Abstract
Immune-checkpoint inhibitors have profoundly changed the treatment landscape for many tumor types. Despite marked improvements in disease control for highly immunogenic cancers, the clinical impact of checkpoint inhibitors in breast cancers to date is limited. Breast cancer is a heterogeneous disease with different levels of PD-L1 expression and variable tumor microenvironment (TME) composition according to molecular subtype. With emerging evidence of the role of different factors involved in immune evasion, there are promising new immunotherapy targets that will reshape early drug development for metastatic breast cancer. This review examines the available evidence for existing and emerging immuno-oncology (IO) approaches including small molecules targeting different regulators of the cancer-immunity cycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune-checkpoint inhibitors (ICIs) that target programmed cell death protein-1 (PD-1) or its ligand (PD-L1), as well as cytotoxic T-lymphocyte antigen 4 (CTLA-4) are now approved to treat multiple tumor types [1,2,3,4,5]. However, the overall response rate (ORR) as monotherapy in pre-treated metastatic breast cancer with PD-1 or PD-L1 ICI monotherapy is generally less than 20% [6, 7]. Breast cancer has historically been considered a non-immunogenic tumor [8]. Accordingly, the prevalence of predictive biomarkers of IO sensitivity, such as PD-L1 expression in tumor and/or immune cells and tumor mutation burden (TMB) is low, with differences according to molecular subtype, testing based on primary versus metastatic tumor tissue and assay characteristics [9,10,11].
Many factors are linked to the immunological quiescence observed in breast cancer, suggesting that more extensive biomarker profiling beyond TMB, tumor infiltrating lymphocytes (TILs) density and PD-L1 expression may be required to individualize immunotherapy treatments. Immunosuppressive cytokines including IL-6, IL-8 and TGF-β and tumor epithelial-to-mesenchymal transition (EMT) may have a role in tumor progression, metastatic spread, and immune resistance. Furthermore, different cells such as tumor-associated M2 macrophages, regulatory T (Treg) and B cells, myeloid derived suppressor cells (MDSC), or tumor-associated fibroblasts may also play a role in immune evasion [8]. Altogether, this may explain the limited response rates observed with single agent ICI in metastatic triple negative breast cancer (mTNBC). Immunotherapy combinations targeting different immunosuppressive elements within the TME may expand clinical responses to ICIs in metastatic breast cancer [12].
Thus far, PD-1 or PD-L1 ICIs in combination with chemotherapy are approved for the first-line treatment of PD-L1 expressing mTNBC. IMpassion130 showed a progression free survival (PFS) improvement in the intention-to-treat population for nab-paclitaxel and atezolizumab as compared to chemotherapy alone. Although a numerically higher median overall survival (OS) was observed among patients with PD-L1 positive tumors by immunohistochemistry, no significant differences were observed in OS in the intention-to-treat population [13, 14]. Notably, the recently reported negative results of the combination of atezolizumab and paclitaxel in the IMpassion131 raises the question of the best chemotherapy backbone to use with ICIs [15]. KEYNOTE-355 showed a PFS improvement for the combination of pembrolizumab plus either paclitaxel, nab-paclitaxel, or gemcitabine plus carboplatin as compared to placebo-chemotherapy in mTNBC patients with a PD-L1 immunohistochemistry combined positive score (CPS) ≥ 10% (CPS ≥ 10%) [16].
While these results are an important advance for patients with PD-L1 positive mTNBC, there are many mTNBC patients who do not benefit from ICI monotherapy or ICI+ chemotherapy. This situation, together with the fact that most patients with mTNBC receive poly-agent chemotherapy in the (neo)-adjuvant setting, has motivated the development of clinical trials testing ICIs in combination with molecularly targeted therapies to improve efficacy [13, 16]. There is current enthusiasm in the field for PARP inhibitor and ICIs combinations based upon promising early phase data for patients with germline BRCA1/2 mutations [17]. Larger studies are ongoing to examine PARP inhibitor and ICIs combinations as maintenance treatment in mTNBC [18, 19]. Androgen blockade has additionally been a focus of attention due to the negative role of the androgen pathway in T-cell activation [20,21,22]. Examples of combination treatments with androgen blockade and immunotherapy include (NCT03650894) and (NCT02971761). The PI3K/AKT/mTOR pathway plays a central role in the biology of specific molecular subtypes of TNBC [23, 24]. Moreover, the activation of this axis leads to recruitment of MDSCs, Tregs, and PD-L1 upregulation [25, 26]. IPI-549 is an inhibitor of the phosphoinositide-3-kinase (PI3K)-gamma that is currently being evaluated in addition to atezolizumab and nab-paclitaxel for patients with mTNBC [27]. Investigation of the AKT inhibitor ipatasertib in combination with chemotherapy, with or without atezolizumab was also pursued in mTNBC. Despite promising results in the Phase 1b (NCT03800836) with an ORR of 73% for the combination of ipatasertib, atezolizumab, and chemotherapy, the Phase III Ipatunity 170 (NCT04177108) did not reach its primary endpoint and has been terminated [28]. Similarly, the BEGONIA trial is currently studying the role of capivasertib, a small molecule inhibiting AKT, in combination with durvalumab and paclitaxel [29]. Another potential partner for ICI combinations are MEK inhibitors. In preclinical models, MEK inhibition has been shown to increase the cytotoxic effects of paclitaxel [30]. Furthermore, Ras/MAP pathway activation has been associated with immune evasion in mTNBC supporting combination strategies with ICIs [31]. Despite the preclinical rationale for combination testing, the COLET phase 2 trial did not show additive effect for the addition of cobimetinib to paclitaxel as compared to paclitaxel single agent or an increase in ORR for the combination of atezolizumab and cobimetinib with either paclitaxel or nab-paclitaxel [32, 33]. Lastly, different trials are studying the potential additive effects of antibody drug conjugates (ADCs) and ICIs combinations. In this respect, the initial results from the arm 6 of the BEGONIA trial testing durvalumab in combination with trastuzumab deruxtecan, were recently presented. Confirmed responses were observed in 8/12 evaluable patients [34]. Sacituzumab govitecan, an ADC against the Trop-2 antigen and ladiratuzumab vedotin in combination with pembrolizumab are currently being tested for mTNBC in two clinical trials (NCT04468061) and (NCT03310957).
The estrogen receptor (ER) positive (ER+)/HER2-negative (HER2−) and HER2-positive (HER2+) subtypes account for up to 90% of BC and lack effective FDA-approved immunotherapies [35]. An increased understanding of the specific genomic and molecular pathways associated with these subtypes has enabled the development of different immunotherapy combinations. To date, the efficacy of HER2-targeted therapies in combination with ICIs for metastatic HER2+ BC has been limited [36,37,38]. For ER+/HER2− breast tumors, lower responsiveness to IO agents have been observed as compared to mTNBC which could be secondary to lower TIL infiltration, lower values of TMB and PD-L1 expression [39,40,41,42]. While ICI+ chemotherapy is being investigated in ER+, disease specific treatment approaches in this molecular subtype include combinations of ICIs with either endocrine therapy or cyclin dependent kinase 4/6 (CDK-4/6) inhibitors [43,44,45,46].
All these examples suggest that more in-depth understanding of the molecular underpinnings leading to immune evasion in breast cancer may lead to the development of co-inhibitory or co-stimulatory agents that in addition to ICIs may help to overcome intrinsic immune resistance [8]. Selected results of clinical trials involving ICIs in combination with other targeted agents in later phases of development are summarized in Table 1 and are not the focus of this review. Herein, we describe emerging IO approaches in breast cancer, including novel therapeutic strategies based on the PD-1/PD-L1 axis and small molecules targeting different points of the cancer-immunity cycle in early stages of drug development.
Novel immunotherapies as single agents or in combination with ICIs
Drugs targeting co-inhibitory or co-stimulatory pathways
Co-inhibitory immune pathways
Anti-tumor responses are regulated by both stimulatory and inhibitory pathways that dictate antigen recognition by T-cells. Theoretically, the blockade of existent co-inhibitory pathways in the tumor and TME may enable responses in non-immunoreactive tumors [47]. In addition to PD-1/PD-L1 and cytotoxic T-lymphocyte-associated antigen 4 (CTLA4), other inhibitory molecules are being explored as potential targets in breast cancer. Indoleamine 2,3 dioxygenase 1 (IDO) is a tryptophan (Trp)-catabolizing enzyme released by both MDSC and tumor cells negatively impacting in T-cell function [48]. Pembrolizumab in combination with the IDO1 inhibitor epacadostat was tested in mTNBC where a 10% ORR was observed, not substantially different from the expected pembrolizumab monotherapy response rate [49].
CD73 is a cell surface enzyme that is expressed in TNBC and is associated with chemotherapy resistance, metastatic potential, and T-cell impairment. CD73 releases adenosine acting as a potent immunosuppressor in the TME [50,51,52]. There are ongoing studies evaluating CD73 inhibitors for mTNBC (NCT03454451, NCT04148937, and NCT03549000). Adenosine can increase in the TME due to the action of CD73. Subsequently, activation of the adenosine A2A receptor (A2AR) can eventually lead to inhibition of the effector function of NK and T-cells [53,54,55,56,57]. CPI-444 is an adenosine A2A receptor inhibitor currently under investigation as single agent or with atezolizumab in selected solid tumors including mTNBC [58]. Also, the phase I/II SYNERGY trial is evaluating the safety and efficacy of the combination of chemotherapy (paclitaxel + carboplatin) with durvalumab ± the anti-CD73 antibody oleclumab for patients with mTNBC [59]. Additionally, for early-stage luminal B breast cancer, oleclumab in addition to chemotherapy and radiotherapy is being investigated in the neoadjuvant setting prior to surgery [60]. Other A2AR inhibitors under investigation in TNBC include NIR178 and IPI-549 (NCT03742349 and NCT03719326, respectively).
Lymphocyte-Activation Gene 3 (LAG-3) is a marker of exhausted CD8+ T-cells [61, 62], which has generated substantial interest. LAG-3 binds to MHC Class II with more affinity than CD4+, acting as a negative regulator for T-cell expansion and promoting Treg function [63, 64]. The co-expression of both PD-L1 and LAG-3 in TILs for metastatic breast cancer supports the rational for treatment combinations [62]. Ongoing phase I clinical trials are evaluating anti-PD-1/L1 ICIs combined with LAG-3 inhibitors (NCT03250832). LAG525 (anti-LAG-3) is being explored in combination with the anti-PD-1 spartalizumab and carboplatin or with carboplatin alone for mTNBC (NCT03499899). A soluble dimeric recombinant form of LAG-3 (IMP321) is being explored in addition to standard chemotherapy in subjects with hormone receptor positive (HR+) metastatic breast cancer [65]. Additionally, the I-SPY trial is investigating the anti-LAG-3 REGN3767 with the anti-PD-1 cemiplimab in the neoadjuvant setting for operable breast cancer (NCT01042379).
TIGIT is a receptor of the Ig superfamily that when activated downregulates both adaptive and innate immune responses [66]. The phase I trial (NCT03628677) is investigating AB154 (domvanalimab) a monoclonal antibody targeting TIGIT alone or in combination with zimberelimab (anti-PD-1). B7-H4 is a PD-L1 family member that is also being tested as a rational partner for checkpoint inhibition. FPA150 is an anti-B7H4 antibody currently under investigation [67]. TIM-3 is an inhibitory receptor that binds to galectin-9 negatively impacting in IFN-gamma production by CD4+ T-cells. Preclinical data have shown synergistic effects by combining anti-PD-1 and TIM-3 blockade [68]. The phase I clinical trial (NCT02608268) studied the combination of MBG453 and spartalizumab in patients with solid tumors. The combination was well tolerated, but with an ORR of only 5% [69].
Co-stimulatory immune pathways
Multiple co-stimulatory molecules regulate T-cell responses against cancer cells [70]. Immunotherapy combinations including ICIs and agents providing agonistic signals through co-stimulatory molecules could re-establish immune surveillance [47]. OX40 and 4-1BB are members of the TNF receptor superfamily which contributes to T-cell activation and survival [71]. OX40 agonists in combination with anti-PD-1/PD-L1 agents are under investigation for mTNBC (NCT03971409). 4-1BB (also known as CD137) is a co-stimulatory immune checkpoint expressed in T-cells. The interaction with its ligand protects CD8+ T-cells from apoptosis, enhances effector functionalities and promotes memory cell differentiation ultimately leading to enhanced anti-tumor responses. Urelumab is a monoclonal antibody currently being tested in early phase clinical trials as single agent (NCT00309023) or in combination with nivolumab (NCT02253992 and NCT02534506) [72]. PRS-343 is a HER2/4-1BB bispecific construct that was investigated in a phase I clinical trial (NCT03330561) involving refractory HER2 + breast cancer. Treatment with PRS-343 was well tolerated with a 11% ORR [73].
STING (stimulator of interferon genes) has been identified as an important mediator of immune response, and STING agonists have been developed for the treatment of solid tumors. In breast cancer, STING agonists enhance innate and adaptive immune responses by driving the production of cytokines such as Type I interferons [74, 75]. Until now, mainly intratumoral administration of STING agonists has been explored given ubiquitous expression of cGAS/STING, narrow therapeutic index, and poor metabolic stability [76]. Intratumoral vaccination with 2′3′-cGAMP led to accumulation of macrophages and repolarization toward a M1 phenotype in preclinical models [77]. The phase I clinical trial CA046-006 (NCT03956680) is investigating the combination of an intratumoral STING agonist with nivolumab and ipilimumab in patients with solid tumors [78], including breast cancer. Additionally, ongoing Phase 1 trials have begun to explore intravenous STING agonists in different solid tumors including breast cancer (NCT04420884).
Dectin-1 is a pattern recognition receptor expressed in dendritic cells. Dectin-1 activation is fundamental for NK-mediated killing of tumor cells and polarization of CD4+ T-cells into Th9 cells [79]. Mechanistically, Th9 cells secrete interleukin (IL)-9 improving CD8+ T-cells responses [80]. Imprime PGG is an agonist of the dectin receptor. The IMPRIME 1 trial studied pembrolizumab and Imprime PGG in patients with mTNBC following progression on at least two prior lines of treatment. Mechanistically, Imprime PGG requires anti-beta glucan antibodies (ABA) to enhance anti-tumor immunity. Therefore, eligibility was restricted to patients with ABA IgG ≥ 20 mcg/ml. The ORR was 15.9% and the median OS was 16.4 months. Intriguingly, there appeared to be greater benefit for patients who were initially HR+ with 50% ORR, though given the small sample size and clinical heterogeneity of the cohort, the implications of this finding remain to be further characterized [81].
Oncolytic viruses
Oncolytic viruses are a promising approach to enhance T-cell infiltration. Oncolytic viruses are specifically engineered to replicate and lyse tumor cells minimizing their effects on normal tissues. Thus, expected effects include increasing T-cell priming, homing, and antagonism of immunosuppressive signals in the TME. Various non-pathogenic viruses have been employed for this purpose [82, 83]. Talimogene Laherparepvec (T-VEC) is a human herpes virus that induces the expression of colony-stimulating factor (GM-CSF) through viral replication in cancer cells producing anti-tumor immune responses [84, 85]. The combination of pembrolizumab and T-VEC was feasible in metastatic melanoma patients with a promising 62% ORR, exceeding the responses observed with either T-VEC or pembrolizumab as single agents [86, 87]. In localized TNBC, the addition of T-VEC to neoadjuvant chemotherapy based on weekly paclitaxel followed by doxorubicin/cyclophosphamide was safe, with a promising pathological complete response observed in five out of nine patients enrolled [88]. The ongoing SOLTI-1503 PROMETEO evaluates the combination of T-VEC with atezolizumab in patients with operable TNBC or Luminal B-like/HER2- breast cancer with residual disease after neoadjuvant chemotherapy. The primary end point of this study is to evaluate whether this strategy increases the expression of a T-cell gene signature [89].
Adoptive cell therapies
Adoptive cell therapy involves the transfer of therapeutic effector cells into a patient. One approach involves isolating TILs from the host, expansion and eventually infusion after lymphocyte-depleting chemotherapy. Proof-of-concept for this approach was demonstrated for a patient with refractory HR+ breast cancer who received TILs in combination with pembrolizumab [90]. The phase 1 clinical trial (NCT00027807) explored anti-CD3-activated T-cells in combination with IL-2 and anti-CD3/anti-HER2 bispecific antibody achieving a limited 5% ORR and manageable toxicity profile in HER2+ breast cancer [91]. Lastly, the (NCT00228358) study explored T-cell infusion primed with a HER2/neu vaccine. A promising ORR of 43% was observed [92]. The second approach within adoptive cell strategies, involves the use of chimeric antigen receptor (CAR)-T therapies. CAR-T cells are genetically modified T-cells to express either a tumor specific T-cell receptor (TCR) or a synthetic chimeric antigen receptor (CAR) that are able to identify specific antigens [93]. Several cancer antigens are currently being evaluated as potential targets [94]. For example, mesothelin is overexpressed in 36% of TNBC [95]. Clinical trials involving mesothelin-CAR-T cells enrolling breast cancer patients are ongoing (NCT02792114) and (NCT02414269) [96].
Interleukins in breast cancer
Cytokines are small peptides or glycoproteins with a short half-life released in response to different stimuli resulting in intracellular signaling and transcription modifications. Cytokines can have either tumor promoting or anti-cancer effects [97]. Recent optimization of the pharmacodynamic and pharmacokinetic profiles of cytokine-based agents has led to an increased interest in the development of clinical trials of cytokines in combination with ICIs or other immunotherapy agents [98]. There are a variety of novel therapeutic cytokines that are being evaluated in breast cancer clinical trials. The phase Ib/II (NCT01131364) explored the safety and tolerability of the F16-IL2 an antibody-cytokine fusion protein in combination with doxorubicin in metastatic breast cancer with an observed clinical benefit rate (CBR) defined as disease stability at 8 months of 57%. Targeting IL-7 has also been studied [99]. In the phase Ib/II KEYNOTE-899 trial, GX-I7, a long-acting IL-7, was evaluated in combination with pembrolizumab mTNBC. The toxicity profile was favorable, however, ORR was only 5.9%. IRX-, a biologic of physiologically derived T-helper type 1 cytokines, was studied as intratumoral injection in 16 patients with early-stage breast cancer before surgical resection. This approach was feasible and upregulation of RNA inflammatory signatures and TILs were observed [100].
Bispecific antibodies
Bispecific antibodies are proteins with at least two epitope recognizing sequences [101]. Molecular characterization of breast cancer subtypes has facilitated the development of bispecific antibodies engineered to bind to tumor specific antigens and receptors on T-cell surface [102]. TGF- β is a pleiotropic cytokine with a role in immune evasion in breast cancer [103]. M7824 targeting PD-L1 and TGF-β, is currently under investigation for localized HER2+ breast cancer (NCT03620201). KN046 is a bispecific antibody that blocks PD-L1 and CTLA-4 by interaction with PD-1 and CD80/86. The preliminary results of the (NCT03733951) were presented in ASCO 2020 for patients with nasopharynx cancer or non-small cell lung cancer. Objective responses were observed in 3 patients out of 26 (12%) [104]. Currently, NCT03872791 is evaluating KN046 alone or in combination with nab-paclitaxel in patients with mTNBC. MGD013 is a dual-affinity retargeting protein (DART) targeting both PD-1 and LAG-3 [105]. Out of 23 patients with mTNBC, 2 partial responses were observed [106].
Probody
Probody therapeutics are proteolytically activated antibody prodrugs designed to be activated and to target specific receptors in the TME rather than in peripheral tissues [107]. In this regard, the results of the PROCLAIM-CX-072 phase I clinical trial described the safety and efficacy of the CX-072 a PD-L1 probody therapeutic as single agent or in combination with Ipilimumab in patients with solid tumors. 3 out of 12 patients with mTNBC responded when used as monotherapy, confirming single agent activity, and with response rate similar to established PD-1/L1 inhibitors. CX-072 both in monotherapy and in combination with ipilimumab was well tolerated with few immune related adverse events reported [108].
Cancer vaccines
T-cell priming, an initial step in the cancer-immunity cycle, can be enhanced by the use of cancer vaccines. Breast cancer vaccines have been designed by using different platforms with the ultimate goal of activating T-cells to recognize cancer cells in a variety of settings including disease prevention, interception of minimal residual disease in the adjuvant setting, and treatment of metastatic disease [109, 110]. MAG-Tn3 glycopeptide vaccine was engineered to activate CD4+ T-cell responses by binding to a wide range of HLA-DRB molecules. MAG-Tn3 was well tolerated and induced humoral responses [111]. The P10s-PADRE vaccine was designed to induce functional antibodies against carbohydrate antigens (TACAs) for patients with metastatic breast cancer. In the (NCT01390064) phase 1 clinical trial, IgG and IgM anti-P10 were long lasting and persisted high one year after vaccination [112]. Additional trials have explored using HER2 as a target for vaccine design including strategies based in the HER2 protein [113, 114] or relying in viral vectors to HER2-specific cell-mediated or humoral immunity [115, 116]. Recently, synthetic mRNA has emerged as novel vaccine format [117]. In this respect, TNBC-MERIT (NCT02316457) is a phase 1 clinical trial assessing the safety and tolerability of a liposome-formulated intravenous RNA vaccine encoding different tumor antigens in patients with early-stage TNBC [118].
Epigenetic drugs
Some described mechanisms of resistance to ICIs may be reverted with the use of epigenetic modulation [119]. Lysine-specific histone demethylase 1A (LSD1) inhibitors, DNA methyl-transferase (DNMT) inhibitors, and histone deacetylase (HDAC) inhibitors can impact on adaptive immunity by inducing dsRNA production from endogenous retrovirus genes leading to a type I interferon response [120,121,122]. Moreover, DNMT inhibitors are also known to influence chemokine production increasing the expression of CXCL12, a key regulator of T-cell homing [123]. Other examples include increased production of CXCL9 and CXCL10 chemokines by enhancer of zeste homologue 2 (EZH2) modulation [124].
Different approaches combining epigenetic modifiers and immunotherapy have been tested in breast cancer [125]. In ER+ endocrine-resistant metastatic breast cancer, a Phase II trial (NCT02395627) tested the combination of tamoxifen, vorinostat (HDAC inhibitor), and pembrolizumab. CBR defined as the sum of partial response and stable disease for more than 6 months was observed in 5 out of 28 patients (18%) [126]. The ENCORE 602 (TRIO025) Phase II trial tested atezolizumab with or without entinostat, an oral class I-selective HDAC. This trial did not show a benefit for entinostat and atezolizumab compared to atezolizumab alone (HR: 0.87, 95% CI 0.52–1.46; p = 0.59) [127]. Lastly, the investigator initiated METADUR trial studied the combination of the PD-L1 ICI durvalumab and the hypomethylating agent oral azacitidine (CC-486) in ER+/HER2- breast cancer. This combination was shown to be safe, however, no clinical activity and limited pharmacodynamic changes were observed. Consequently, the underlying basis for the combination strategy could not be evaluated [128].
Immune targeted kinase inhibitors
Small molecules targeting pathways related to T-cell inhibitory signals or immunosuppressive cells in the TME are a feasible approach and a very attractive partner for immunotherapy combinations. As compared to ICIs, small molecules can penetrate into the TME and be directed to different intracellular proteins. Several tyrosine kinase inhibitors (TKIs) in combination with ICIs are currently under investigation [129]. Among them, HPK1 inhibitors have the potential to become a successful approach by acting in multiple steps of the cancer-immunity cycle [130,131,132,133]. HPK1 activation is linked to the MAPK/NfKB pathways and plays a negative regulatory role in T-cell activation and cell adhesion regulation. Notably, HPK1 inhibition resulted in increased IL-2 production in mouse T-cells [131]. CFI-402411 is an oral immunomodulatory kinase inhibitor currently being tested in a Phase 1 trial (NCT04521413) alone or in combination with pembrolizumab in patients with advanced solid tumors. BGB-15025 is another example of HPK1 inhibitor currently under study as single agent and in combination with anti-PD-1 (NCT04649385).
Conclusions
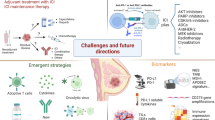
While PD-1/L1 inhibitors added to chemotherapy have established the first clinical role for immunotherapy in PD-L1 + mTNBC and high risk early TNBC, this strategy is currently relevant only to a minority of breast cancer patients [13, 16, 134, 135]. Substantial unmet need remains for advanced disease with lower level or absent PD-L1 expression, and other subtypes of breast cancer where checkpoint inhibitor therapy, alone or in combination with standard chemotherapy, has not been shown to improve treatment outcomes. As novel IO compounds enter into the clinical arena of breast cancer drug development, there are important questions about whether personalized interventions with doublets or triplets may be capable of overcoming immune resistance. There are many steps in the cancer-immunity cycle that might be susceptible to modification by novel IO compounds seeking to restore immune surveillance Fig. 1. Considering the complexity and variability of tumor-host immune interactions, as well as the observed differences in anti-tumor activity with ICIs in advanced and early-stage disease breast cancer according to PD-L1 expression, “one-size-fits-all” treatment approaches are unlikely to be successful. The development of robust predictive biomarkers will be required to individualize IO interventions in breast cancer.
Selected novel therapeutics currently being tested in the immuno-oncology field and its interaction with elements of the immune-cell cycle ([138].
The recent success of immunotherapy and chemotherapy combinations and the relatively limited efficacy of ICIs as monotherapy in breast cancer provides many important lessons to guide future drug development. First, better outcomes have been observed in advanced TNBC with both ICIs as monotherapy or in combination with chemotherapy in patients selected by PD-L1 expression and this marker may be considered a surrogate of ongoing immunologic activation [13, 136, 137]. Second, the additive benefit of immunotherapy appears to be greater in early-stage disease and may not differ based upon PD-L1 expression [134]. Third, there are clinical factors, such as absence of liver metastases, low lactate dehydrogenase (LDH) levels, and lymph node predominant metastatic disease, associated with an increased responsiveness to immunotherapy in the metastatic setting [7]. However, several unanswered questions remain from the available clinical data, such as whether loss of hormone receptor expression during evolution from localized to metastatic disease converts a non-immunogenic microenvironment to an immunogenic microenvironment and whether single-agent PD-1/L1 inhibitors without chemotherapy might be effective in a subset of patients with immunogenic TNBC to minimize chemotherapy-associated side effects. Moreover, mechanisms of both intrinsic resistance and acquired resistance to ICIs in breast cancer are largely unknown. Particularly with the adoption of immunotherapy for early disease, it will be important to understand which approaches may be relevant in second line settings for patients who initially respond to the standard first-line treatments. Biomarkers of acquired resistance are critical to develop rational combinations for the second line setting and beyond.
All of the above suggest that future immunotherapy combinations should take into account the highly complex TME in breast cancer and incorporate biomarkers for patient selection. Establishing clear signals of activity for novel combinations with PD-1 or PD-L1 ICIs is challenging. To date, most studies include small, single-arm cohorts of highly selected patients with refractory breast cancer. With few responses observed, it is difficult to determine whether there is synergistic or even additive activity when the expected response rate to PD-1 or PD-L1 ICI monotherapy is 10–20% and is highly dependent on patient selection. Establishing proof of mechanism a priori benchmarks of pharmacodynamic activity will be critical to determine which combinations should be accelerated to randomized clinical trials.
We envision a future immunotherapy landscape in breast cancer with greater individualization of treatment based upon characterization of targetable tumor alterations, understanding of host-related factors and TME interactions, ongoing response monitoring and identification of adaptive resistance mechanisms.
Data Availability
NA.
Code availability
NA.
References
Burtness B, Harrington KJ, Greil R et al (2019) Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. https://doi.org/10.1016/S0140-6736(19)32591-7
Reck M, Rodríguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833. https://doi.org/10.1056/nejmoa1606774
Larkin J, Chiarion-Sileni V, Gonzalez R et al (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373(1):23–34. https://doi.org/10.1056/nejmoa1504030
Motzer RJ, Tannir NM, McDermott DF et al (2018) Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378(14):1277–1290. https://doi.org/10.1056/NEJMoa1712126
Powles T, Durán I, van der Heijden MS et al (2018) Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 391(10122):748–757. https://doi.org/10.1016/S0140-6736(17)33297-X
Adams S, Schmid P, Rugo HS et al (2019) Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann Oncol 30(3):397–404. https://doi.org/10.1093/annonc/mdy517
Emens LA, Cruz C, Eder JP et al (2019) Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol 5(1):74–82. https://doi.org/10.1001/jamaoncol.2018.4224
Gatti-Mays ME, Balko JM, Gameiro SR et al (2019) If we build it they will come: targeting the immune response to breast cancer. npj Breast Cancer. https://doi.org/10.1038/s41523-019-0133-7
Cimino-Mathews A, Thompson E, Taube JM et al (2016) PD-L1 (B7–H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol 47(1):52–63. https://doi.org/10.1016/j.humpath.2015.09.003
Sobral-Leite M, Van de Vijver K, Michaut M et al (2018) Assessment of PD-L1 expression across breast cancer molecular subtypes, in relation to mutation rate, BRCA1 -like status, tumor-infiltrating immune cells and survival. Oncoimmunology 7(12):e1509820. https://doi.org/10.1080/2162402X.2018.1509820
Cristescu R, Mogg R, Ayers M et al (2018) Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. https://doi.org/10.1126/science.aar3593
Page DB, Bear H, Prabhakaran S et al (2019) Two may be better than one: PD-1/PD-L1 blockade combination approaches in metastatic breast cancer. npj Breast Cancer 5(1):1–9. https://doi.org/10.1038/s41523-019-0130-x
Schmid P, Adams S, Rugo HS et al (2018) Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379(22):2108–2121. https://doi.org/10.1056/nejmoa1809615
Emens LA, Adams S, Barrios CH et al (2020) LBA16 IMpassion130: final OS analysis from the pivotal phase III study of atezolizumab + nab-paclitaxel vs placebo + nab-paclitaxel in previously untreated locally advanced or metastatic triple-negative breast cancer. Ann Oncol 31:S1148. https://doi.org/10.1016/j.annonc.2020.08.2244
Miles D, Gligorov J, André F et al (2021) Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol 32(8):994–1004. https://doi.org/10.1016/j.annonc.2021.05.801
Cortes J, Cescon DW, Rugo HS et al (2020) Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396(10265):1817–1828. https://doi.org/10.1016/S0140-6736(20)32531-9
Vinayak S, Tolaney SM, Schwartzberg LS et al (2018) TOPACIO/Keynote-162: Niraparib + pembrolizumab in patients (pts) with metastatic triple-negative breast cancer (TNBC), a phase 2 trial. J Clin Oncol 36(15_suppl):1011–1011. https://doi.org/10.1200/JCO.2018.36.15_suppl.1011
Rugo HS, Llombart-Cussac A, Andre F et al (2020) KEYLYNK-009: A phase II/III, open-label, randomized study of pembrolizumab (pembro) plus olaparib vs pembro plus chemotherapy after induction with first-line pembro plus chemotherapy in patients with locally recurrent inoperable or metastatic triple-negative breast cancer (TNBC). J Clin Oncol 38(15_suppl):TPS596–TPS596. https://doi.org/10.1200/JCO.2020.38.15_suppl.TPS596
Sammons S, Tan TJY, Traina TA et al (2019) Dora: A randomized phase II multicenter maintenance study of olaparib alone or olaparib in combination with durvalumab in platinum responsive advanced triple-negative breast cancer (aTNBC). J Clin Oncol 37:1113. https://doi.org/10.1200/JCO.2019.37.15_suppl.TPS1113
Velardi E, Tsai JJ, Holland AM et al (2014) Sex steroid blockade enhances thymopoiesis by modulating Notch signaling. J Exp Med 211(12):2341–2349. https://doi.org/10.1084/jem.20131289
Bishop JL, Sio A, Angeles A et al (2015) PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget 6(1):234–242. https://doi.org/10.18632/oncotarget.2703
Kwilas AR, Ardiani A, Gameiro SR, Richards J, Hall AB, Hodge JW (2016) Androgen deprivation therapy sensitizes triple negative breast cancer cells to immune-mediated lysis through androgen receptor independent modulation of osteoprotegerin. Oncotarget 7(17):23498–23511. https://doi.org/10.18632/oncotarget.8274
Walsh S, Flanagan L, Quinn C et al (2012) mTOR in breast cancer: differential expression in triple-negative and non-triple-negative tumors. Breast 21(2):178–182. https://doi.org/10.1016/j.breast.2011.09.008
Kriegsmann M, Endris V, Wolf T et al (2014) Mutational profiles in triple-negative breast cancer defined by ultradeep multigene sequencing show high rates of PI3K pathway alterations and clinically relevant entity subgroup specific differences. Oncotarget 5(20):9952–9965. https://doi.org/10.18632/oncotarget.2481
Valabrega G, Montemurro F, Aglietta M (2007) Trastuzumab: Mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol 18(6):977–984. https://doi.org/10.1093/annonc/mdl475
Groth C, Hu X, Weber R et al (2019) Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer 120(1):16–25. https://doi.org/10.1038/s41416-018-0333-1
Hamilton E, Lee A, Swart R et al (2021) Abstract PS11-32: Mario-3 phase II study safety run-in evaluating a novel triplet combination of eganelisib (formerly IPI-549), atezolizumab (atezo), and nab-paclitaxel (nab-pac) as first-line (1L) therapy for locally advanced or metastatic triple-negative breast cancer (TNBC). Poster Session Abstracts. https://doi.org/10.1158/1538-7445.SABCS20-PS11-32
No Title (2021) https://www.roche.com/dam/jcr:3bc1f151-1def-483b-868c-8bd1ef59ccc4/en/irp210204-a.pdf
Schmid P, Nunes A, Lall R, D’Cruz C, Grinsted L, Lanasa M (2019) Abstract OT3–01–01: BEGONIA: Phase Ib/II open-label, platform study of safety and efficacy of durvalumab, paclitaxel and other novel oncology therapy agents as first-line (1L) therapy in patients with metastatic triple negative breast cancer (mTNBC). Ongoing Clin Trials. https://doi.org/10.1158/1538-7445.SABCS18-OT3-01-01
McDaid HM, Horwitz SB (2001) Selective potentiation of paclitaxel (taxol)-induced cell death by mitogen-activated protein kinase kinase inhibition in human cancer cell lines. Mol Pharmacol 60(2):290–301. https://doi.org/10.1124/mol.60.2.290
Loi S, Dushyanthen S, Beavis PA et al (2016) RAS/MAPK activation is associated with reduced tumor-infiltrating lymphocytes in triple-negative breast cancer: therapeutic cooperation between MEK and PD-1/PD-L1 immune checkpoint inhibitors. Clin Cancer Res 22(6):1499–1509. https://doi.org/10.1158/1078-0432.CCR-15-1125
Brufsky A, Kim S-B, Zvirbule Z et al (2019) Phase II COLET study: Atezolizumab (A) + cobimetinib (C) + paclitaxel (P)/nab-paclitaxel (nP) as first-line (1L) treatment (tx) for patients (pts) with locally advanced or metastatic triple-negative breast cancer (mTNBC). J Clin Oncol 37:1013–1013. https://doi.org/10.1200/JCO.2019.37.15_suppl.1013
Brufsky A, Kim SB, Zvirbule Ž et al (2021) A phase II randomized trial of cobimetinib plus chemotherapy, with or without atezolizumab, as first-line treatment for patients with locally advanced or metastatic triple-negative breast cancer (COLET): primary analysis. Ann Oncol. https://doi.org/10.1016/j.annonc.2021.01.065
Schmid P, Im S-A, Armstrong A et al (2021) BEGONIA: Phase 1b/2 study of durvalumab (D) combinations in locally advanced/metastatic triple-negative breast cancer (TNBC)—Initial results from arm 1, d+paclitaxel (P), and arm 6, d+trastuzumab deruxtecan (T-DXd). J Clin Oncol 39:1023–1023. https://doi.org/10.1200/JCO.2021.39.15_suppl.1023
Kong X, Liu Z, Cheng R et al (2020) Variation in breast cancer subtype incidence and distribution by race/ethnicity in the United States from 2010 to 2015. JAMA Netw open 3(10):e2020303. https://doi.org/10.1001/jamanetworkopen.2020.20303
Loi S, Giobbie-Hurder A, Gombos A et al (2019) Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b–2 trial. Lancet Oncol 20(3):371–382. https://doi.org/10.1016/S1470-2045(18)30812-X
Chia S, Bedard PL, Hilton J et al (2019) A Phase Ib Trial of Durvalumab in Combination with Trastuzumab in HER2-Positive Metastatic Breast Cancer (CCTG IND229). Oncologist 24(11):1439–1445. https://doi.org/10.1634/theoncologist.2019-0321
Emens LA, Esteva FJ, Beresford M et al (2020) Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol 21(10):1283–1295. https://doi.org/10.1016/S1470-2045(20)30465-4
Terranova-Barberio M, Pawlowska N, Dhawan M et al (2020) Exhausted T cell signature predicts immunotherapy response in ER-positive breast cancer. Nat Commun. https://doi.org/10.1038/s41467-020-17414-y
Denkert C, von Minckwitz G, Darb-Esfahani S et al (2018) Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 19(1):40–50. https://doi.org/10.1016/S1470-2045(17)30904-X
Luen S, Virassamy B, Savas P, Salgado R, Loi S (2016) The genomic landscape of breast cancer and its interaction with host immunity. Breast 29:241–250. https://doi.org/10.1016/j.breast.2016.07.015
Mittendorf EA, Philips AV, Meric-Bernstam F et al (2014) PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res 2(4):361–370. https://doi.org/10.1158/2326-6066.CIR-13-0127
Tolaney SM, Barroso-Sousa R, Keenan T et al (2020) Effect of eribulin with or without pembrolizumab on progression-free survival for patients with hormone receptor-positive, ERBB2-negative metastatic breast cancer: a randomized clinical trial. JAMA Oncol 6(10):1598–1605. https://doi.org/10.1001/jamaoncol.2020.3524
Yardley D, Abu-Khalaf M, Boni V et al (2019) Abstract OT2–06–04: MORPHEUS: a phase Ib/II trial platform evaluating the safety and efficacy of multiple cancer immunotherapy combinations in patients with hormone receptor–positive and triple-negative breast cancer. Ongoing Clin Trials. https://doi.org/10.1158/1538-7445.SABCS18-OT2-06-04
Rugo HS, Kabos P, Beck JT et al (2020) A phase Ib study of abemaciclib in combination with pembrolizumab for patients with hormone receptor positive (HR+), human epidermal growth factor receptor 2 negative (HER2-) locally advanced or metastatic breast cancer (MBC) (NCT02779751): Interim result. J Clin Oncol 38:1051–1051. https://doi.org/10.1200/JCO.2020.38.15_suppl.1051
Yuan Y, Yost SE, Lee JS, et al (2020) Abstract P3–11–04: A phase II study of pembrolizumab, letrozole and palbociclib in patients with metastatic estrogen receptor positive breast cancer. In: Poster Session Abstracts. American Association for Cancer Research; 2020:P3–11–04-P3–11–04. https://doi.org/10.1158/1538-7445.SABCS19-P3-11-04
Sharma P, Allison JP (2015) The future of immune checkpoint therapy. Science 348(6230):56–61. https://doi.org/10.1126/science.aaa8172
Schafer CC, Wang Y, Hough KP et al (2016) Indoleamine 2,3-dioxygenase regulates anti-tumor immunity in lung cancer by metabolic reprogramming of immune cells in the tumor microenvironment. Oncotarget 7(46):75407–75424. https://doi.org/10.18632/oncotarget.12249
Spira AI, Hamid O, Bauer TM et al (2017) Efficacy/safety of epacadostat plus pembrolizumab in triple-negative breast cancer and ovarian cancer: phase I/II ECHO-202 study. J Clin Oncol 35:1103–1103. https://doi.org/10.1200/JCO.2017.35.15_suppl.1103
Stagg J, Divisekera U, Duret H et al (2011) CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res 71(8):2892–2900. https://doi.org/10.1158/0008-5472.CAN-10-4246
Loi S, Pommey S, Haibe-Kains B et al (2013) CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci USA 110(27):11091–11096. https://doi.org/10.1073/pnas.1222251110
Stagg J, Divisekera U, McLaughlin N et al (2010) Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci USA 107(4):1547–1552. https://doi.org/10.1073/pnas.0908801107
Ohta A, Gorelik E, Prasad SJ et al (2006) A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA 103(35):13132–13137. https://doi.org/10.1073/pnas.0605251103
Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z (2011) Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol 187(2):676–683. https://doi.org/10.4049/jimmunol.1003884
Novitskiy SV, Ryzhov S, Zaynagetdinov R et al (2008) Adenosine receptors in regulation of dendritic cell differentiation and function. Blood 112(5):1822–1831. https://doi.org/10.1182/blood-2008-02-136325
Csóka B, Himer L, Selmeczy Z et al (2008) Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function. FASEB J 22(10):3491–3499. https://doi.org/10.1096/fj.08-107458
Willingham SB, Ho PY, Hotson A et al (2018) A2AR Antagonism with CPI-444 induces antitumor responses and augments efficacy to anti-PD-(L)1 and anti-CTLA-4 in preclinical models. Cancer Immunol Res 6(10):1136–1149. https://doi.org/10.1158/2326-6066.CIR-18-0056
Fong L, Forde PM, Powderly JD et al (2017) Safety and clinical activity of adenosine A2a receptor (A2aR) antagonist, CPI-444, in anti-PD1/PDL1 treatment-refractory renal cell (RCC) and non-small cell lung cancer (NSCLC) patients. J Clin Oncol 35:3004–3004. https://doi.org/10.1200/JCO.2017.35.15_suppl.3004
Eiger D, Maurer C, Brandao M et al (2020) 348P First findings from SYNERGY, a phase I/II trial testing the addition of the anti-CD73 oleclumab (O) to the anti-PD-L1 durvalumab (D) and chemotherapy (ChT) as first line therapy for patients (pts) with metastatic triple-negative breast cancer (mTNBC). Ann Oncol 31:S386–S387. https://doi.org/10.1016/j.annonc.2020.08.450
De Caluwé A, Buisseret L, Poortmans P et al (2021) Neo-CheckRay: radiation therapy and adenosine pathway blockade to increase benefit of immuno-chemotherapy in early stage luminal B breast cancer, a randomized phase II trial. BMC Cancer 21(1):899. https://doi.org/10.1186/s12885-021-08601-1
Goldberg MV, Drake CG (2011) LAG-3 in cancer immunotherapy. Curr Top Microbiol Immunol 344:269–278. https://doi.org/10.1007/82_2010_114
Burugu S, Gao D, Leung S, Chia SK, Nielsen TO (2017) LAG-3+ tumor infiltrating lymphocytes in breast cancer: clinical correlates and association with PD-1/PD-L1+ tumors. Ann Oncol 28(12):2977–2984. https://doi.org/10.1093/annonc/mdx557
Workman CJ, Cauley LS, Kim I-J, Blackman MA, Woodland DL, Vignali DAA (2004) Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol 172(9):5450–5455. https://doi.org/10.4049/jimmunol.172.9.5450
Workman CJ, Vignali DAA (2005) Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223). J Immunol 174(2):688–695. https://doi.org/10.4049/jimmunol.174.2.688
Dirix L, Wildiers H, Huizing MT, et al (2020) Abstract OT1–01–05: AIPAC (Active Immunotherapy PAClitaxel): A randomized, double blind, placebo controlled, multinational phase IIb trial evaluating the efficacy of eftilagimod alpha (a soluble LAG-3 fusion protein) in combination with paclitaxel in hormone receptor positive metastatic breast cancer. American Association for Cancer Research:OT1–01–05-OT1–01–05. https://doi.org/10.1158/1538-7445.SABCS19-OT1-01-05
Chauvin J-M, Zarour HM (2020) TIGIT in cancer immunotherapy. J Immunother Cancer 8(2):e000957. https://doi.org/10.1136/jitc-2020-000957
Sachdev JC, Bauer TM, Chawla SP et al (2019) Phase 1a/1b study of first-in-class B7–H4 antibody, FPA150, as monotherapy in patients with advanced solid tumors. J Clin Oncol 37:2529–2529. https://doi.org/10.1200/JCO.2019.37.15_suppl.2529
Wolf Y, Anderson AC, Kuchroo VK (2020) TIM3 comes of age as an inhibitory receptor. Nat Rev Immunol 20(3):173–185. https://doi.org/10.1038/s41577-019-0224-6
Curigliano G, Gelderblom H, Mach N et al (2019) Phase (Ph) I/II study of MBG453± spartalizumab (PDR001) in patients (pts) with advanced malignancies. Am Assoc Cancer Res. https://doi.org/10.1158/1538-7445.AM2019-CT183
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4):252–264. https://doi.org/10.1038/nrc3239
Murphy K, Weaver C (2017) Janeway ’ S 9 Th Edition
Segal NH, Logan TF, Hodi FS et al (2017) Results from an integrated safety analysis of urelumab, an agonist anti-CD137 monoclonal antibody. Clin Cancer Res 23(8):1929–1936. https://doi.org/10.1158/1078-0432.CCR-16-1272
Piha-Paul S, Bendell J, Tolcher A et al (2020) O82 A phase 1 dose escalation study of PRS-343, a HER2/4–1BB bispecific molecule, in patients with HER2-positive malignancies. J Immunother Cancer. https://doi.org/10.1136/LBA2019.2
Chin EN, Yu C, Vartabedian VF et al (2020) Antitumor activity of a systemic STING-activating non-nucleotide cGAMP mimetic. Science 369(6506):993–999. https://doi.org/10.1126/science.abb4255
Pan B-S, Perera SA, Piesvaux JA et al (2020) An orally available non-nucleotide STING agonist with antitumor activity. Science 369(6506):6098. https://doi.org/10.1126/science.aba6098
Gajewski TF, Higgs EF (2020) Immunotherapy with a sting. Science 369(6506):921–922. https://doi.org/10.1126/science.abc6622
Su T, Zhang Y, Valerie K, Wang X-Y, Lin S, Zhu G (2019) STING activation in cancer immunotherapy. Theranostics 9(25):7759–7771. https://doi.org/10.7150/thno.37574
Cheng N, Watkins-Schulz R, Junkins RD et al (2018) A nanoparticle-incorporated STING activator enhances antitumor immunity in PD-L1–insensitive models of triple-negative breast cancer. JCI Insight. https://doi.org/10.1172/jci.insight.120638
Chiba S, Ikushima H, Ueki H et al (2014) Recognition of tumor cells by Dectin-1 orchestrates innate immune cells for anti-tumor responses. Elife 3:e04177. https://doi.org/10.7554/eLife.04177
Zhao Y, Chu X, Chen J et al (2016) Dectin-1-activated dendritic cells trigger potent antitumour immunity through the induction of Th9 cells. Nat Commun 7:12368. https://doi.org/10.1038/ncomms12368
O’Day S, Borges VF, Chmielowski B et al (2019) An open label, multicenter phase II study combining imprime PGG (PGG) with pembrolizumab ( P ) in previously treated metastatic triple-negative breast cancer (mTNBC). J Clin Oncol 37:2550–2550. https://doi.org/10.1200/JCO.2019.37.15_suppl.2550
Twumasi-Boateng K, Pettigrew JL, Kwok YYE, Bell JC, Nelson BH (2018) Oncolytic viruses as engineering platforms for combination immunotherapy. Nat Rev Cancer 18(7):419–432. https://doi.org/10.1038/s41568-018-0009-4
Kaufman HL, Kohlhapp FJ, Zloza A (2016) Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov 15(9):660. https://doi.org/10.1038/nrd.2016.178
Senzer NN, Kaufman HL, Amatruda T et al (2009) Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol 27(34):5763–5771. https://doi.org/10.1200/JCO.2009.24.3675
U. S. Food and Drug Administration (2019) Vaccine and related biological product guidances. https://www.fda.gov/vaccines-blood-biologics/biologics-guidances/vaccine-and-related-biological-product-guidances accessed 1 June 2019
Andtbacka RHI, Kaufman HL, Collichio F et al (2015) Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 33(25):2780–2788. https://doi.org/10.1200/JCO.2014.58.3377
Robert C, Schachter J, Long GV et al (2015) Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372(26):2521–2532. https://doi.org/10.1056/NEJMoa1503093
Soliman H, Hogue D, Han H et al (2021) A phase I trial of talimogene laherparepvec in combination with neoadjuvant chemotherapy for the treatment of nonmetastatic triple-negative breast cancer. Clin Cancer Res 27(4):1012–1018. https://doi.org/10.1158/1078-0432.CCR-20-3105
Pascual T, Villagrasa P, Vidal MJ, et al (2020) Abstract OT1–01–01: SOLTI-1503 PROMETEO: Combination of talimogene laherparepvec (T-VEC) with atezolizumab in patients with residual breast cancer after standard neoadjuvant multi-agent chemotherapy. American Association for Cancer Research; 2020:OT1–01–01-OT1–01–01. https://doi.org/10.1158/1538-7445.SABCS19-OT1-01-01
Zacharakis N, Chinnasamy H, Black M et al (2018) Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med 24(6):724–730. https://doi.org/10.1038/s41591-018-0040-8
Lum LG, Thakur A, Al-Kadhimi Z et al (2015) Targeted T-cell therapy in stage IV breast cancer: a phase I clinical trial. Clin Cancer Res 21(10):2305–2314. https://doi.org/10.1158/1078-0432.CCR-14-2280
Disis ML, Dang Y, Coveler AL et al (2014) HER-2/neu vaccine-primed autologous T-cell infusions for the treatment of advanced stage HER-2/neu expressing cancers. Cancer Immunol Immunother 63(2):101–109. https://doi.org/10.1007/s00262-013-1489-4
Lim WA, June CH (2017) The principles of engineering immune cells to treat cancer. Cell 168(4):724–740. https://doi.org/10.1016/j.cell.2017.01.016
Dees S, Ganesan R, Singh S, Grewal IS (2020) Emerging CAR-T cell therapy for the treatment of triple-negative breast cancer. Mol Cancer Ther 19(12):2409–2421. https://doi.org/10.1158/1535-7163.mct-20-0385
Adusumilli PS, Cherkassky L, Villena-Vargas J et al (2014) Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med. 6(261):261ra151. https://doi.org/10.1126/scitranslmed.3010162
Adusumilli PS, Zauderer MG, Rusch VW et al (2019) Regional delivery of mesothelin-targeted CAR T cells for pleural cancers: Safety and preliminary efficacy in combination with anti-PD-1 agent. J Clin Oncol 37:2511–2511. https://doi.org/10.1200/JCO.2019.37.15_suppl.2511
Waldmann TA (2018) Cytokines in cancer immunotherapy. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a028472
Berraondo P, Sanmamed MF, Ochoa MC et al (2019) Cytokines in clinical cancer immunotherapy. Br J Cancer 120(1):6–15. https://doi.org/10.1038/s41416-018-0328-y
Catania C, Maur M, Berardi R et al (2015) The tumor-targeting immunocytokine F16-IL2 in combination with doxorubicin: dose escalation in patients with advanced solid tumors and expansion into patients with metastatic breast cancer. Cell Adh Migr 9(1–2):14–21. https://doi.org/10.4161/19336918.2014.983785
Page DB, Pucilowska J, Sanchez KG et al (2020) A phase Ib study of preoperative, locoregional IRX-2 cytokine immunotherapy to prime immune responses in patients with early-stage breast cancer. Clin Cancer Res 26(7):1595–1605. https://doi.org/10.1158/1078-0432.CCR-19-1119
Hosseini SS, Khalili S, Baradaran B et al (2021) Bispecific monoclonal antibodies for targeted immunotherapy of solid tumors: recent advances and clinical trials. Int J Biol Macromol 167:1030–1047. https://doi.org/10.1016/j.ijbiomac.2020.11.058
Weidanz J (2021) Targeting cancer with bispecific antibodies. Science 371(6533):996–997. https://doi.org/10.1126/science.abg5568
Metelli A, Wu BX, Fugle CW et al (2016) Surface expression of TGFβ docking receptor GARP promotes oncogenesis and immune tolerance in breast cancer. Cancer Res 76(24):7106–7117. https://doi.org/10.1158/0008-5472.CAN-16-1456
Zhao H, Ma Y, Zhang Y et al (2020) The preliminary efficacy and safety data of KN046 in patients failed on prior immune checkpoint inhibitors therapy. J Clin Oncol 38:3020–3020. https://doi.org/10.1200/JCO.2020.38.15_suppl.3020
Kontermann RE, Brinkmann U (2015) Bispecific antibodies. Drug Discov Today 20(7):838–847. https://doi.org/10.1016/j.drudis.2015.02.008
Luke JJ, Patel MR, Hamilton EP et al (2020) A phase I, first-in-human, open-label, dose-escalation study of MGD013, a bispecific DART molecule binding PD-1 and LAG-3, in patients with unresectable or metastatic neoplasms. J Clin Oncol 38:3004–3004. https://doi.org/10.1200/JCO.2020.38.15_suppl.3004
Autio KA, Boni V, Humphrey RW, Naing A (2020) Probody therapeutics: an emerging class of therapies designed to enhance on-target effects with reduced off-tumor toxicity for use in immuno-oncology. Clin Cancer Res 26(5):984–989. https://doi.org/10.1158/1078-0432.CCR-19-1457
Thistlethwaite F, Naing A, Gil-Martin M et al (2020) PROCLAIM-CX-072: Analysis of patients with advanced solid tumors receiving long-term treatment with CX-072, a PD-L1 probody therapeutic, as a single agent or in combination with ipilimumab. J Clin Oncol 38:3005–3005. https://doi.org/10.1200/JCO.2020.38.15_suppl.3005
Soliman H (2010) Developing an effective breast cancer vaccine. Cancer Control 17(3):183–190. https://doi.org/10.1177/107327481001700307
Solinas C, Aiello M, Migliori E, Willard-Gallo K, Emens LA (2020) Breast cancer vaccines: heeding the lessons of the past to guide a path forward. Cancer Treat Rev 84:101947. https://doi.org/10.1016/j.ctrv.2019.101947
Rosenbaum P, Artaud C, Bay S et al (2020) The fully synthetic glycopeptide MAG-Tn3 therapeutic vaccine induces tumor-specific cytotoxic antibodies in breast cancer patients. Cancer Immunol Immunother 69(5):703–716. https://doi.org/10.1007/s00262-020-02503-0
Hutchins LF, Makhoul I, Emanuel PD et al (2017) Targeting tumor-associated carbohydrate antigens: a phase I study of a carbohydrate mimetic-peptide vaccine in stage IV breast cancer subjects. Oncotarget 8(58):99161–99178. https://doi.org/10.18632/oncotarget.21959
Knutson KL, Block MS, Norton N et al (2020) Rapid generation of sustainable HER2-specific T-cell immunity in patients with HER2 breast cancer using a degenerate HLA class II epitope vaccine. Clin Cancer Res 26(5):1045–1053. https://doi.org/10.1158/1078-0432.CCR-19-2123
Limentani SA, Campone M, Dorval T et al (2016) A non-randomized dose-escalation Phase I trial of a protein-based immunotherapeutic for the treatment of breast cancer patients with HER2-overexpressing tumors. Breast Cancer Res Treat 156(2):319–330. https://doi.org/10.1007/s10549-016-3751-x
Kim S-B, Ahn J-H, Kim J, Jung KH (2015) A phase 1 study of a heterologous prime-boost vaccination involving a truncated HER2 sequence in patients with HER2-expressing breast cancer. Mol Ther - Methods Clin Dev 2:15031. https://doi.org/10.1038/mtm.2015.31
Soliman H, Khambati F, Han HS et al (2018) A phase-1/2 study of adenovirus-p53 transduced dendritic cell vaccine in combination with indoximod in metastatic solid tumors and invasive breast cancer. Oncotarget 9(11):10110–10117. https://doi.org/10.18632/oncotarget.24118
Kranz LM, Diken M, Haas H et al (2016) Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534(7607):396–401. https://doi.org/10.1038/nature18300
Schmidt M, Vogler I, Derhovanessian E et al (2020) 88MO T-cell responses induced by an individualized neoantigen specific immune therapy in post (neo)adjuvant patients with triple negative breast cancer. Ann Oncol 31:S276. https://doi.org/10.1016/j.annonc.2020.08.209
Aspeslagh S, Morel D, Soria J-C, Postel-Vinay S (2018) Epigenetic modifiers as new immunomodulatory therapies in solid tumours. Ann Oncol 29(4):812–824. https://doi.org/10.1093/annonc/mdy050
Sheng W, LaFleur MW, Nguyen TH et al (2018) LSD1 Ablation Stimulates Anti-tumor Immunity and Enables Checkpoint Blockade. Cell 174(3):549-563.e19. https://doi.org/10.1016/j.cell.2018.05.052
Stone ML, Chiappinelli KB, Li H et al (2017) Epigenetic therapy activates type I interferon signaling in murine ovarian cancer to reduce immunosuppression and tumor burden. Proc Natl Acad Sci USA 114(51):E10981–E10990. https://doi.org/10.1073/pnas.1712514114
Terranova-Barberio M, Thomas S, Ali N et al (2017) HDAC inhibition potentiates immunotherapy in triple negative breast cancer. Oncotarget 8(69):114156–114172. https://doi.org/10.18632/oncotarget.23169
Li B, Wang Z, Wu H et al (2018) Epigenetic regulation of CXCL12 plays a critical role in mediating tumor progression and the immune response in osteosarcoma. Cancer Res 78(14):3938–3953. https://doi.org/10.1158/0008-5472.CAN-17-3801
Peng D, Kryczek I, Nagarsheth N et al (2015) Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 527(7577):249–253. https://doi.org/10.1038/nature15520
Morel D, Jeffery D, Aspeslagh S, Almouzni G, Postel-Vinay S (2020) Combining epigenetic drugs with other therapies for solid tumours — past lessons and future promise. Nat Rev Clin Oncol 17(2):91–107. https://doi.org/10.1038/s41571-019-0267-4
Terranova Barberio M, Thomas S, Chien AJ et al (2016) Phase II trial with tamoxifen in combination with vorinostat and pembrolizumab in estrogen receptor (+) hormone therapy resistant metastatic breast cancer patients (NCT02395627). J Clin Oncol 34:620. https://doi.org/10.1200/JCO.2016.34.15_suppl.TPS620
O’Shaughnessy J, Moroose RL, Babu S et al (2020) Results of ENCORE 602 (TRIO025), a phase II, randomized, placebo-controlled, double-blinded, multicenter study of atezolizumab with or without entinostat in patients with advanced triple-negative breast cancer (aTNBC). J Clin Oncol 38:1014–1014. https://doi.org/10.1200/JCO.2020.38.15_suppl.1014
Taylor K, Loo Yau H, Chakravarthy A et al (2020) An open-label, phase II multicohort study of an oral hypomethylating agent CC-486 and durvalumab in advanced solid tumors. J Immunother Cancer. https://doi.org/10.1136/jitc-2020-000883
van der Zanden SY, Luimstra JJ, Neefjes J, Borst J, Ovaa H (2020) Opportunities for small molecules in cancer immunotherapy. Trends Immunol 41(6):493–511. https://doi.org/10.1016/j.it.2020.04.004
Patzak IM, Königsberger S, Suzuki A, Mak TW, Kiefer F (2010) HPK1 competes with ADAP for SLP-76 binding and via Rap1 negatively affects T-cell adhesion. Eur J Immunol 40(11):3220–3225. https://doi.org/10.1002/eji.201040313
Sawasdikosol S, Burakoff S (2020) A perspective on hpk1 as a novel immuno-oncology drug target. Elife 9:1–15. https://doi.org/10.7554/ELIFE.55122
Brenner D, Golks A, Kiefer F, Krammer PH, Arnold R (2005) Activation or suppression of NFκB by HPK1 determines sensitivity to activation-induced cell death. EMBO J 24(24):4279–4290. https://doi.org/10.1038/sj.emboj.7600894
Shui J-W, Boomer JS, Han J et al (2007) Hematopoietic progenitor kinase 1 negatively regulates T cell receptor signaling and T cell–mediated immune responses. Nat Immunol 8(1):84–91. https://doi.org/10.1038/ni1416
Schmid P, Cortes J, Pusztai L et al (2020) Pembrolizumab for early triple-negative breast cancer. N Engl J Med 382(9):810–821. https://doi.org/10.1056/NEJMoa1910549
Schmid P, Cortes J, Dent R et al (2021) VP7–2021: KEYNOTE-522: phase III study of neoadjuvant pembrolizumab + chemotherapy vs placebo + chemotherapy, followed by adjuvant pembrolizumab vs placebo for early-stage TNBC. Ann Oncol 32(9):1198–1200. https://doi.org/10.1016/j.annonc.2021.06.014
Adams S, Loi S, Toppmeyer D et al (2019) Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol 30(3):405–411. https://doi.org/10.1093/annonc/mdy518
Cortes J, Cescon DW, Rugo HS et al (2020) Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer double-blind, phase 3 clinical trial. Lancet 396:1817. https://doi.org/10.1016/S0140-6736(20)32531-9
Chen DS, Mellman I (2013) Oncology meets immunology: the cancer-immunity cycle. Immunity 39(1):1–10. https://doi.org/10.1016/j.immuni.2013.07.012
Acknowledgements
Holdem for life Oncology Fellowship 2020-2021 and SEOM/CRIS contra el cancer Grant 2021.
Funding
NA.
Author information
Authors and Affiliations
Contributions
Conception and design: AH-C, DC, PB. Development of methodology: AH-C, DC, PB. Acquisition of data: AH-C. Writing, review, and/or revision of the manuscript: AH-C, DC, PB.
Corresponding author
Ethics declarations
Conflict of interest
Alberto Hernando-Calvo reports travel grants from Kyowa Kirin, Merck Serono, MSD and Bristol Myers. Dave Cescon reports Consulting/Advisory: Agendia, AstraZeneca, Dynamo Therapeutics, Exact Sciences, GlaxoSmithKline, Merck, Novartis, Pfizer, Puma Biotechnology, and Roche. Research funding to institution: GlaxoSmithKline, Pfizer, and Roche. Patent (US62/675,228) for methods of treating cancers characterized by a high expression level of spindle and kinetochore associated complex subunit 3 (ska3) gene. Philippe Bedard reports consulting or advisory role with Seattle Genetics, Lilly, Amgen, Merck, BMS (institution) and Pfizer (institution). Research funding from Bristol-Myers Squibb (institution), Sanofi (institution), Astrazeneca (institution), Genentech/Roche (institution) Servier (institution), GlaxoSmithKline (institution), Novartis(institution), PTC Therapeutics (institution), Nektar (institution), Merck (institution), Merck (institution), Seattle Genetics (institution) Mersana (institution), Immunomedics (institution), Lilly (institution), Amgen (institution) and Bicara (institution).
Ethical approval
NA.
Research involving human and animal rights
Additional declarations for articles in life science journals that report the results of studies involving humans and/or animals.
Consent to participate
All the authors have consented to participate in this review.
Consent for publication
All the authors have consented to participate in this review and confirmed consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hernando-Calvo, A., Cescon, D.W. & Bedard, P.L. Novel classes of immunotherapy for breast cancer. Breast Cancer Res Treat 191, 15–29 (2022). https://doi.org/10.1007/s10549-021-06405-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06405-2