Abstract
To examine ambient air pollutants, specifically polycyclic aromatic hydrocarbons (PAHs), as a factor in the geographic variation of breast cancer incidence seen in the US, we conducted an ecological study involving counties throughout the US to examine breast cancer incidence in relation to PAH emissions in ambient air. Age-adjusted incidence rates of female breast cancer from the surveillance, epidemiology, and end results (SEER) program of the US National Cancer Institute were collected and analyzed using SEER*Stat 8.3.2. PAH emissions data were obtained from the Environmental Protection Agency. Linear regression analysis was performed using SPSS 23 software for Windows to analyze the association between PAH emissions and breast cancer incidence, adjusting for potential confounders. Age-adjusted incidence rates of female breast cancer were found being significantly higher in more industrialized metropolitan SEER regions over the years of 1973–2013 as compared to less industrialized regions. After adjusting for sex, race, education, socioeconomic status, obesity, and smoking prevalence, PAH emission density was found to be significantly associated with female breast cancer incidence, with the adjusted β of 0.424 (95% CI 0.278, 0.570; p < 0.0001) for emissions from all sources and of 0.552 (95% CI 0.278, 0.826; p < 0.0001) for emissions from traffic source. This study suggests that PAH exposure from ambient air could play a role in the increased breast cancer risk among women living in urban areas of the US. Further research could provide insight into breast cancer etiology and prevention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is among the most commonly diagnosed cancers as 1 in 8 women in the US will develop invasive breast cancer during her lifetime [1]. The pathogenesis of breast cancer is complex involving both genetic and environmental aspects. Well-established risk factors include later age at first birth, nulliparity, and first-degree family history of breast cancer [2]. However, these factors only account for less than half of all breast cancer cases in the US population [3]. Additional influences such as hormone replacement therapy, radiation, and chemical exposure as well as lifestyle factors including diet, alcohol consumption, physical activity, and personal behavior have been found to play a role in the development of breast cancer [4,5,6]. However, geographic variation in breast cancer incidence throughout the US exists after accounting for these known risk factors [7,8,9]. Higher incidence rates have been observed in certain geographical regions, particularly in urban and industrialized areas [10, 11]. Multiple studies have proposed environmental pollutants as a factor and provided evidence for the association [12,13,14,15,16]. Specifically, traffic-related air pollution has been shown to be associated with breast cancer as a key factor in the incidence difference between rural and metropolitan areas [17, 18].
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous contaminants in ambient air generated from the incomplete combustion of organic material and are released primarily from industrial process and motor vehicular traffic [19]. They consist of two or more fused aromatic rings made up of carbon and hydrogen atoms and are found in both gas and particulate phases in ambient air. The United States Environmental Protection Agency (EPA) currently monitors 16 PAHs due to health concerns as these have been found to be carcinogenic in experimental animal models while benzo[a]pyrene specifically has been classified as a Group 1 known human carcinogen [20]. PAHs have been shown to disrupt BRCA-1 expression in estrogen receptor-positive breast cancer cells suggesting their role in the etiology of sporadic breast cancer development [19]. Benzo[a]pyrene has also demonstrated an ability to induce mutations and DNA damage while repressing DNA mismatch repair enzymes in human breast cancer cells presenting a role in the initiation of carcinogenesis [21, 22]. Recent population studies have demonstrated that PAH exposure may be a risk factor and suggested additional mechanisms for its association in breast cancer development [15, 17].
To our knowledge, there are no data evaluating PAH exposure as a factor in the nationwide geographic variation in breast cancer incidence. To further assess this difference in incidence throughout the US in relation to air pollution, we examined the association between ambient air emissions of PAHs and the incidence of female breast cancer.
Methods
We conducted an ecological study involving counties throughout the US to examine breast cancer incidence in relation to PAH emissions in ambient air. County-level data on incidence rates of female breast cancer and PAH emissions were obtained from national data sets and analyzed for the association.
Breast cancer incidence
Age-adjusted annual incidence rates of female breast cancer from the Surveillance, Epidemiology, and End Results (SEER) program of the US National Cancer Institute were collected and analyzed using SEER*Stat 8.3.2 [23]. The SEER 9 regions’ research data were utilized, which cover different regions of the US, including the states of Connecticut, Hawaii, Iowa, New Mexico, and Utah, as well as several metropolitan areas (San Francisco–Oakland, Atlanta, Detroit, and Seattle Puget Sound). Both region-level data and county-level data under each region were analyzed. There are a total of 200 counties. For our further analysis, we excluded Alameda, Contra Costa, Marin, San Francisco, and San Mateo counties in the San Francisco–Oakland region due to lack of data on PAH emissions from traffic sources. Additionally, Kalawao County of the Hawaii region was removed due to a possibly inaccurate breast cancer incidence data. The remaining 194 counties of the SEER regions were classified as metropolitan, micropolitan, and rural based on core population size as defined by the US Office of Management and Budget [24]. A metropolitan county contains a core urban area of 50,000 or more population, and a micropolitan county contains an urban core of at least 10,000 (but less than 50,000) population. All other counties, not classified as metropolitan or micropolitan, are designated as rural. Age-adjusted annual incidence rates of female breast cancer for the years 1973–2013 were analyzed based on the 2000 US standard population.
PAHs emissions
Data of PAH emissions from all emissions sectors as well as from traffic sources were collected from the 2008 National Emissions Inventory by the US EPA [25] for each of the 194 counties as designated by the SEER program. The emissions from motor vehicle traffic sources included on-road diesel and non-diesel vehicles. The PAHs measured include 1-methylnaphthalene, 2-methylnaphthalene, 5-methylchrysene, 7,12-dimethylbenz[a]anthracene, acenaphthene, acenaphthylene, anthracene, benz[a]anthracene, benzo(a)fluoranthene, benzo[a]pyrene, benzo[b]fluoranthene, benzo[e]pyrene, benzo[g,h,i,]perylene, benzo[k]fluoranthene, benzofluoranthenes, chrysene, dibenzo[a,h]anthracene, fluoranthene, fluorene, indeno[1,2,3-c,d]pyrene, perylene, phenanthrene, and pyrene. Total emissions of these compounds were summated for each county. Land area was collected from the US Census [26] for the year of 2010. The PAH emission density for each county was calculated as total PAH emissions divided by land area.
Covariates
Certain covariates known to be associated with the development of breast cancer were selected and controlled in order to better assess the relation between PAH emissions and the incidence of female breast cancer. These factors include sex, ethnicity, poverty, education, smoking, and obesity. The 2010 US Census [26] was used to obtain data on the demographic variables of the county, including percentage of females, percentage of non-Hispanic White persons, percentage of those living in poverty, and percentage of those with a high school education for the population of each county as designated by the SEER program [25]. Additionally, data on obesity and smoking prevalence for each county were obtained from the Institute for Health Metrics and Evaluation [27] for the year of 2011.
Statistical analysis
Data were analyzed using SPSS 23 for Windows to assess associations between PAH emissions and breast cancer incidence rates. Linear regression analyses were conducted with breast cancer incidence being the dependent variable and PAH emission density being the independent. Multiple models were constructed to assess the association with and without adjustment for the potential confounders: sex, ethnicity, poverty, education, obesity, and smoking. The regression coefficient (β) for changes in breast cancer incidence in association with PAH emission density was obtained from the analysis. A p value of less than 0.05 was considered statistically significant.
Results
In this study, we examined the difference in female breast cancer incidence among multiple regions in the US and evaluated the role of PAHs in ambient air emissions in the variations in breast cancer incidence in these areas by analyzing the SEER 9 regions’ research data and air pollutant emission data from EPA.
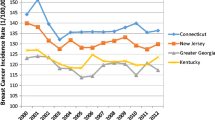
Figure 1 presents the annual incidence rates of female breast cancer for the years of 1973–2013 in the 9 SEER regions. Apart from 1974, overall breast cancer incidence remained low during the early years from 1973 to 1983. Incidence rates began to rise in 1984 until leveling off in 1988. Except for a peak around the year 2000, incidence rates have stayed fairly consistent in each region until 2013. There were statistically significant differences in breast cancer incidence among many of the regions from 1973 to 2013 (Table 1). The more industrialized metropolitan regions, San Francisco–Oakland, Connecticut, Atlanta, Detroit, and Seattle, had a significantly higher incidence of female breast cancer, as compared to less industrialized regions, Hawaii, Iowa, New Mexico, and Utah. Large variations in PAH emission density were also seen among the SEER regions, with a much higher emission density in metropolitan regions than the less industrialized regions (Table 1).
We then investigated the association between PAH emission density and breast cancer incidence in 194 counties of the SEER regions’ data. Linear regression analyses (Table 2) revealed a positive association between PAH emissions from all sources and breast cancer incidence with a regression coefficient β of 0.448 (95% CI 0.303, 0.594; p < 0.0001) in the unadjusted model (Model 1). After adjusting for possible demographic confounders including percentage of non-Hispanic White, percentage of female, poverty status, and percentage of high school education (Model 2), the association between PAH emission density and breast cancer incidence remained statistically significant (β = 0.377; 95% CI 0.231, 0.522; p < 0.0001). After further adjustment for smoking and obesity prevalence (Model 3), the β was 0.424 (95% CI 0.278, 0.570; p < 0.0001).
Since traffic is considered the major source of PAH emission, we further evaluated the association of PAH emissions from traffic sources with the incidence of breast cancer (Table 2). Linear regression analysis showed that the association of breast cancer incidence with PAH traffic emissions seemed stronger than PAH emissions from all sources. The β was 0.639 (95% CI 0.363, 0.914; p < .0001) in the unadjusted model, 0.476 (95% CI 0.206, 0.746; p = 0.001) after adjusting for demographic variables, and 0.552 (95% CI 0.278, 0.826; p < 0.0001) after additionally adjusting for smoking and obesity prevalence.
We further classified the 194 counties of the SEER regions into metropolitan, micropolitan, and rural groups as designated by the US Office of Management and Budget. It can be seen that there has been a large difference in the annual age-adjusted incidence rate of female breast cancer over the time span of 1973–2013 among the three sub-groups, with a higher incidence seen in the metropolitan area compared to the micropolitan and rural areas (Fig. 2). Figure 3 shows scatter plots of the correlation between PAH emission density and breast cancer incidence among the 194 counties sub-classified into metropolitan, micropolitan, and rural groups.
Discussion
The pathogenesis of breast cancer is known to be multifactorial with established risk factors including age, family history, parity, age at menstruation, and age at menopause, accounting for less than half of breast cancer cases [2, 4, 28]. Lifestyle factors such as diet, alcohol consumption, physical activity, and personal behavior have been implicated, but these additional factors cannot fully explain the geographic variation in breast cancer incidence seen in the US [5,6,7,8,9, 29]. Our study was conducted to analyze the association between environmental pollutants and breast cancer in the US to examine the role of PAHs in ambient air in the geographic variation in female breast cancer incidence. Our results support the findings of previous epidemiologic studies on the positive association between PAH exposure and breast cancer risk in women [12,13,14, 16, 17]. Traffic is a major source of PAH exposure in ambient air. Consistent with several studies [13, 16, 17], we found that PAH emissions specifically from motor vehicle traffic to be significantly associated with increased incidence of female breast cancer throughout the US.
As with previous studies, we observed a significant difference in breast cancer incidence geographically [15, 30]. Our study showed a consistently higher average incidence in regions designated as metropolitan from the years 1973–2013. These regions are known to be more industrialized and more heavily polluted than the micropolitan or rural regions analyzed. PAHs are ubiquitous contaminants in ambient air released through industrial process, motor vehicle traffic, residential heating, waste incineration, and natural sources [19]. The industrialized metropolitan areas were observed to have higher levels of PAH emission density and a higher incidence of female breast cancer when compared to the other regions. A population-based, case–control study conducted in New York found a similar association between early life exposure to total suspended particles, a proxy measure of PAH exposure, and an increased risk of breast cancer [12]. Additional studies in Montreal and Long Island, New York, observed an association between breast cancer incidence and PAH emissions specifically from vehicular traffic emissions [13, 17]. Several studies have proposed mechanisms of action for the role of PAH exposure in breast cancer development using in vitro, animal, and human models. These studies have demonstrated PAHs to be associated with PAH–DNA adducts, gene-specific promoter methylation, and altered Ah receptor signaling in breast tumors and potential mechanisms [19, 22, 31]. PAH exposure has been shown to increase cytochrome P4501A1 activation of chemical carcinogens with additional effects on COMT target genes [32]. Benzo[a]pyrene in particular is a group 1 known human carcinogen which has been shown to prevent BMP2-mediated differentiation of human mammary epithelial stem cells promoting the development of breast cancer [33]. Additionally, the lipophilic nature of aromatic compounds such as PAHs increases the amount of deposition in breast tissue further increasing the risk of breast cancer development [34]. Serologic studies have also found increased titers of antibodies against PAHs in patients with breast cancer [35].
This ecological study by analyzing county-level data on breast cancer incidence and emissions of PAHs provides more insight on the role of ambient air PAHs in the increased incidence of female breast cancer in the more industrialized urban areas. However, due to the limitations of an ecological study, we are unable to establish a causal relationship. Exposure to PAHs cannot be assessed at the individual level, and the potential confounding variables such as genetic background, socioeconomic status, and lifestyle factors, could not be controlled at individual levels when analyzing the association between PAH emissions and breast cancer incidence. As a result, we cannot rule out the effect of other factors playing a role in the differences in incidence between metropolitan, micropolitan, and rural areas. While our sample size was relatively large with 194 counties throughout the country, it is possible that these regions are not an accurate representation of urban and rural areas in the US as a whole. Finally, the National Emissions Inventory is based on voluntary reporting by states which potentially allows for inconsistencies in data collection state by state. Recent studies have shown geographic variation in breast cancer incidence to exist after accounting for known risk factors [9]. Thus, our current findings, together with previous studies [13,14,15, 18, 36] suggest that environmental air pollutants in the form of PAHs may contribute to these regional differences in breast cancer incidence.
Conclusions
Our study demonstrates an association between PAH emissions in ambient air and female breast cancer, which suggests PAH exposure to be a factor in the regional variation in breast cancer incidence seen in the US. Additional epidemiologic studies are needed to better evaluate the role of potentially carcinogenic air pollutants and the risk of breast cancer specifically in the metropolitan areas with higher emissions. Further research could provide insight into breast cancer etiology and prevention.
References
US Breast Cancer Statistics. 2016. http://www.breastcancer.org/symptoms/understand_bc/statistics.
Kelsey JL, Bernstein L. Epidemiology and prevention of breast cancer. Ann Rev Public Health. 1996;17:47–67.
Madigan MP, Ziegler RG, Benichou J, Byrne C, Hoover RN. Proportion of breast cancer cases in the United States explained by well-established risk factors. J Natl Cancer Inst. 1995;87:1681–5.
Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer: epidemiologic studies. Cancer. 2007;109:2667–711.
Brody JG, Rudel RA. Environmental pollutants and breast cancer. Environ Health Perspect. 2003;111:1007–19.
Johnson-Thompson MC, Guthrie J. Ongoing research to identify environmental risk factors in breast carcinoma. Cancer. 2000;88:1224–9.
Blot WJ, Fraumeni JF Jr, Stone BJ. Geographic patterns of breast cancer in the United States. J Natl Cancer Inst. 1977;59:1407–11.
Reynolds P, Hurley S, Goldberg DE, Anton-Culver H, Bernstein L, Deapen D, et al. Regional variations in breast cancer among california teachers. Epidemiology. 2004;15:746–54.
Boulos DN, Ghali RR, Ibrahim EM, Boulos MN, AbdelMalik P. An eight-year snapshot of geospatial cancer research (2002–2009): clinico-epidemiological and methodological findings and trends. Med Oncol. 2011;28:1145–62.
Parkin DM, Muir CS. Cancer Incidence in Five Continents. Comparability and quality of data. IARC Sci Publ. 1992(120):45–173.
Wei Y, Davis J, Bina WF. Ambient air pollution is associated with the increased incidence of breast cancer in US. Int J Environ Health Res. 2012;22:12–21.
Bonner MR, Han D, Nie J, Rogerson P, Vena JE, Muti P, et al. Breast cancer risk and exposure in early life to polycyclic aromatic hydrocarbons using total suspended particulates as a proxy measure. Cancer Epidemiol Biomarkers Prev. 2005;14:53–60.
Crouse DL, Goldberg MS, Ross NA, Chen H, Labreche F. Postmenopausal breast cancer is associated with exposure to traffic-related air pollution in Montreal, Canada: a case-control study. Environ Health Perspect. 2010;118:1578–83.
Garcia E, Hurley S, Nelson DO, Hertz A, Reynolds P. Hazardous air pollutants and breast cancer risk in California teachers: a cohort study. Environ Health. 2015;14:14.
Grant WB. Air pollution in relation to U.S. cancer mortality rates: an ecological study; likely role of carbonaceous aerosols and polycyclic aromatic hydrocarbons. Anticancer Res. 2009;29:3537–45.
Nie J, Beyea J, Bonner MR, Han D, Vena JE, Rogerson P, et al. Exposure to traffic emissions throughout life and risk of breast cancer: the Western New York Exposures and Breast Cancer (WEB) study. Cancer Causes Control. 2007;18:947–55.
Mordukhovich I, Beyea J, Herring AH, Hatch M, Stellman SD, Teitelbaum SL, et al. Vehicular traffic-related polycyclic aromatic hydrocarbon exposure and breast cancer incidence: The Long Island Breast Cancer Study Project (LIBCSP). Environ Health Perspect. 2016;124:30–8.
Parikh PV, Wei Y, PAHs. and PM2.5 emissions and female breast cancer incidence in metro Atlanta and rural Georgia. Int J Environ Health Res. 2016;26:458–66.
Jeffy BD, Chirnomas RB, Romagnolo DF. Epigenetics of breast cancer: polycyclic aromatic hydrocarbons as risk factors. Environ Mol Mutagen. 2002;39:235–44.
Dybing ESP, Nafstad P, Victorin K, Penning TM. Chapter 7. Polycyclic aromatic hydrocarbons in ambient air and cancer. IARC Sci Publ. 2013(161):75–94.
Chen Y, Huang C, Bai C, Gao H, Ma R, Liu X, et al. Benzo[alpha]pyrene repressed DNA mismatch repair in human breast cancer cells. Toxicology. 2013;304:167–72.
Roepstorff V, Ostenfeldt N, Autrup H. Extracts of airborne particulates collected at different locations in the Copenhagen area induce the expression of cytochrome P-450IA1. J Toxicol Environ Health. 1990;30:225–37.
Surveillance. Epidemiology, and end results (SEER) program. 2016. SEER*Stat Database: incidence—SEER 9 regs limited-use, Nov 2015 Sub (1973–2013).
Office of Management and Budget. 2010 Standards for delineating metropolitan and micropolitan statistical areas. Fed Reg. 2010;75:37246–52.
Environmental Protection Agency. National Emissions Inventory. 2008. https://www.epa.gov/air-emissions-inventories/2008-national-emissions-inventory-nei-data. Accessed June 2016.
US Census. 2010. https://www.census.gov/quickfacts/fact/table/US/PST045216. Accessed June 2016.
Institute for Health Metrics and Evaluation. 2012. http://www.healthdata.org/us-county-profiles. Accessed June 2016.
Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2:133–40.
Key TJ, Reeves GK. Alcohol, diet, and risk of breast cancer. BMJ. 2016;353:i2503.
Dey S, Soliman AS, Hablas A, Seifeldein IA, Ismail K, Ramadan M, et al. Urban-rural differences in breast cancer incidence in Egypt (1999–2006). Breast. 2010;19:417–23.
White AJ, Chen J, McCullough LE, Xu X, Cho YH, Teitelbaum SL, et al. Polycyclic aromatic hydrocarbon (PAH)-DNA adducts and breast cancer: modification by gene promoter methylation in a population-based study. Cancer Causes Control. 2015;26:1791–802.
Zajda K, Ptak A, Rak A, Fiedor E, Grochowalski A, Milewicz T, et al. Effects of human blood levels of two PAH mixtures on the AHR signalling activation pathway and CYP1A1 and COMT target genes in granulosa non-tumor and granulosa tumor cell lines. Toxicology. 2017;389:1–12.
Clement F, Xu X, Donini CF, Clement A, Omarjee S, Delay E, et al. Long-term exposure to bisphenol A or benzo(a)pyrene alters the fate of human mammary epithelial stem cells in response to BMP2 and BMP4, by pre-activating BMP signaling. Cell Death Differ. 2017;24:155 – 66.
Perera FP, Estabrook A, Hewer A, Channing K, Rundle A, Mooney LA, et al. Carcinogen-DNA adducts in human breast tissue. Cancer Epidemiol Biomarkers Prev. 1995;4:233–8.
Pouns O, Mangas A, Covenas R, Geffard M. Circulating antibodies directed against “polycyclic aromatic hydrocarbon-like” structures in the sera of cancer patients. Cancer Epidemiol. 2009;33:3–8.
Large C, Wei Y. Geographic variations in female breast cancer incidence in relation to ambient air emissions of polycyclic aromatic hydrocarbons. Environ Sci Pollut Res Int. 2017;24:17874–80.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Stults, W.P., Wei, Y. Ambient air emissions of polycyclic aromatic hydrocarbons and female breast cancer incidence in US. Med Oncol 35, 88 (2018). https://doi.org/10.1007/s12032-018-1150-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-018-1150-3