):1268–1279
Harris HR et al (2017) An adolescent and early adulthood dietary pattern associated with inflammation and the incidence of breast cancer. Cancer Res 77(5):1179–1187
Gonzalez-Nahm S, et al (2017) Low maternal adherence to a Mediterranean diet is associated with increase in methylation at the MEG3-IG differentially methylated region in female infants. Environ Epigenet 3(2):dvx007
Godfrey KM, Gluckman PD, Hanson MA (2010) Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol Metab 21(4):199–205
Gluckman PD et al (2008) Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359(1):61–73
Boekelheide K et al (2012) Predicting later-life outcomes of early-life exposures. Environ Health Perspect 120(10):1353–1361
McCullough LE et al (2015) Genetic polymorphisms in DNA repair and oxidative stress pathways may modify the association between body size and postmenopausal breast cancer. Ann Epidemiol 25(4):263–269
Rose DP, Haffner SM, Baillargeon J (2007) Adiposity, the metabolic syndrome, and breast cancer in African-American and white American women. Endocr Rev 28(7):763–777
McCullough LE, et al (2015) Gene-specific promoter methylation status in hormone-receptor-positive breast cancer associates with postmenopausal body size and recreational physical activity. Int J Cancer Clin Res 2(1)
Weinsier RL et al (2000) Energy expenditure and free-living physical activity in black and white women: comparison before and after weight loss. Am J Clin Nutr 71(5):1138–1146
Arcidiacono B et al (2012) Insulin resistance and cancer risk: an overview of the pathogenetic mechanisms. Exp Diabetes Res 2012:789174
Henderson KD et al (2006) Ethnic disparity in the relationship between obesity and plasma insulin-like growth factors: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev 15(11):2298–2302
Key TJ et al (2001) Energy balance and cancer: the role of sex hormones. Proc Nutr Soc 60(1):81–89
Muti P (2004) The role of endogenous hormones in the etiology and prevention of breast cancer: the epidemiological evidence. Ann N Y Acad Sci 1028:273–282
Speakman JR, Goran MI (2010) Tissue-specificity and ethnic diversity in obesity-related risk of cancer may be explained by variability in insulin response and insulin signaling pathways. Obesity (Silver Spring) 18(6):1071–1078
Moore SC et al (2016) Association of Leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med 176(6):816–825
McCullough LE et al (2015) Associations between prenatal physical activity, birth weight, and DNA methylation at genomically imprinted domains in a multiethnic newborn cohort. Epigenetics 10(7):597–606
Rodgers KM et al (2018) Environmental chemicals and breast cancer: an updated review of epidemiological literature informed by biological mechanisms. Environ Res 160:152–182
White AJ, Bradshaw PT, Hamra GB (2018) Air pollution and breast cancer: a review. Curr Epidemiol Rep 5(2):92–100
Stults WP, Wei Y (2018) Ambient air emissions of polycyclic aromatic hydrocarbons and female breast cancer incidence in US. Med Oncol 35(6):88
White AJ et al (2016) Sources of polycyclic aromatic hydrocarbons are associated with gene-specific promoter methylation in women with breast cancer. Environ Res 145:93–100
Callahan CL et al (2018) Lifetime exposure to ambient air pollution and methylation of tumor suppressor genes in breast tumors. Environ Res 161:418–424
White AJ et al (2019) Airborne metals and polycyclic aromatic hydrocarbons in relation to mammographic breast density. Breast Cancer Res 21(1):24
Johansson A, Flanagan JM (2017) Epigenome-wide association studies for breast cancer risk and risk factors. Trends Cancer Res 12:19–28
Zhao QY et al (2016) Global histone modification profiling reveals the epigenomic dynamics during malignant transformation in a four-stage breast cancer model. Clin Epigenetics 8:34
Blackburn EH (1991) Structure and function of telomeres. Nature 350(6319):569–573
Monaghan P (2010) Telomeres and life histories: the long and the short of it. Ann N Y Acad Sci 1206:130–142
Olsson M, Wapstra E, Friesen CR (2017) Evolutionary ecology of telomeres: a review. Ann N Y Acad Sci 1422:5–28
von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27(7):339–344
Jennings BJ, Ozanne SE, Hales CN (2000) Nutrition, oxidative damage, telomere shortening, and cellular senescence: individual or connected agents of aging? Mol Genet Metab 71(1–2):32–42
Monaghan P (2014) Organismal stress, telomeres and life histories. J Exp Biol 217(Pt 1):57–66
Barrett EL, Richardson DS (2011) Sex differences in telomeres and lifespan. Aging Cell 10(6):913–921
Campisi J (2005) Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120(4):513–522
Dantzer B, Fletcher QE (2015) Telomeres shorten more slowly in slow-aging wild animals than in fast-aging ones. Exp Gerontol 71:38–47
Harley CB et al (1992) The telomere hypothesis of cellular aging. Exp Gerontol 27(4):375–382
Blackburn EH, Epel ES, Lin J (2015) Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science 350(6265):1193–1198
Koorstra JB et al (2008) Pancreatic carcinogenesis. Pancreatology 8(2):110–125
Londono-Vallejo JA (2008) Telomere instability and cancer. Biochimie 90(1):73–82
Salpea KD et al (2010) Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis 209(1):42–50
Sanders JL, Newman AB (2013) Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol Rev 35:112–131
Wentzensen IM et al (2011) The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev 20(6):1238–1250
Drury SS et al (2014) The association of telomere length with family violence and disruption. Pediatrics 134(1):e128–e137
Geronimus AT et al (2015) Race-ethnicity, poverty, urban stressors, and telomere length in a detroit community-based sample. J Health Soc Behav 56(2):199–224
Mitchell C et al (2014) Social disadvantage, genetic sensitivity, and children’s telomere length. Proc Natl Acad Sci USA 111(16):5944–5949
Needham BL et al (2015) Leukocyte telomere length and mortality in the National Health and Nutrition Examination Survey, 1999–2002. Epidemiology 26(4):528–535
Park M et al (2015) Where you live may make you old: the association between perceived poor neighborhood quality and leukocyte telomere length. PLoS ONE 10(6):e0128460
Shalev I et al (2013) Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol Psychiatry 18(5):576–581
Theall KP et al (2013) Neighborhood disorder and telomeres: connecting children’s exposure to community level stress and cellular response. Soc Sci Med 85:50–58
Frias C et al (2012) Telomere dysfunction and genome instability. Front Biosci (Landmark Ed) 17:2181–2196
DePinho RA (2000) The age of cancer. Nature 408(6809):248–254
Ennour-Idrissi K, Maunsell E, Diorio C (2017) Telomere length and breast cancer prognosis: a systematic review. Cancer Epidemiol Biomarkers Prev 26(1):3–10
Kammori M et al (2015) Telomere shortening in breast cancer correlates with the pathological features of tumor progression. Oncol Rep 34(2):627–632
Ennour-Idrissi K et al (2016) Association of telomere length with breast cancer prognostic factors. PLoS ONE 11(8):e0161903
Samulin Erdem J et al (2017) Mechanisms of breast cancer risk in shift workers: association of telomere shortening with the duration and intensity of night work. Cancer Med 6(8):1988–1997
Gomez SL et al (2015) The impact of neighborhood social and built environment factors across the cancer continuum: current research, methodological considerations, and future directions. Cancer 121(14):2314–2330
Lynch SM, Rebbeck TR (2013) Bridging the gap between biologic, individual, and macroenvironmental factors in cancer: a multilevel approach. Cancer Epidemiol Biomarkers Prev 22(4):485–495
Warnecke RB et al (2008) Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health 98(9):1608–1615
Zahnd WE, McLafferty SL (2017) Contextual effects and cancer outcomes in the United States: a systematic review of characteristics in multilevel analyses. Ann Epidemiol 27(11):739–748
Polite BN et al (2017) Charting the Future of cancer health disparities research: a position statement from the american association for cancer research, the American Cancer Society, the American Society of Clinical Oncology, and the National Cancer Institute. J Clin Oncol 35(26):3075–3082
Schootman M et al (2017) Geospatial approaches to cancer control and population sciences. Cancer Epidemiol Biomarkers Prev 26(4):472–475
Jackson JW, VanderWeele TJ (2019) Intersectional decomposition analysis with differential exposure, effects, and construct. Soc Sci Med 1:1–10. https://doi.org/10.1016/j.socscimed.2019.01.033
VanderWeele TJ, Jackson JW, Li S (2016) Causal inference and longitudinal data: a case study of religion and mental health. Soc Psychiatry Psychiatr Epidemiol 51(11):1457–1466
Acknowledgments
This work was supported by grants to RA from the National Cancer Institute, including U01 CA179671 and R01 CA169127.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saini, G., Ogden, A., McCullough, L.E. et al. Disadvantaged neighborhoods and racial disparity in breast cancer outcomes: the biological link. Cancer Causes Control 30, 677–686 (2019). https://doi.org/10.1007/s10552-019-01180-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-019-01180-4