Abstract
The racial/ethnic disparities in cancer incidence and outcome are partially due to the inequities in neighborhood advantage. Mounting evidences supported a link between neighborhood deprivation and cancer outcomes including higher mortality. In this review, we discuss some of the findings related to work on area-level neighborhood variables and cancer outcomes, and the potential biological and built/natural environmental mechanisms that might explain this link. Studies have also shown that residents of deprived neighborhoods or of racially or economically segregated neighborhoods have worse health outcomes than residents of more affluent neighborhoods and/or less racially or economically segregated neighborhoods, even after adjusting for the individual-level socioeconomic status. To date, little research has been conducted investigating the biological mediators that may play roles in the associations of neighborhood deprivation and segregation with cancer outcomes. The psychophysiological stress induced by neighborhood disadvantage among people living in these neighborhoods could be a potential underlying biological mechanism. We examined a number of chronic stress-related pathways that may potentially mediate the relationship between area-level neighborhood factors and cancer outcomes, including higher allostatic load, stress hormones, altered epigenome and telomere maintenance and biological aging. In conclusion, the extant evidence supports the notion that neighborhood deprivation and racial segregation have unfavorable impacts on cancer. Understanding how neighborhood factors influence the biological stress response has the potential to inform where and what types of resources are needed within the community to improve cancer outcomes and reduce disparities. More studies are warranted to directly assess the role of biological and social mechanisms in mediating the relationship between neighborhood factors and cancer outcomes.
Similar content being viewed by others
Introduction
In the United States, historic and present-day residential segregation has shifted access to neighborhood and community resources such that a greater percentage of minoritized individuals are living in more disadvantaged neighborhoods. The racial/ethnic disparities in cancer incidence and outcome are believed to be, in part, due to the inequities in neighborhood advantage and the community stress this induces among people in these neighborhoods. Thus, where one grows up and resides matters to one’s cancer risk and one’s ability to recover from the disease. This interest in neighborhood factors and how they relate to health has been a cornerstone in the field of social epidemiology for several years. Fueled by the availability of geospatial data and advanced analytics, increased interest in this area has emerged rapidly over the past 20 years. While neighborhood determinants can refer to and include the natural environment (e.g., pollution and exposures to toxins), green space (e.g., access to parks), and retail environments (e.g., food deserts) herein we focus on the social, systemic, and structural characteristics of the neighborhood and the ways in which these area-level characteristics have been examined in relation to cancer.
A consistent finding across several studies is that residents of deprived neighborhoods or of racially or economically segregated neighborhoods have worse health outcomes than residents of more affluent neighborhoods and/or less racially or economically segregated neighborhoods, even after adjusting for the individual-level socioeconomic status (SES) [1,2,3]. Characteristics of neighborhood deprivation have included either area-level components (e.g., low SES of residents, high concentration of rental homes, low home property value, poverty, neighborhood crime/violence) or indices of these components (e.g., Neighborhood Deprivation Index) [1, 4, 5]. Measures of segregation have included measures such as the percent of residents in a geographic area who belong to a racial or ethnic minority group, and other measures, such dissimilarity (uneven distribution of individuals from African American (AA) and Caucasian backgrounds), isolation (probability of AA individual encountering another AA individual), concentration (density of AA individuals), centralization (degree to which AA individuals are located in urban centers) or combinations of these characteristics, such as hyper segregation (simultaneous occurrence of these) and the Index of Concentration at the Extremes, which combines area-level residential household income and racial segregation data [6]. Unfavorable neighborhood conditions related to deprivation and segregation are associated with an increased risk of morbidity and mortality of chronic diseases, including diabetes, hypertension, cardiovascular diseases, depression, and, as highlighted in this review, cancer [7,8,9].
In this review, we discuss some of the previous literature and more recent findings related to work on area-level neighborhood variables and cancer outcomes along the cancer-control continuum. By cancer-control continuum we mean papers that have examined outcomes related to early detection (screening), incidence and stage of diagnosis, mortality and survivorship. We organize our discussion around papers that have examined (i) characteristics of neighborhood deprivation and (ii) racial and economic segregation and cancer outcomes and then (iii) evaluate several potential (iii) biological and (iv) social mechanisms linking neighborhood variables to cancer outcomes. We conclude with a discussion of next steps for future research.
Neighborhood deprivation
The relationship that neighborhood deprivation has with cancer outcomes has been highlighted by Gomez et al. the authors reviewed published papers between 2010 and 2014 (n = 34) and concluded that a majority of these papers support a link between a harmful social or built environment attributes and a cancer outcome, including a higher incidence, later stage of diagnosis, poorer treatment outcomes, poorer quality of life and higher mortality [10].
More recent articles have emerged showing similar findings as those in this previous review. For instance, recommended screenings for cancers (e.g., breast, cervical, and colorectal) have been shown to be lower for individuals living in the most deprived neighborhoods compared with the least deprived [11]. Neighborhood deprivation has also been linked to a higher incidence of lung cancer, especially among black men who are current or former smokers [12] and a higher incidence of liver cancer among individuals identifying as Hispanic [13]. Examining triple-negative breast cancer (TNBC) data, Hossain et al. found that neighborhood deprivation was related to disparities between AA and Caucasian women in stage at diagnosis and survival (later stage and poor survival among AA women). Notably, disparities in incidence were not observed with respect to neighborhood deprivation [14]. Poorer patient reported outcomes and health related quality of life among cancer survivors has also been associated with greater neighborhood deprivation [15, 16].
Of note, area-level socioeconomic status and neighborhood deprivation in relation to cancer outcomes has been examined across several different countries. Registry data from England for women diagnosed with breast cancer found wide disparities in survival by neighborhood deprivation, regardless of whether they were up-to-date on screening or not, suggesting that the neighborhood effects were independent of access [17]. In a Swedish population study, increased incidence of lung cancer incidence and mortality have been observed in the most deprived neighborhood [18]. Increased incidence of head and neck cancers have also been found in relation to the European Deprivation Index in a French study [19]. Using British Columbia cancer registry data on oral cancers collected between 1981 and 2009, greater proportions of oral cavity cancer cases were diagnosed at later-stage disease for both sexes residing in deprived neighborhoods [20].
Related to neighborhood deprivation are emerging studies of persistent poverty in relation to cancer outcomes. Persistent poverty has been defined as areas where at least 20% of residents have lived below the federal poverty line for several decades. These areas often designated rural and/or have higher percent of individuals from minoritized backgrounds. In two recent papers, Moss and colleagues demonstrated a 12% higher county-level cancer mortality rate in counties designated as persistently poor and 7% higher cancer mortality rate in counties designated as currently poor [21]. In a follow-on study, the team investigated how the intersection between race and poverty relates to these mortality rates by showing that rural black residents had some of the highest cancer mortality rates for several of the more common types of cancers (colorectal, oropharyngeal, breast, cervical and prostate) [22]. Recent executive orders [23], as well as the National Cancer Institute’s recent strategic budget, have highlighted a need for more research on the “systemic traits of persistent poverty that lead to cancer disparities.” [24] Continued research examining the different aspects and intersection of neighborhood socioeconomic conditions are needed to improve our understanding of how best to tackle the iniquities on cancer outcomes we observe here in the US and elsewhere.
Discrimination, racial segregation, and redlining and cancer outcomes
Studies of racial segregation in relation to cancer outcomes have been highlighted in two reviews, one by Landrine et al. [25] and another by Fang and Tseng [26]. Landrine et al. reviewing papers primarily focused on breast cancer (n = 17), noted several papers in their review supported a link between residential segregation and Black-white cancer disparities (higher likelihood of later-stage diagnosis, higher mortality rates, and lower survival rates) [25]. Fang and Tseng reviewed papers examining racial and ethnic minority density—the percentage of residents in a geographic area who belong to a racial or ethnic minority group [26]. Minority density has been examined as a cancer risk factor, as it can be a proxy for neighborhood segregation. In some instances, however, these “ethnic enclaves” may be protective, as they preserve social-cultural cohesion and support. From their review, these authors concluded that racial and ethnic density was related to a higher incidence for cancers of an infectious origin (e.g., liver, cervical) but a lower risk for breast and colorectal cancers among Hispanic/Latinx and Asian Americans [26]. Also, Hispanic density was related to later-stage diagnosis and Black density was related to a higher cancer mortality.
Discrimination
Discrimination can come in the form of implicit and explicit biases and discrimination in health care access and delivery of quality care may lead to disparities in cancer treatments and, consequently, outcomes [27]. Using data from the California Cancer Registry collected during 2004 to 2016, Black patients and those on Medicaid were less likely to receive guideline-concordant medications, compared with white patients and those who had managed care insurance plans [28]. Patients of lower socioeconomic status were also less likely to receive NCCN-adherent care across all cancer types except cervical cancer (P < 0.0001). Studies have also shown that discrimination may influence certain behavioral risk factors for cancer, through heightened levels of stress and depressive symptoms [29, 30]. For example, Shariff‐Marco et al. reported that community residents who reported experiencing more racism (being treated unfairly or receiving poorer medical care because of race) were more likely to smoke, binge drink, and be overweight [30]. In another study, men who reported experiencing more racism in the health care system were less likely to be up-to-date on antigen screening for prostate cancer [31].
Racial segregation and redlining
Residential segregation in the U.S. is one indicator of structural racism [32,33,34]. Structural racism like this operates such that institutions and governmental systems on the federal, state, and local level develop, implement, and enforce laws and policies that explicitly or implicitly advantage whites and disadvantage Blacks and other racial or ethnic minority groups [35, 36]. For decades, starting at least in the 1930s, low-income and minority communities were intentionally cut off from lending and investment through a system known as redlining [37]. The practice of redlining was explicit in its targeting of African Americans. While Latino or Hispanic residents, low-income white residents, noncitizens, communists, and other populations the federal government deemed “risky” were often included in redlining, they were not targeted in the same manner as Black residents. Today, neighborhoods that fall within once-redlined areas are more likely to have a higher concentration of Black residents as well as lower incomes, lower home values, and greater social vulnerability. Those same neighborhoods also suffer from lower life expectancy and higher incidence of chronic diseases [38,39,40,41].
Racial segregation has been made operational in geospatial and area-level studies in a number of different ways. As mentioned above, one method has been to examine racial and ethnic density of an area-level variable. Yet other methods have proposed examining racial isolation, or the probability of contact between Black and white residents across neighborhoods [42]. The relationship between racial isolation or ethnic density and cancer has been discussed in a previous review by Fang and Tseng [26]. Since that review, additional studies have been published further assessing the relationship between segregation and cancer [43, 44]. In the Mississippi Delta region, a U-shaped relationship was found between racial segregation and colorectal cancer mortality rates among Black residents in urban counties indicating that for Black residents living in highest and least segregated areas were most at risk [43] In Florida, in a large sample of racially diverse women diagnosed with malignant epithelial ovarian cancer (EOC), Westrick et al. reported that the influence of economic and racialized economic residential segregations on EOC survival was more significant than racial segregation in both non-Hispanic Black and Hispanic women [44]. In further race specified model, Hispanic women had a statistically significant increased hazard of death after controlling for covariates in neighborhood segregations. Examining racial and economic segregation using the Index of Concentration at the Extremes (ICE), an estimate of racial and economic segregation, have also been found to be associated with higher cancer mortality at the county level [45] and a higher hazard of breast cancer mortality at the individual level [46].
Though still very limited, a few studies have explored the relationship between historical redlining and cancer outcomes. In a recent study of residence at Greater Atlanta area, living in redlined census tracts was found associated with a nearly 1.6-fold increase in breast cancer mortality [47]. In another study using data from the Massachusetts Cancer Registry between 2001 and 2015, residing in a previously redlined area imposed an elevated risk for late-stage cervical, breast, lung, or colorectal cancer diagnosis, even for residents of census tracts with present-day economic and racial privilege. The best historical grade was not protective for residents of census tracts without current privilege [48].
Psychophysiological and biological stress pathways
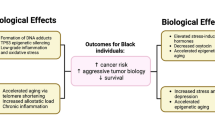
To date, most of the studies about the influence of neighborhood deprivation and segregation on cancer have ignored potential pathways and little research has been conducted investigating the biological and psychophysiological mediators that may play a role in these association. In the broader epidemiologic literature, the biological imbedding of neighborhood disadvantage and the ways it impacts physiological stress pathways has been highlighted by Krieger and Smith and others [49]. Below, we discuss several potential biological mechanisms that could be the focus of further study within the cancer literature, including higher allostatic load (AL), stress hormones, altered epigenome and telomere maintenance and cellular aging.
Allostatic load
Several conceptual frameworks have been proposed to delineate how neighborhood deprivation may be biologically imbedded and, consequently, influence cancer risk, mortality, and disparities. The major theme of those frameworks is that disadvantaged neighborhoods elicit chronic stress, resulting in weathering of endocrine and inflammatory response systems in the body. Living in residential areas that have been systematically devalued may increase stress by long-term direct exposure to environmental, physiological, and psychological stressors associated with the neighborhood environment, and by triggering unhealthy behavioral responses (e.g., increased smoking, alcohol consumption, lack of sleep and exercise, and poor diet). Normally, stress will activate the sympathetic nervous system and the hypothalamic–pituitary–adrenal axis—the classic stress systems—which release chemicals to combat the perceived threat [50]. However, when challenged with prolonged or exaggerated stress stimuli, the normal physiological regulatory systems will be disrupted and consequently cause greater “wear and tear” on the body. Studies have attempted to capture levels of the body’s responses to chronic stress using an AL score, which is a multi-system, multi-dimensional composite index, usually involving neuroendocrine, immunological, cardiovascular, and metabolic components.
Though AL has been used in other chronic diseases, its application in cancer research is still limited. Using National Health and Nutrition Examination Survey (NHANES) data, a history of breast cancer was found to be associated with elevated AL in Black women, but not in white women [51]. In our own study, AL was found to be higher in Black and Hispanic than white breast cancer patients (P = 0.001 and 0.032, respectively) [52]. AL was also found associated with poorer tumor grade and estrogen receptor negative (ER-) tumors. These findings were similar to those reported recently by Xing et al. [53]. In another recent study within the REGARDS cohort, Blacks were found to have a higher AL compared with whites (P < 0.001) at baseline [54]. Then, during the follow-up, a higher baseline AL score was associated with increased risk of all-cause and cancer-specific mortality among both Black and white participants. Similarly, in the NHANES III study, individuals in the highest quartile of multi-systemic biological risk (MSBR), a proxy for AL, had a 64% increased risk of cancer mortality, compared to those in the lowest quartile of MSBR [55].
Stress hormones
Studies in the past decade have shown that living in disadvantage neighborhood is not only associated with lower serum or saliva cortisol but also altered cortisol response to stressors [56,57,58,59]. For example, Dublin-Keita et al. reported that higher neighborhood disorder exposure resulted in lower serum cortisol over time compared to individuals in socially ordered neighborhoods among children [56]. Interestingly, the association is seemingly modified by race and gender [56, 57]. Also, disadvantaged neighborhood was found associated with a flatter rate of cortisol decline throughout the day [58]. In terms of stress response, Hackman et al. found that neighborhood disadvantage was associated with cortisol reactivity and this relationship was moderated by gender, such that higher disadvantage predicted higher cortisol reactivity and steeper recovery in boys but not in girls [59].
Chronic stress and excessive levels of stress hormones promote carcinogenesis through several different molecular pathways. First, they can directly affect tumor suppressor genes (e.g., p53) or oncogenes (et al., MDM2, c-myc), damage DNA, and compromise DNA repair capacity [60]. Second, excessive stress hormones lead to inflammation and suppress immunity, thereby disrupting immune surveillance [50, 61]. Third, excessive stress hormones can act on tumor and stromal cells in the tumor microenvironment to promote tumor growth, invasion, and metastasis [62]. Fourth, emerging evidence suggests that chronic stress may affect the microbiota-gut-brain axis [63, 64], and disrupt the metabolic homeostasis. However, we need to be cautious to interpret the findings since to date most of them were from cell line or animal-based studies. There are few human studies, but not in the context of neighborhood deprivation.
Excessive stress hormones (e.g., catecholamines and glucocorticoids) have been shown to promote tumorigenesis through distinct signaling pathways. For example, catecholamines can trigger the cAMP-protein kinase A (PKA) signaling pathway [65, 66], which further leads to inducing DNA damage, degrading p53, and up-regulating vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMP-2 and MMP-9). Chronic stress has also been involved in angiogenesis. In stressed animals, significantly increased tumor blood vessel formation was observed [67].
Chronic stress and stress hormones can induce the expression of stress-related pro-inflammatory genes, thus increasing the release of pro-inflammatory cells and the production of pro-inflammatory cytokines, which results in the activation of inflammatory responses and leads to tumor initiation, promotion, and metastasis [68]. Norepinephrine (a catecholamine-family hormone) is known to increase levels of C-reactive protein (CRP) and the cytokine interleukin 6 (IL-6), both of which function as pro-inflammatory molecules and tumorigenesis. Elevated levels of corticosteroids during stress induce immune suppression via the pro-inflammatory nuclear factor (NF-κB) signaling [69], which helps tumor initiation and progression [70]. Moreover, research shows that stress management in patients with early-stage breast cancer can reverse the up-regulation of the stress-related pro-inflammatory genes in white blood cells [71].
Chronic stress may also activate pro-tumorigenic immune cells (e.g., tumor-associated macrophages (TAMs), dendritic cells (DCs), myeloid-derived suppressor cells (MDSCs), and tumor-infiltrating lymphocytes (TILs)). These cells can further promote tumorigenesis [72]. Moreover, activated inflammatory cells produce excess reactive oxygen species (ROS) to drive inflammation and mutagenesis through different pathways [73]. The released cytokines can activate key transcription factors such as NF-κB [74,75,76] and STAT3 [77], and further promote tumor progression.
Epigenome
Interest in the epigenome has grown rapidly in recent years because it is exquisitely plastic—particularly in early life—and can be programmed or reprogrammed by environmental experience [78]. The epigenome also represents a potential mechanism by which social exposures early in life are embodied at the molecular level, affecting phenotypic expression. Disadvantaged neighborhoods have the potential to disproportionally expose members to community stress and multiple other environmental assaults, which will consequently become imprinted in different epigenomic signatures and affect biological factors underlying multiple disease pathways [79]. In the Multi-Ethnic Study of Atherosclerosis, multiple neighborhood indexes were found to influence DNA methylation and subsequent gene expression of stress- and inflammation-related genes, even after accounting for individual socioeconomic factors [80]. In another study, children raised in more socioeconomically disadvantaged neighborhoods appeared exhibited greater differential DNA methylation in genes involved in inflammation relative to their peers living in more advantaged settings as they entered young adulthood [81].
Recently developed “epigenetic clocks” are a class of biological age estimators that use DNA methylation at predetermined CpG sites to estimate biological variation among those with the same chronologic age [82]. These clocks may be a more sensitive measure of biological aging and are better at estimating biological age than other markers. Interestingly, in a recent study of 2630 women who had a sister with breast cancer but had not had breast cancer themselves, those with the greatest (>75th percentile) neighborhood deprivation had higher epigenetic age acceleration [83].
Telomere length and cellular aging
Telomeres naturally shorten with age [84, 85], but also shorten prematurely in response to stress [86, 87]. Paradoxically, both shorter and longer telomere length has been associated with various types of chronic diseases, including cancer, diabetes, depression, and cardiovascular disease [86,87,88,89,90,91]. Several studies have shown that telomere length is shorter among African Americans relative to their Caucasian counterparts, suggesting a putative biological stress response to discrimination and inequities [92,93,94,95]. Telomere length has also been shown to differ by level of poverty and interact with race and ethnicity to predict TL differently across racial and ethnic groups [96]. Studies also show associations between shorter telomere length and other individual-level exposures that correlate with poor neighborhood circumstances and psychosocial stressors [86, 87, 96].
An inverse relationship between shorter telomere length and a number of area-level neighborhood deprivation factors has been observed, including neighborhood socioeconomic status [97,98,99], neighborhood disadvantage [100,101,102], unfavorable social environment [97, 103] and perceived neighborhood quality [103, 104]. In a recent study using the data from the 1999–2002 NHANES, neighborhood deprivation was inversely associated with leukocyte telomere length among individuals living in neighborhoods with medium neighborhood deprivation index (NDI) (β = −0.043, P = 0.0005) and high NDI (β = −0.039, P = 0.003) [105]. Telomere shortening in high deprivation neighborhoods represented 7.5 years of accelerated aging. And the association was more evident among men than women. In another study among breast cancer survivors, higher levels of everyday discrimination were associated with longer telomere length, adjusting for age, race, ethnicity, breast cancer stage, and breast cancer subtype [106]. The opposing direction of associations is interesting, though it may simply reflect differences across the study populations. Clearly, more research is needed.
Built and natural environmental pathways
Neighborhood deprivation and segregation have restructured aspects of the built and natural environment which may represent mechanisms through which deprivation and segregation impact cancer outcomes. Indeed, research has documented a link between food deserts and colorectal cancer incidence [107] and breast and colorectal cancer mortality [108] and food insecurity has been associated with being up-to-date for cancer screening practices [109]. Pollution (e.g., area-level PM2.5) has also been associated with a higher incidence of all types of cancer [110] and specific types such as breast cancer [111,112,113,114] and breast density [111], a putative risk factor for breast cancer. Green space, on the other hand, has been associated with reduced cancer incidence of prostate and lung cancer [110]. Also, as highlighted across a number of studies, the tobacco retail environment has been associated with tobacco use, a behavioral risk factor for cancer.
Although often overlooked, there is likely a great deal of correlation between neighborhood deprivation and segregation and other built and natural environmental drivers of health. Research in this area has tended to examine many of these factors in isolation without taking into consideration how structural characteristics of neighborhoods related to segregation, access to educational opportunities, and poverty may drive other aspects of the built and natural environment. Additional work is need to model and understand what aspects of the community environment relate most strongly to cancer outcomes. Given the potential multicollinearity of area-level variables, modeling procedures such as Bayesian index regression models, can be useful in estimating area-level components of neighborhood deprivation along with aspects of the built environment may be most relevant in predicting particular outcomes [115]. For instance, in some of our work, we have found that when considering the importance of both the built environment (tobacco retail environment) and neighborhood deprivation in relation to prenatal smoke exposure, it is aspects of neighborhood deprivation that have the strongest association [116]. Bringing together multiple area-level variables to examine independent, moderating and mediating roles of neighborhood deprivation along with built and natural environmental variables has the potential to improve our efforts at addressing the inequities that arise in relation to these drivers.
Discussion and future directions
In this review we highlighted the role of neighborhood factors on cancer outcomes, with an eye toward describing the potential biological and built/natural environmental mechanisms that might explain this link. Convincing evidence has supported the notion that neighborhood deprivation has an unfavorable impact on cancer, including lower screening rates, heightened cancer lifestyle risk factors, higher cancer incidence of some types of cancer, more challenging tumor characteristics, higher mortality, and worse survival rates. Though, there are some exceptions where studies find that “ethnic enclave” may serve a protective factor, economic and racial segregation have deleterious associations with cancer outcomes. We further reviewed the literature examining the roles of discrimination, racial segregation, and redlining with cancer outcomes. Though the number of existing studies is still limited, the extant evidence shows that racial segregation and redlining are associated with increased mortality among cancer patients.
Departing from previous reviews, this paper examined a number of mechanisms worth exploring that may mediate the relationship between area-level neighborhood factors and cancer outcomes. The broader scientific literature has highlighted the embodiment hypothesis to explain how neighborhood conditions get “under the skin” and alter psychophysiological stress, immune, and epigenetic pathways. In this review, we highlight how some of these mechanisms are also clearly linked with cancer biology underlying tumorigeneses and progression. In particular, AL, stress hormones, and epigenetics (including telomere biology) are all linked with cancer biomarkers and could be examined further as mediating mechanisms linking neighborhood factor to cancer outcomes, such as stage, tumor progression, and survival. Indeed, as we have highlighted, emerging literature is beginning to show how some of these biomarkers are linked with cancer outcomes. Thus, the logical next step is to examine within existing or new cancer cohorts the link between neighborhood stressors, biomarkers and cancer outcomes. Such findings would highlight more clearly which aspects of neighborhoods relate most to perturbations in which biomarker to impact which outcomes. Honing down on these processes has the potential to then begin to think about how to alter these pathways in favor of preventing and controlling cancer effectively within the population.
While we have highlighted biomarkers that have been linked in independent analyses to upstream structural factors and downstream to cancer progression and outcomes, there may be others to consider. For instance, in ongoing studies, members of our group are exploring protein arginine methyl transferases 6 (PRMT6). PRMT6 expression is increased in AA men compared to Caucasian Men, is stimulated by smoking, and is overexpressed in in vivo models driving lung cancer development. In one of the largest cohorts of black men being screened for lung cancer to date, the team is examining to what degree neighborhood stressors relate to PRMT6 expression and interact with smoking to increase the risk of lung cancer. The findings hold promise at identifying the combination of biomarkers, behavior and neighborhood conditions that could be evaluated to improve early detection of lung cancer among this group of men who have higher rates of lung cancer mortality.
In addition to understanding the biological pathways, aspects such as the retail environment and environmental toxics have been linked to cancer outcomes. It is important to note, however, that these types of environmental factors do not randomly emerge. The structural conditions related to neighborhood deprivation and racial segregation precondition other area-level factors that increase unhealthy lifestyles and exposure to environmental toxins. While continued research is clearly needed, these structural characteristics of our society may be more clearly important to the psychophysiological and biological response than other conditions, like clustering of tobacco outlets or exposure to pollution, that result because of them. While there has been increasing attention in the public health and cancer prevention literature to developing policies that correct for certain types of built and natural environmental conditions (e.g., reducing tobacco retail outlet density, reducing food deserts, minimizing city pollution, etc.), less work has been dedicated to thinking through how to begin to modify the historical and structural conditions that underly neighborhood disadvantage. Given the growing evidence highlighted in this review that neighborhood deprivation and segregation are clearly linked to cancer outcomes, it is imperative to now consider how, we as a field, can begin to correct these systemic injustices. Policies that were once thought to be outside the field of cancer prevention, such as reducing food insecurity, improving stable housing, advancing education equity, addressing systemic racism where it occurs, implementing universal basic income strategies, and other structural interventions could be considered [117]. Addressing these structural factors is not without challenges and will require successful community engagement and partnership. Such efforts are also disease agnostic and do not fit neatly into the National Cancer Institute’s funding models which prioritize clear focus on cancer biology and outcomes. Thus, bottom up and top down efforts will be needed to increase our field’s focus on addressing these more systemic conditions that clearly matter to multiple health outcomes, including cancer.
It is important to be cautious in interpreting the results from these studies, because few studies have directly assessed the role of molecular mechanisms in mediating the relationship between neighborhood factors and cancer outcomes. Thus, the results from those studies need to be further confirmed. In the future, large cancer cohort studies with detailed information at neighborhood (e.g., neighborhood deprivation), individual (e.g., healthy behaviors and demographics), and molecular levels (e.g., biomarkers) are needed to better understand how unfavorable neighborhood factors may become embedded biologically to influence cancer outcomes [118, 119]. We will also need to consider how exposure to neighborhoods change over time, by incorporating residential histories.
Reducing cancer disparities remains at the top of the national agenda for the National Cancer Institute as well as many other organizations (American Cancer Society, American Society of Clinical Oncology, etc.), and there is an increasing recognition that a radical approach is needed to get to the root of problem. While there is a need to understand ancestry and how it may influence cancer risk or response to treatment, modern cancer disparities are likely largely rooted in the historical and present-day racist practices and structural factors, such as neighborhood deprivation [120]. Moving forward to solve iniquities in cancer outcomes requires looking back to better understand how discriminatory practices have contributed to social determinates of health, fair access to health care and the quality of care delivery. Thus, to advance work in this area, we need to study how neighborhood deprivation and segregation have contributed to cancer outcomes and explore the potential biological and social/behavioral mechanisms that may be driving these effects [121].
The driving force of the modern cancer center is to learn from and partner with members of the community to improve cancer outcomes. Thus, understanding how neighborhood factors influence stress and the biological stress response has the potential to inform the cancer center’s “place-based” outreach strategies, and such efforts have the potential to reduce cancer disparities [122]. The findings from such studies can also help inform where and what types of resources are needed within the community to improve cancer outcomes and reduce disparities. In addition, the data from such studies have the potential to inform local policy strategies by identifying structural factors that create disparities. Shining a light, with data, on the ecosystems that are detrimental to cancer outcomes can inform local community and health system efforts that can be leveraged to correct disparities—a laudable mission of any cancer center.
References
Gee GC, Payne-Sturges DC. Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environ Health Perspect. 2004;112:1645–53.
Meijer M, Rohl J, Bloomfield K, Grittner U. Do neighborhoods affect individual mortality? A systematic review and meta-analysis of multilevel studies. Soc Sci Med. 2012;74:1204–12.
Cozier YC, Albert MA, Castro-Webb N, Coogan PF, Ridker P, Kaufman HW, et al. Neighborhood Socioeconomic Status in Relation to Serum Biomarkers in the Black Women’s Health Study. J Urban Health. 2016;93:279–91.
Mitchell R, Blane D, Bartley M. Elevated risk of high blood pressure: climate and the inverse housing law. Int J Epidemiol. 2002;31:831–8.
Blane D, Mitchell R, Bartley M. The “inverse housing law” and respiratory health. J Epidemiol Community Health. 2000;54:745–9.
Krieger N, Feldman JM, Kim R, Waterman PD. Cancer Incidence and Multilevel Measures of Residential Economic and Racial Segregation for Cancer Registries. JNCI Cancer Spectr. 2018;2:pky009.
Brown AF, Ang A, Pebley AR. The relationship between neighborhood characteristics and self-rated health for adults with chronic conditions. Am J Public Health. 2007;97:926–32.
Kim D, Glazier RH, Zagorski B, Kawachi I, Oreopoulos P. Neighbourhood socioeconomic position and risks of major chronic diseases and all-cause mortality: a quasi-experimental study. BMJ Open. 2018;8:e018793.
Ross CE, Mirowsky J. Neighborhood disadvantage, disorder, and health. J Health Soc Behav. 2001;42:258–76.
Gomez SL, Shariff-Marco S, DeRouen M, Keegan TH, Yen IH, Mujahid M, et al. The impact of neighborhood social and built environment factors across the cancer continuum: Current research, methodological considerations, and future directions. Cancer. 2015;121:2314–30.
Kurani SS, McCoy RG, Lampman MA, Doubeni CA, Finney Rutten LJ, Inselman JW, et al. Association of Neighborhood Measures of Social Determinants of Health With Breast, Cervical, and Colorectal Cancer Screening Rates in the US Midwest. JAMA Netw Open. 2020;3:e200618.
Sanderson M, Aldrich MC, Levine RS, Kilbourne B, Cai Q, Blot WJ. Neighbourhood deprivation and lung cancer risk: a nested case-control study in the USA. BMJ Open. 2018;8:e021059.
Ortiz AG, Wiese D, Sorice KA, Nguyen M, Gonzalez ET, Henry KA, et al. Liver Cancer Incidence and Area-Level Geographic Disparities in Pennsylvania-A Geo-Additive Approach. Int J Environ Res Public Health. 2020;17:7526.
Hossain F, Danos D, Prakash O, Gilliland A, Ferguson TF, Simonsen N, et al. Neighborhood Social Determinants of Triple Negative Breast Cancer. Front Public Health. 2019;7:18.
Li CC, Matthews AK, Asthana A, Shah RC. The impact of neighborhood disadvantage on health-related quality of life among African American and White cancer survivors. Transl Cancer Res. 2019;8:S313–22.
Rosenzweig MQ, Althouse AD, Sabik L, Arnold R, Chu E, Smith TJ, et al. The Association Between Area Deprivation Index and Patient-Reported Outcomes in Patients with Advanced Cancer. Health Equity. 2021;5:8–16.
Morris M, Woods LM, Rogers N, O’Sullivan E, Kearins O, Rachet B. Ethnicity, deprivation and screening: survival from breast cancer among screening-eligible women in the West Midlands diagnosed from 1989 to 2011. Br J Cancer. 2015;113:1640.
Li X, Sundquist J, Zoller B, Sundquist K. Neighborhood deprivation and lung cancer incidence and mortality: a multilevel analysis from Sweden. J Thorac Oncol. 2015;10:256–63.
Bryere J, Menvielle G, Dejardin O, Launay L, Molinie F, Stucker I, et al. Neighborhood deprivation and risk of head and neck cancer: A multilevel analysis from France. Oral Oncol. 2017;71:144–9.
Auluck A, Walker BB, Hislop G, Lear SA, Schuurman N, Rosin M. Socio-economic deprivation: a significant determinant affecting stage of oral cancer diagnosis and survival. BMC Cancer. 2016;16:569.
Moss JL, Pinto CN, Srinivasan S, Cronin KA, Croyle RT. Persistent Poverty and Cancer Mortality Rates: An Analysis of County-Level Poverty Designations. Cancer Epidemiol Biomark Prev. 2020;29:1949–54.
Moss JL, Pinto CN, Srinivasan S, Cronin KA, Croyle RT. Enduring Cancer Disparities by Persistent Poverty, Rurality, and Race: 1990-1992 to 2014-2018. J Natl Cancer Inst. 2022;114:829–36.
Executive order on advancing racial equity and support for underserved communities through the federal government. The White House. https://www.whitehouse.gov/briefing-room/presidential-actions/2021/01/20/executive-order-advancing-racial-equity-and-support-for-underserved-communities-through-the-federal-government/. 2021. Accessed Date 2021.
NCI annual plan and budget proposal for fiscal year 2024. National Cancer Institute. https://www.cancer.gov/research/annual-plan. 2023. Accessed Date 2023.
Landrine H, Corral I, Lee JGL, Efird JT, Hall MB, Bess JJ. Residential Segregation and Racial Cancer Disparities: A Systematic Review. J Racial Ethn Health Disparities. 2017;4:1195–205.
Fang CY, Tseng M. Ethnic density and cancer: A review of the evidence. Cancer. 2018;124:1877–903.
Nelson B. How structural racism can kill cancer patients: Black patients with breast cancer and other malignancies face historical inequities that are ingrained but not inevitable. In this article, the second of a 2-part series, we explore the consequences of and potential solutions to racism and inequality in cancer care. Cancer Cytopathol. 2020;128:83–4.
Clair K, Chang J, Ziogas A, Tanjasiri SP, Kansal KJ, Gin GE, et al. Disparities by race, socioeconomic status, and insurance type in the receipt of NCCN guideline concordant care for select cancer types in California. J Clin Oncol. 2020;38:7031.
Cuevas AG, Reitzel LR, Adams CE, Cao Y, Nguyen N, Wetter DW, et al. Discrimination, affect, and cancer risk factors among African Americans. Am J Health Behav. 2014;38:31–41.
Shariff-Marco S, Klassen AC, Bowie JV. Racial/ethnic differences in self-reported racism and its association with cancer-related health behaviors. Am J Public Health. 2010;100:364–74.
Crittendon DR, LaNoue M, George B. Does Perceived Racism Affect Prostate Cancer Screening Rates and Patient-Provider Shared Discussions Among Black and White Men? J Health Care Poor Underserved. 2022;33:5–19.
Riley AR. Neighborhood Disadvantage, Residential Segregation, and Beyond-Lessons for Studying Structural Racism and Health. J Racial Ethn Health Disparities. 2018;5:357–65.
Chambers BD, Erausquin JT, Tanner AE, Nichols TR, Brown-Jeffy S. Testing the Association Between Traditional and Novel Indicators of County-Level Structural Racism and Birth Outcomes among Black and White Women. J Racial Ethn Health Disparities. 2018;5:966–77.
Harrell CJ, Burford TI, Cage BN, Nelson TM, Shearon S, Thompson A, et al. Multiple Pathways Linking Racism to Health Outcomes. Du Bois Rev. 2011;8:143–57.
Wallace M, Crear-Perry J, Richardson L, Tarver M, Theall K. Separate and unequal: Structural racism and infant mortality in the US. Health Place. 2017;45:140–4.
Bailey ZD, Krieger N, Agenor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453–63.
Jackson KT. Crabgrass Frontier The Suburbanization of the United States. New York: Oxford University Press; 1987. p. 432.
Nardone A, Casey JA, Morello-Frosch R, Mujahid M, Balmes JR, Thakur N. Associations between historical residential redlining and current age-adjusted rates of emergency department visits due to asthma across eight cities in California: an ecological study. Lancet Planet Health. 2020;4:e24–31.
McClure E, Feinstein L, Cordoba E, Douglas C, Emch M, Robinson W, et al. The legacy of redlining in the effect of foreclosures on Detroit residents’ self-rated health. Health Place. 2019;55:9–19.
Lynch EE, Malcoe LH, Laurent SE, Richardson J, Mitchell BC, Meier HCS. The legacy of structural racism: Associations between historic redlining, current mortgage lending, and health. SSM Popul Health. 2021;14:100793.
Krieger N, Van Wye G, Huynh M, Waterman PD, Maduro G, Li W, et al. Structural Racism, Historical Redlining, and Risk of Preterm Birth in New York City, 2013-7. Am J Public Health. 2020;110:1046–53.
Anthopolos R, James SA, Gelfand AE, Miranda ML. A spatial measure of neighborhood level racial isolation applied to low birthweight, preterm birth, and birthweight in North Carolina. Spat Spatiotemporal Epidemiol. 2011;2:235–46.
Kruse-Diehr AJ, McDaniel JT, Lewis-Thames MW, James AS, Yahaya M. Racial Residential Segregation and Colorectal Cancer Mortality in the Mississippi Delta Region. Prev Chronic Dis. 2021;18:E14.
Westrick AC, Bailey ZD, Schlumbrecht M, Hlaing WM, Kobetz EE, Feaster DJ, et al. Residential segregation and overall survival of women with epithelial ovarian cancer. Cancer. 2020;126:3698–707.
Zhang L, Gong R, Shi L, Wen M, Sun X, Yabroff KR, et al. Association of Residential Racial and Economic Segregation With Cancer Mortality in the US. JAMA Oncol. 2023;9:122–6.
Connor AE, Kaur M, Dibble KE, Visvanathan K, Dean LT, Hayes JH. Racialized Economic Segregation and Breast Cancer Mortality among Women in Maryland. Cancer Epidemiol Biomark Prev. 2022;31:413–21.
Collin LJ, Gaglioti AH, Beyer KM, Zhou Y, Moore MA, Nash R, et al. Neighborhood-Level Redlining and Lending Bias Are Associated with Breast Cancer Mortality in a Large and Diverse Metropolitan Area. Cancer Epidemiol Biomark Prev. 2021;30:53–60.
Krieger N, Wright E, Chen JT, Waterman PD, Huntley ER, Arcaya M. Cancer Stage at Diagnosis, Historical Redlining, and Current Neighborhood Characteristics: Breast, Cervical, Lung, and Colorectal Cancers, Massachusetts, 2001-2015. Am J Epidemiol. 2020;189:1065–75.
Krieger N, Davey Smith G. “Bodies count,” and body counts: social epidemiology and embodying inequality. Epidemiol Rev. 2004;26:92–103.
Liu YZ, Wang YX, Jiang CL. Inflammation: The Common Pathway of Stress-Related Diseases. Front Hum Neurosci. 2017;11:316.
Howard JT, Sparks PJ. Does allostatic load calculation method matter? Evaluation of different methods and individual biomarkers functioning by race/ethnicity and educational level. Am J Hum Biol. 2016;28:627–35.
Zhao H, Song R, Ye Y, Chow WH, Shen J. Allostatic score and its associations with demographics, healthy behaviors, tumor characteristics, and mitochondrial DNA among breast cancer patients. Breast Cancer Res Treat. 2021;187:587–96.
Xing CY, Doose M, Qin B, Lin Y, Plascak JJ, Omene C, et al. Prediagnostic Allostatic Load as a Predictor of Poorly Differentiated and Larger Sized Breast Cancers among Black Women in the Women’s Circle of Health Follow-Up Study. Cancer Epidemiol Biomark Prev. 2020;29:216–24.
Akinyemiju T, Wilson LE, Deveaux A, Aslibekyan S, Cushman M, Gilchrist S, et al. Association of Allostatic Load with All-Cause andCancer Mortality by Race and Body Mass Index in theREGARDS Cohort. Cancers. 2020;12:1695.
Acheampong T, Jiang L, Ziogas A, Odegaard AO. Multi-Systemic Biological Risk and Cancer Mortality: The NHANES III Study. Sci Rep. 2020;10:5047.
Dulin-Keita A, Casazza K, Fernandez JR, Goran MI, Gower B. Do neighbourhoods matter? Neighbourhood disorder and long-term trends in serum cortisol levels. J Epidemiol Community Health. 2012;66:24–9.
Finegood ED, Rarick JRD, Blair C. Family Life Project I. Exploring longitudinal associations between neighborhood disadvantage and cortisol levels in early childhood. Dev Psychopathol. 2017;29:1649–62.
Karb RA, Elliott MR, Dowd JB, Morenoff JD. Neighborhood-level stressors, social support, and diurnal patterns of cortisol: the Chicago Community Adult Health Study. Soc Sci Med. 2012;75:1038–47.
Hackman DA, Betancourt LM, Brodsky NL, Hurt H, Farah MJ. Neighborhood disadvantage and adolescent stress reactivity. Front Hum Neurosci. 2012;6:277.
Hara MR, Kovacs JJ, Whalen EJ, Rajagopal S, Strachan RT, Grant W, et al. A stress response pathway regulates DNA damage through beta2-adrenoreceptors and beta-arrestin-1. Nature. 2011;477:349–53.
Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5:617–25.
Dai S, Mo Y, Wang Y, Xiang B, Liao Q, Zhou M, et al. Chronic Stress Promotes Cancer Development. Front Oncol. 2020;10:1492.
Vodicka M, Ergang P, Hrncir T, Mikulecka A, Kvapilova P, Vagnerova K, et al. Microbiota affects the expression of genes involved in HPA axis regulation and local metabolism of glucocorticoids in chronic psychosocial stress. Brain Behav Immun. 2018;73:615–24.
Farzi A, Frohlich EE, Holzer P. Gut Microbiota and the Neuroendocrine System. Neurotherapeutics. 2018;15:5–22.
Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–44.
Renz BW, Takahashi R, Tanaka T, Macchini M, Hayakawa Y, Dantes Z, et al. beta2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer. Cancer Cell. 2018;33:75–90.e77.
Landen CN Jr., Lin YG, Armaiz Pena GN, Das PD, Arevalo JM, Kamat AA, et al. Neuroendocrine modulation of signal transducer and activator of transcription-3 in ovarian cancer. Cancer Res. 2007;67:10389–96.
Bondar T, Medzhitov R. The origins of tumor-promoting inflammation. Cancer Cell. 2013;24:143–4.
Nagaraja AS, Sadaoui NC, Dorniak PL, Lutgendorf SK, Sood AK. SnapShot: Stress and Disease. Cell Metab. 2016;23:388–8.e1.
Ling J, Kumar R. Crosstalk between NFkB and glucocorticoid signaling: a potential target of breast cancer therapy. Cancer Lett. 2012;322:119–26.
Antoni MH, Lutgendorf SK, Blomberg B, Carver CS, Lechner S, Diaz A, et al. Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biol Psychiatry. 2012;71:366–72.
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99.
El-Kenawi A, Ruffell B. Inflammation, ROS, and Mutagenesis. Cancer Cell. 2017;32:727–9.
Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59.
Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6.
Taniguchi K, Karin M. NF-kappaB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18:309–24.
Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–12.
Francis DD. Conceptualizing child health disparities: a role for developmental neurogenomics. Pediatrics. 2009;124:S196–202.
Olden K, Olden HA, Lin YS. The Role of the Epigenome in Translating Neighborhood Disadvantage Into Health Disparities. Curr Environ Health Rep. 2015;2:163–70.
Smith JA, Zhao W, Wang X, Ratliff SM, Mukherjee B, Kardia SLR, et al. Neighborhood characteristics influence DNA methylation of genes involved in stress response and inflammation: The Multi-Ethnic Study of Atherosclerosis. Epigenetics. 2017;12:662–73.
Reuben A, Sugden K, Arseneault L, Corcoran DL, Danese A, Fisher HL, et al. Association of Neighborhood Disadvantage in Childhood With DNA Methylation in Young Adulthood. JAMA Netw Open. 2020;3:e206095.
Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19:371–84.
Lawrence KG, Kresovich JK, O’Brien KM, Hoang TT, Xu Z, Taylor JA, et al. Association of Neighborhood Deprivation With Epigenetic Aging Using 4 Clock Metrics. JAMA Netw Open. 2020;3:e2024329.
Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–6.
Meena J, Rudolph KL, Gunes C. Telomere Dysfunction, Chromosomal Instability and Cancer. Recent Results Cancer Res. 2015;200:61–79.
Sanders JL, Newman AB. Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol Rev. 2013;35:112–31.
Starkweather AR, Alhaeeri AA, Montpetit A, Brumelle J, Filler K, Montpetit M, et al. An integrative review of factors associated with telomere length and implications for biobehavioral research. Nurs Res. 2014;63:36–50.
Mair C, Diez Roux AV, Galea S. Are neighbourhood characteristics associated with depressive symptoms? A review of evidence. J Epidemiol Community Health. 2008;62:940–6.
Freedman VA, Grafova IB, Rogowski J. Neighborhoods and chronic disease onset in later life. Am J Public Health. 2011;101:79–86.
Xu C, Wang Z, Su X, Da M, Yang Z, Duan W, et al. Association between leucocyte telomere length and cardiovascular disease in a large general population in the United States. Sci Rep. 2020;10:80.
Vakonaki E, Tsiminikaki K, Plaitis S, Fragkiadaki P, Tsoukalas D, Katsikantami I, et al. Common mental disorders and association with telomere length. Biomed Rep. 2018;8:111–6.
Needham BL, Salerno S, Roberts E, Boss J, Allgood KL, Mukherjee B. Do black/white differences in telomere length depend on socioeconomic status? Biodemography Soc Biol. 2019;65:287–312.
Rewak M, Buka S, Prescott J, De Vivo I, Loucks EB, Kawachi I, et al. Race-related health disparities and biological aging: does rate of telomere shortening differ across blacks and whites? Biol Psychol. 2014;99:92–9.
Hamad R, Tuljapurkar S, Rehkopf DH. Racial and Socioeconomic Variation in Genetic Markers of Telomere Length: A Cross-Sectional Study of U.S. Older Adults. EBioMedicine. 2016;11:296–301.
Selvaraju V, Phillips M, Fouty A, Babu JR, Geetha T. Telomere Length as a Biomarker for Race-Related Health Disparities. Genes. 2021;12:78.
Geronimus AT, Pearson JA, Linnenbringer E, Schulz AJ, Reyes AG, Epel ES, et al. Race-Ethnicity, Poverty, Urban Stressors, and Telomere Length in a Detroit Community-based Sample. J Health Soc Behav. 2015;56:199–224.
Needham BL, Carroll JE, Diez Roux AV, Fitzpatrick AL, Moore K, Seeman TE. Neighborhood characteristics and leukocyte telomere length: the Multi-Ethnic Study of Atherosclerosis. Health Place. 2014;28:167–72.
Lynch SM, Mitra N, Ravichandran K, Mitchell J, Spangler E, Zhou W, et al. Telomere Length and Neighborhood Circumstances: Evaluating Biological Response to Unfavorable Exposures. Cancer Epidemiol Biomark Prev. 2017;26:553–60.
Alexeeff SE, Schaefer CA, Kvale MN, Shan J, Blackburn EH, Risch N, et al. Telomere length and socioeconomic status at neighborhood and individual levels among 80,000 adults in the Genetic Epidemiology Research on Adult Health and Aging cohort. Environ Epidemiol. 2019;3:e049.
Mitchell C, Hobcraft J, McLanahan SS, Siegel SR, Berg A, Brooks-Gunn J, et al. Social disadvantage, genetic sensitivity, and children’s telomere length. Proc Natl Acad Sci USA. 2014;111:5944–9.
Theall KP, Brett ZH, Shirtcliff EA, Dunn EC, Drury SS. Neighborhood disorder and telomeres: connecting children’s exposure to community level stress and cellular response. Soc Sci Med. 2013;85:50–8.
Massey DS, Wagner B, Donnelly L, McLanahan S, Brooks-Gunn J, Garfinkel I, et al. Neighborhood Disadvantage and Telomere Length: Results from the Fragile Families Study. RSF. 2018;4:28–42.
Park M, Verhoeven JE, Cuijpers P, Reynolds CF III, Penninx BW. Where You Live May Make You Old: The Association between Perceived Poor Neighborhood Quality and Leukocyte Telomere Length. PLoS ONE. 2015;10:e0128460.
Ellaway A, Dundas R, Robertson T, Shiels PG. More miles on the clock: Neighbourhood stressors are associated with telomere length in a longitudinal study. PLoS ONE. 2019;14:e0214380.
Powell-Wiley TM, Gebreab SY, Claudel SE, Ayers C, Andrews MR, Adu-Brimpong J, et al. The relationship between neighborhood socioeconomic deprivation and telomere length: The 1999-2002 National Health and Nutrition Examination Survey. SSM Popul Health. 2020;10:100517.
Aghaee S, Allen A, Ramirez J, Shariff-Marco S, Allen L, DeRouen M, et al. Everyday discrimination and telomere length in a multiethnic cohort of breast cancer survivors. Ethn Health. 2022;27:542–53.
Masdor NA, Mohammed Nawi A, Hod R, Wong Z, Makpol S, Chin SF. The Link between Food Environment and Colorectal Cancer: A Systematic Review. Nutrients. 2022;14:3954.
Fong AJ, Lafaro K, Ituarte PHG, Fong Y. Association of Living in Urban Food Deserts with Mortality from Breast and Colorectal Cancer. Ann Surg Oncol. 2021;28:1311–9.
Mendoza JA, Miller CA, Martin KJ, Resnicow K, Iachan R, Faseru B, et al. Examining the Association of Food Insecurity and Being Up-to-Date for Breast and Colorectal Cancer Screenings. Cancer Epidemiol Biomark Prev. 2022;31:1017–25.
Huang YJ, Lee PH, Chen LC, Lin BC, Lin C, Chan TC. Relationships among green space, ambient fine particulate matter, and cancer incidence in Taiwan: A 16-year retrospective cohort study. Environ Res. 2022;212:113416.
Kotake R, Yamauchi H, Kimura T, Tsunoda H, Lee M. An association between mammographic breast density and fine particulate matter among postmenopausal women. Environ Sci Pollut Res Int. 2022. https://doi.org/10.1007/s11356-022-23529-0. Online ahead of print.
Tomczak A, Miller AB, Weichenthal SA, To T, Wall C, van Donkelaar A, et al. Long-term exposure to fine particulate matter air pollution and the risk of lung cancer among participants of the Canadian National Breast Screening Study. Int J Cancer. 2016;139:1958–66.
White AJ, Keller JP, Zhao S, Carroll R, Kaufman JD, Sandler DP. Air Pollution, Clustering of Particulate Matter Components, and Breast Cancer in the Sister Study: A U.S.-Wide Cohort. Environ Health Perspect. 2019;127:107002.
Cheng I, Tseng C, Wu J, Yang J, Conroy SM, Shariff-Marco S, et al. Association between ambient air pollution and breast cancer risk: The multiethnic cohort study. Int J Cancer. 2020;146:699–711.
Wheeler DC, Boyle J, Barsell DJ, Maguire RL, Dahman B, Murphy SK, et al. Neighborhood Deprivation is Associated with Increased Risk of Prenatal Smoke Exposure. Prev Sci. 2022;23:1078–89.
Wheeler DC, Boyle J, Jeremy Barsell D, Maguire RL, Zhang JJ, Oliver JA, et al. Tobacco Retail Outlets, Neighborhood Deprivation and the Risk of Prenatal Smoke Exposure. Nicotine Tob Res. 2022;24:2003–10.
Brown AF, Ma GX, Miranda J, Eng E, Castille D, Brockie T, et al. Structural Interventions to Reduce and Eliminate Health Disparities. Am J Public Health. 2019;109:S72–8.
Krieger N. Embodiment: a conceptual glossary for epidemiology. J Epidemiol Community Health. 2005;59:350–5.
Lynch SM, Rebbeck TR. Bridging the gap between biologic, individual, and macroenvironmental factors in cancer: a multilevel approach. Cancer Epidemiol Biomark Prev. 2013;22:485–95.
Borrell LN, Elhawary JR, Fuentes-Afflick E, Witonsky J, Bhakta N, Wu AHB, et al. Race and Genetic Ancestry in Medicine - A Time for Reckoning with Racism. N Engl J Med. 2021;384:474–80.
Wheeler DC, Ward MH, Waller LA. Spatial-temporal Analysis of Cancer Risk in Epidemiologic Studies with Residential Histories. Ann Assoc Am Geogr. 2012;102:1049–52.
Marmot M, Friel S, Bell R, Houweling TA, Taylor S. Commission on Social Determinants of H. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet. 2008;372:1661–9.
Author information
Authors and Affiliations
Contributions
Statement: BFF provided an initial conceptualization and outline for the review. JS conducted initial and ongoing literature reviews. BFF, JS, and HZ contributed equally to drafts of the paper. RW and BFF jointly supervised the work and provided final editorial review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fuemmeler, B.F., Shen, J., Zhao, H. et al. Neighborhood deprivation, racial segregation and associations with cancer risk and outcomes across the cancer-control continuum. Mol Psychiatry 28, 1494–1501 (2023). https://doi.org/10.1038/s41380-023-02006-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02006-1
- Springer Nature Limited
This article is cited by
-
Prevalence and correlates of fear of recurrence among oral and oropharyngeal cancer survivors
Journal of Cancer Survivorship (2023)