Abstract
Purpose
BRCA1/2 mutations influence the molecular characteristics and the effects of systemic treatment of breast cancer. This study investigates the impact of germline BRCA1/2 mutations on pathological complete response and prognosis in patients receiving neoadjuvant systemic chemotherapy.
Methods
Breast cancer patients were tested for a BRCA1/2 mutation in clinical routine work and were treated with anthracycline-based or platinum-based neoadjuvant chemotherapy between 1997 and 2015. These patients were identified in the tumor registry of the Breast Center of the University of Erlangen (Germany). Logistic regression and Cox regression analyses were performed to investigate the associations between BRCA1/2 mutation status, pathological complete response, disease-free survival, and overall survival.
Results
Among 355 patients, 59 had a mutation in BRCA1 or in BRCA2 (16.6%), 43 in BRCA1 (12.1%), and 16 in BRCA2 (4.5%). Pathological complete response defined as “ypT0; ypN0” was observed in 54.3% of BRCA1/2 mutation carriers, but only in 22.6% of non-carriers. The adjusted odds ratio was 2.48 (95% CI 1.26–4.91) for BRCA1/2 carriers versus non-carriers. Patients who achieved a pathological complete response had better disease-free survival and overall survival rates compared with those who did not achieve a pathological complete response, regardless of BRCA1/2 mutation status.
Conclusions
BRCA1/2 mutation status leads to better responses to neoadjuvant chemotherapy in breast cancer. Pathological complete response is the main predictor of disease-free survival and overall survival, independently of BRCA1/2 mutation status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the last few decades, the connection between the patients’ response to neoadjuvant therapy and the prognosis has been investigated in multiple studies. For patients with triple-negative breast cancer (TNBC) and HER2-positive breast cancer, there is a strong association between pathological complete response (pCR) and prognosis, while in hormone receptor-positive patients, the pCR rate is low and the association between pCR and prognosis is rather weak [1, 2]. Identifying further breast cancer subtypes with a strong or weak correlation between pCR and the prognosis should therefore be helpful for directing future therapies. The present study is concerned with BRCA1/2 mutations in this context.
The data on BRCA1/2 mutation status and prognosis are scarce and contradictory. Studies comparing BRCA1 mutation carriers and non-carriers have reported better [3,4,5,6], poorer [7], or similar survival [6, 8, 9]. BRCA2 mutation carriers appear to have a similar prognosis in comparison with non-carriers [5, 7, 8]. A meta-analysis did not show any major differences in survival, either [10].
With regard to pCR, reports have demonstrated that a germline mutation in BRCA1 is associated with excellent pCR rates in breast cancer patients [4, 11,12,13]. The data for patients with BRCA2 mutations are not as clear, and the pCR rates in this group were similar to those in patients without a mutation [4, 5].
A recent report questioned whether triple-negative BRCA1 carriers benefit from pCR at all. Although a clear association was found between pCR and a good prognosis in patients without mutations, no benefit from pCR was noted in patients with a BRCA1 mutation [14].
The aim of the present study was therefore to investigate pCR in patients with primary breast cancer, with or without BRCA1/2 mutations. A further aim was to analyze how pCR affects the prognosis in BRCA1/2 mutation carriers in comparison with non-carriers.
Methods
Study design and selection of patients
This analysis was conducted in a group of patients included in the “Erlangen Neoadjuvant Study Breast” (ERNEST-B) study. ERNEST-B represents a consecutive cohort of primary breast cancer patients who were treated with neoadjuvant chemotherapy at the University Breast Center at Erlangen University Hospital between 1997 and 2015. During that time, 6801 patients with primary invasive breast cancer were treated, 1575 of whom received neoadjuvant chemotherapy. BRCA1/2 testing was performed in 401 of these patients. All patients with primary metastases (n = 25) and with bilateral cases (n = 21) were excluded. The study population ultimately consisted of 355 breast cancer patients (Fig. 1). The Ethics Committee of the Medical Faculty of Friedrich Alexander University Erlangen–Nuremberg approved this retrospective study.
Data collection
As required for certification by the German Cancer Society and German Society of Mastology, each breast cancer case was documented, including patient and tumor characteristics, detailed treatment, and epidemiological data [15]. Follow-up data were collected for up to 10 years after the primary diagnosis, and documentation quality was audited annually.
Therapies
In the neoadjuvant setting, most patients received the following anthracycline-based therapies: four cycles of epirubicin (80–90 mg/m2) and cyclophosphamide (600 mg/m2) every 3 weeks, followed by 12 cycles of paclitaxel (80–90 mg/m2) weekly. Platinum-based therapies were administered using the following standard protocol: six cycles of carboplatin AUC5 on day 1 and paclitaxel (80–90 mg/m2) on days 1, 8, and 15 every 3 weeks. Platinum-based treatment was not administered before 2007. The documentation only included whether anthracyclines, cyclophosphamide, taxanes, platinum, HER2 therapies, or other treatments were administered. Precise information on the exact treatment scheme for every patient is therefore not available.
Definition of pCR and molecular subtypes
Data on pathological complete responses were drawn from the original pathology reports. pCR was defined as the complete eradication of invasive tumor cells from the breast and lymph nodes after chemotherapy at the time of surgery (ypT0; ypN0). The tumor’s molecular subtype is defined by hormone receptor status, HER2 status, and cellular proliferation rate (Ki-67). Luminal A-like tumors were estrogen receptor (ER)-positive or progesterone receptor (PR)-positive, HER2-negative, and had low Ki-67 values (≤ 14%). Luminal B-like tumors were ER-positive or PR-positive, and HER2-negative with high Ki-67 values (> 14%). TNBCs were required to be negative or weakly positive (≤ 10%) for ER and PR, consistent with the guidelines applicable in different treatment years, and constantly negative for HER2 [16, 17]. All HER2-positive tumors were grouped together, regardless of hormone receptor status. ER, PR, and Ki-67 were assessed with immunohistochemistry by individual breast center pathologists, as described previously [18]. HER2 was positive when immunohistochemical staining indicated a 3 + result or the tumor showed HER2 amplification, as determined by chromogene in situ hybridization (CISH; ZytoDot, 2C SPEC HER2/CEN17, Zyto Vision Ltd., Bremerhaven, Germany). The immunohistochemical evaluation was quality-controlled and validated in annual round robin tests.
Mutation screening
Mutations in BRCA1/2 were analyzed as part of routine clinical testing. Genotyping was performed in patients with a positive family history who met the criteria for BRCA1/2 diagnostic testing established by the German Consortium for Hereditary Breast and Ovarian Cancer [19] and/or in patients with TNBC, independently of their age. All patients received genetic counseling and provided written informed consent for diagnostic genetic testing and scientific use of their data.
Genomic DNA was extracted with an automated chemagic MSM I system (PerkinElmer, Baesweiler, Germany). Up to 2012, Sanger sequencing was performed, and primers for all coding exons of both genes were designed to cover 100% of coding and flanking sequences. The fragments were sequenced using BigDye version 3.1 (Applied Biosystems, part of Thermo Fisher, Darmstadt, Germany). The polymerase chain reaction products and sequencing reactions were purified automatically with the AMPure and CleanSEQ kits (Agencourt, Beverly, MA, USA) on a Biomek pipetting robot (Beckman Coulter, Fullerton, CA, USA). Purified sequences were separated on an ABI3730 sequencer (Applied Biosystems) and were analyzed using the Sequence Pilot software (JSI Medical Systems, Kippenheim, Germany).
After 2012, the Illumina TruSight Cancer Panel was used as a targeted resequencing kit for library preparation and sequenced on a MiSeq platform (Illumina, San Diego, CA, USA). Library preparation was done using 50 ng of genomic DNA per sample. The prepared libraries were applied to 2 × 150 bp paired-end sequencing, and the reads obtained were mapped to human genome reference GRCh37/hg19 using BWA-MEM version 0.7.7 [20]. BRCA1/2 and other genes were analyzed using the SeqNext module of the Sequence Pilot software program (JSI Medical Systems). Mutations were classified as deleterious in accordance with the breast cancer information core [21]. Using both of these methods resulted in 100% of the targets being covered.
Statistical analysis
A logistic regression model (basic model) was fitted with pCR (“yes” vs. “no”) as the outcome and the following predictors for pCR: age at diagnosis (continuous), body mass index (BMI, continuous), clinical tumor size (categorical; T1–T4), molecular tumor type (categorical; TNBC, luminal A-like, luminal B-like, HER2-positive), therapy type (categorical; anthracycline-based therapy, platinum-based therapy). Subsequently, an additional logistic regression model was fitted containing BRCA1/2 mutation (categorical, “yes” vs. “no”), the predictors from the previous basic model, and the interaction between BRCA1/2 mutation and therapy type (interaction model). The two models were compared using the likelihood ratio test. A significant test result indicates that BRCA1/2 mutation influenced pCR beyond the other predictors, either across all patients or at least within one of the therapy types. No further analyses were conducted if the test result was not significant, in order to avoid false-positive results. However, if the p value was significant, the interaction model was compared with a reduced logistic regression model without the interaction term (reduced model), using the likelihood ratio test again. In case of significance, therapy type-specific odds ratios for BRCA1/2 mutation adjusted for the other predictors were calculated, using the interaction model. If the result was not significant, an adjusted overall odds ratio for BRCA1/2 mutation was calculated, using the reduced model. No molecular tumor type-specific analyses (e.g., molecular tumor type as an interaction term) were performed, due to the small sample sizes in these subgroups.
The discrimination and calibration achieved by the logistic regression models were assessed using the area under the receiver operating characteristic curve (AUC) and the Hosmer–Lemeshow χ2 test comparing predicted and observed pCR events, as done recently in [22]. A large p value indicates satisfactory calibration. To address overfitting, cross-validated AUC values were also calculated, as described in [23]. The smaller the difference between the cross-validated AUC and the original AUC, the lower the amount of overfitting.
A Cox regression model was fitted with disease-free survival as outcome and with the following predictors: age at diagnosis, BMI, clinical tumor size, molecular tumor type, BRCA1/2 mutation status, and pCR. This Cox model was compared with an extended Cox model additionally including the interaction between BRCA1/2 mutation status and pCR, using a likelihood ratio test. In case of significance, BRCA1/2 mutation-specific hazard ratios for pCR were calculated using the interaction model. Otherwise, an overall hazard ratio for pCR was calculated using the model without the interaction term. The proportional hazards assumptions were checked using the Grambsch–Therneau method. The results of the survival analyses are also illustrated using Kaplan–Meier curves for patient groups defined by pCR status, BRCA1/2 mutation status, and therapy type. A similar analysis was performed for overall survival. Sensitivity analyses with time from genetic testing to event, instead of time from diagnosis to event, were performed to address a possible bias due to immortal time between the date of diagnosis and the date of genetic testing.
Patients with missing outcomes were excluded from the analyses. Missing predictor values were imputed, and continuous predictors were used as natural cubic spline functions, as done in [24]. All of the tests were two-sided, and a p value of < 0.05 was regarded as statistically significant. Calculations were carried out using the R system for statistical computing, version 3.0.1.
Results
Patient and tumor characteristics relative to BRCA1/2 mutation status
Fifty-nine patients had a mutation in either BRCA1 or BRCA2 (16.6%). Forty-three mutations were detected in BRCA1 (12.1%) and 16 in BRCA2 (4.5%). The BRCA1/2 mutation rates were 24.6% in all tested patients with TNBC, 10.3% in HER2-positive patients, 16.5% in patients with luminal B-like tumors, and 0.0% in patients with luminal A-like tumors.
Patient characteristics relative to BRCA1/2 mutation status are shown in Table 1. BRCA1/2 mutation carriers were on average 8 years younger than non-carriers. Among mutation carriers, 49.0% had at least one first-degree relative with breast and/or ovarian cancer, while this rate was 20.0% in non-mutation patients. Among the BRCA1/2 mutation carriers, 57.6% of the tumors were triple-negative, 10.2% were HER2-positive, 32.2% were luminal B-like, and none was luminal A-like. BRCA1/2-related breast cancer was also more likely to have a histological grading of 3 (91.5 vs. 58.1%) and a higher Ki-67 level (57.7 vs. 41.4%).
Therapies
In the total study population, 24.5% of the patients received carboplatin. Relative to molecular subtype, 49.3% of patients with TNBC, 8.6% of those with HER2-positive breast cancer, 12.2% of those with luminal B-like breast cancer, and none with luminal A-like breast cancer received carboplatin-based therapy, regardless of mutation status. All patients with HER2-positive breast cancer were treated with trastuzumab.
Across all breast cancer types, 35.6% of patients with a BRCA1/2 mutation received carboplatin, while the rest received anthracycline-based treatments. Carboplatin treatments relative to molecular subtype in the group of mutation carriers represented 44.1% of treatments for patients with TNBC, 16.7% for HER2-positive patients, and 26.3% for patients with luminal B-like tumors.
pCR relative to BRCA1/2 mutation status
In patients without a mutation, the pCR rate was 22.6% (n = 67). In mutation carriers, the pCR rate was 54.3% (n = 32). With regard to subgroups of patients with either BRCA1 or BRCA2 mutations, the pCR rates were 58.1% in BRCA1 carriers and 43.7% in BRCA2 carriers (Table 2).
The pCR rates were further analyzed in relation to mutation status and treatment administered in the subsets of luminal A/B-like tumors, in HER2-positive tumors, in triple-negative tumors, and in overall breast cancer. Among TNBC patients (n = 138) who received anthracycline-based treatment, the pCR rate was 27.5% in non-carriers and 47.4% in BRCA1/2 mutation carriers, while for patients who received platinum-based treatments, the rates were 58.5% and 73.3% (Fig. 2). In addition, for BRCA1/2 mutation carriers, the pCR rates were higher across all breast cancer subtypes if carboplatin was administered. The responses to neoadjuvant chemotherapy for non-mutation carriers, for BRCA1 mutation carriers, and for BRCA1/2 mutation carriers are shown in Table 3. However, sample sizes in patients with luminal A/B-like tumors and HER2-positive tumors were too low for further conclusions to be drawn.
Comparison of prediction models with and without BRCA1/2 mutation status showed that BRCA1/2 mutation status significantly influenced pCR additionally to the predictors considered (p = 0.03, first likelihood ratio test). The interaction between BRCA1/2 mutation and therapy type, however, was not significant (p = 0.84, second likelihood ratio test). Thus, it was not demonstrated that the effect of BRCA1/2 differed between patients who received anthracycline-based treatments and those who received platinum-based treatments. The adjusted odds ratio for BRCA1/2 carriers versus non-carriers was 2.48 (95% CI 1.26–4.91).
The reduced logistic regression model used to estimate the odds ratio was well calibrated. The difference between actual and predicted events was very low (p = 0.71, Hosmer–Lemeshow test). Discrimination was also satisfactory, at AUC = 0.828. The cross-validated AUC was 0.811, indicating some overfitting. The cross-validated AUC values for the basic model and the interaction model were lower (0.807 and 0.806, respectively), confirming the main result that BRCA1/2 mutation status is predictive without differences between therapy types.
Influence of pCR and BRCA1/2 mutation status on prognosis
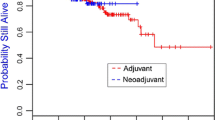
The prognostic effect of pCR on the disease-free survival did not differ between patients with or without BRCA1/2 mutations (p = 0.55, likelihood ratio test). The adjusted hazard ratio for pCR status in the overall patient population was 0.30 (95% CI 0.15–0.60). Kaplan–Meier curves relative to pCR and mutation status are shown in Fig. 3a. Disease-free survival did not differ relative to mutation status and type of therapy (Fig. 3b).
The overall survival analysis yielded similar results. It was not shown that overall survival depended on BRCA1/2 mutation status (p = 0.13, likelihood ratio test). Again, patients benefited from pCR (hazard ratio 0.20; 95% CI 0.07–0.60).
Time interval between breast cancer diagnosis and genetic test results
Genetic test results were available at a median of 5.8 months (interquartile range 1.7–29.8 months; mean 23.1 months) after breast cancer diagnosis. In patients with a BRCA1/2-negative test result, the median time from diagnosis to genetic testing was 6.0 months (interquartile range 1.6–33.5 months; mean 24.3 months). In patients with a BRCA1/2-positive test result, the median time from diagnosis to genetic testing was 4.8 months (interquartile range 2.1–20.8 months; mean 17.3 months). Sensitivity analyses with date of genetic testing as the start of the observation time showed results similar to the main analyses. It was not shown that disease-free and overall survival were influenced by BRCA1/2 test results, and patients with pCR had a better prognosis than patients without pCR (data not shown).
Discussion
This study shows that patients with BRCA1/2 mutations have higher pCR rates than patients without mutations, and that pCR is associated with a better prognosis regardless of BRCA1/2 mutation status. Although it was not shown that BRCA1/2 status had a different effect on the pCR rate in patients treated with anthracyclines and those receiving carboplatin, the highest pCR rates were observed in BRCA1/2 mutation carriers with TNBC who received platinum-based therapy.
With pCR having an influence on the prognosis regardless of BRCA1/2 mutation status, the present study is in line with findings from the GeparSixto study [25]. In that study, pCR was clearly associated with a favorable 3-year disease-free survival (96.1% for patients with wild-type mutations and 95.5% for patients with BRCA1/2 mutations). After chemotherapy without pCR, the 3-year disease-free survival rates were 65.4% for patients with wild-type mutations and 62.7% for BRCA1/2 mutation carriers [25]. The GeparSixto study had a different chemotherapy regimen, with pegylated doxorubicin and bevacizumab for all TNBCs [25], but the present study is very similar to the one by Paluch-Shimon et al. [14]. In that study, neoadjuvant chemotherapy with doxorubicin and cyclophosphamide followed by paclitaxel yielded a similar survival after a median follow-up of 44 months, although the pCR rate for BRCA1 mutation carriers versus non-carriers was nearly as twice as high (68 vs. 37%; p = 0.01) [14]. It remains unclear what the population effects were that led to the results of the study by Paluch-Shimon et al. The results could be due to chance, but an analysis of the GeparQuinto study also reported that in patients with BRCA1/2 mutations, the effect of pCR on the prognosis may be weaker than in patients with wild-type mutations [26].
Identifying patient subgroups with a clear association, or lacking an association, between pCR and the prognosis is of great importance for understanding how neoadjuvant study results can be translated into the adjuvant setting. While a clear association between pCR and prognosis has been established in patients with TNBC and HER2-positive breast cancer, this effect appears to be weaker in patients with hormone receptor-positive breast cancer [1, 2]. With regard to BRCA1/2, this might be an important piece of information in the near future as genetic testing becomes increasingly integrated into clinical practice [27], and neoadjuvant poly(adenosine diphosphate ribose) polymerase (PARP) inhibitor studies are under way [28]. However, there is one more aspect that needs to be taken into consideration: With regard to therapies, there have also been a few examples from randomized trials suggesting that the randomization did not result in any difference in pCR, although the patients still benefited from one of the therapies more than the other [29, 30]. Whether BRCA1/2 mutation status may be helpful, probably as one of the many factors that are of importance in this context, will require further research.
Otherwise, the results of the present study are very similar to the published data, with patients with a BRCA1/2 mutation having clearly higher pCR rates than patients without a mutation [4, 11,12,13,14, 25, 26, 31]. The greater efficacy of chemotherapy in these tumors is apparently related to a reduced capacity for DNA repair and/or higher tumor proliferation [32,33,34,35].
The present study also investigated whether the type of treatment administered influences pCR in BRCA1/2 mutation carriers differently from non-carriers. Although it was not shown in this retrospective study that the type of chemotherapy has a different influence on pCR rates in patients with or without BRCA1/2 mutation, the highest pCR rates were achieved in triple-negative BRCA1/2 mutation carriers who were treated with carboplatin. The comparable pCR rate in the GeparSixto trial was 61.5% in TNBC patients receiving platinum-containing chemotherapy [25]. Another recent study by Narod et al. investigated 10-year all-cause survival in 372 Polish women with breast cancer and a BRCA1 mutation. The best survival rates were observed if women received cisplatin and an oophorectomy, in comparison with those who had neither of these two treatments (10-year survival, 94.4 vs. 65.4%; p < 0.01) [36]. These data and the present study therefore support the use of platinum-containing chemotherapies for patients with TNBC and a BRCA1/2 mutation, based on extraordinarily high pCR rates that appear to translate into a favorable prognosis.
Emerging data on the efficacy of platinum in TNBC and BRCA1/2 mutation carriers encouraged the implementation of platinum treatment in the present study population after 2007. Platinum treatment has shown very promising efficacy in several trials [11, 25, 26, 31, 36,37,38,39,40,41,42] and has been under discussion for certain subgroups of TNBC as an alternative to anthracycline-based treatment [43]. Irrespective of BRCA1/2 mutation status, subgroup analyses from the GeparSixto trial demonstrated higher pCR rates in TNBC patients with G3 tumors in comparison with G1/G2 tumors [41]. In addition, platinum has been successfully used in several trials in patients with HER2-positive breast cancer [41, 42, 44]. Early results from these studies prompted us subsequently to include platinum in the treatment of patients with TNBC and/or BRCA1/2 mutation carriers.
Major limitations of this retrospective study are the small sample size for BRCA1/2 carriers and the heterogeneity of tumor types and different treatments, sometimes with different dosages. Moreover, carboplatin was not administered before 2007. The subgroups become too small for subgroup analyses to be performed in relation to mutation status, molecular subtype, treatment, and pCR. These questions cannot be adequately answered in this study and require further investigation in larger trials. Due to the study’s retrospective nature, the analyses of disease-free survival and overall survival are limited. However, no unexpected associations and no immortal time bias were noted.
In conclusion, this study shows higher pCR rates after neoadjuvant chemotherapy in patients with BRCA1/2 mutations than in patients without mutations. It also shows that pCR in BRCA1/2 mutation carriers has similar effects on the prognosis to those seen in patients with a wild-type genotype. High pCR rates in mutation carriers with TNBC after platinum-containing chemotherapy support the use of this chemotherapy regimen in this patient population.
References
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172
von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J et al (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30(15):1796–1804
Cortesi L, Masini C, Cirilli C, Medici V, Marchi I, Cavazzini G, Pasini G, Turchetti D, Federico M (2010) Favourable ten-year overall survival in a Caucasian population with high probability of hereditary breast cancer. BMC Cancer 10:90
Arun B, Bayraktar S, Liu DD, Gutierrez Barrera AM, Atchley D, Pusztai L, Litton JK, Valero V, Meric-Bernstam F, Hortobagyi GN et al (2011) Response to neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers: a single-institution experience. J Clin Oncol 29(28):3739–3746
Rennert G, Bisland-Naggan S, Barnett-Griness O, Bar-Joseph N, Zhang S, Rennert HS, Narod SA (2007) Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. New Engl J Med 357(2):115–123
Copson ER, Maishman TC, Tapper WJ, Cutress RI, Greville-Heygate S, Altman DG, Eccles B, Gerty S, Durcan LT, Jones L et al (2018) Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol 19(2):169–180
Zhong Q, Peng HL, Zhao X, Zhang L, Hwang WT (2015) Effects of BRCA1- and BRCA2-related mutations on ovarian and breast cancer survival: a meta-analysis. Clin Cancer Res 21(1):211–220
Goodwin PJ, Phillips KA, West DW, Ennis M, Hopper JL, John EM, O’Malley FP, Milne RL, Andrulis IL, Friedlander ML et al (2012) Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an International Prospective Breast Cancer Family Registry population-based cohort study. J Clin Oncol 30(1):19–26
Huzarski T, Byrski T, Gronwald J, Gorski B, Domagala P, Cybulski C, Oszurek O, Szwiec M, Gugala K, Stawicka M et al (2013) Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J Clin Oncol 31(26):3191–3196
van den Broek AJ, Schmidt MK, van ‘t Veer LJ, Tollenaar RA, van Leeuwen FE (2015) Worse breast cancer prognosis of BRCA1/BRCA2 mutation carriers: what’s the evidence? A systematic review with meta-analysis. PLoS ONE 10(3):e0120189
Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M, Mierzwa T, Szwiec M, Wisniowski R, Siolek M et al (2010) Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol 28(3):375–379
Byrski T, Huzarski T, Dent R, Gronwald J, Zuziak D, Cybulski C, Kladny J, Gorski B, Lubinski J, Narod SA (2009) Response to neoadjuvant therapy with cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat 115(2):359–363
Byrski T, Huzarski T, Dent R, Marczyk E, Jasiowka M, Gronwald J, Jakubowicz J, Cybulski C, Wisniowski R, Godlewski D et al (2014) Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat 147(2):401–405
Paluch-Shimon S, Friedman E, Berger R, Papa M, Dadiani M, Friedman N, Shabtai M, Zippel D, Gutman M, Golan T et al (2016) Neo-adjuvant doxorubicin and cyclophosphamide followed by paclitaxel in triple-negative breast cancer among BRCA1 mutation carriers and non-carriers. Breast Cancer Res Treat 157(1):157–165
Beckmann MW, Brucker C, Hanf V, Rauh C, Bani MR, Knob S, Petsch S, Schick S, Fasching PA, Hartmann A et al (2011) Quality assured health care in certified breast centers and improvement of the prognosis of breast cancer patients. Onkologie 34(7):362–367
Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ (2009) Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol 20(8):1319–1329
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M et al (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med 134(6):907–922
Fasching PA, Heusinger K, Haberle L, Niklos M, Hein A, Bayer CM, Rauh C, Schulz-Wendtland R, Bani MR, Schrauder M et al (2011) Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer 11:486
Meindl A, Ditsch N, Kast K, Rhiem K, Schmutzler RK (2011) Hereditary breast and ovarian cancer: new genes, new treatments, new concepts. Dtsch Arztebl Int 108(19):323–330
Li H (2014) Toward better understanding of artifacts in variant calling from high-coverage samples. Bioinformatics 30(20):2843–2851
Szabo C, Masiello A, Ryan JF, Brody LC (2000) The breast cancer information core: database design, structure, and scope. Hum Mutat 16(2):123–131
Haberle L, Fasching PA, Brehm B, Heusinger K, Jud SM, Loehberg CR, Hack CC, Preuss C, Lux MP, Hartmann A et al (2016) Mammographic density is the main correlate of tumors detected on ultrasound but not on mammography. Int J Cancer 139(9):1967–1974
Burghaus S, Haberle L, Schrauder MG, Heusinger K, Thiel FC, Hein A, Wachter D, Strehl J, Hartmann A, Ekici AB et al (2015) Endometriosis as a risk factor for ovarian or endometrial cancer - results of a hospital-based case-control study. BMC Cancer 15:751
Salmen J, Neugebauer J, Fasching PA, Haeberle L, Huober J, Wockel A, Rauh C, Schuetz F, Weissenbacher T, Kost B et al (2014) Pooled analysis of the prognostic relevance of progesterone receptor status in five German cohort studies. Breast Cancer Res Treat 148(1):143–151
Hahnen E, Lederer B, Hauke J, Loibl S, Krober S, Schneeweiss A, Denkert C, Fasching PA, Blohmer JU, Jackisch C et al (2017) Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: Secondary analysis of the GeparSixto randomized clinical trial. JAMA Oncol 3(10):1378–1385
Fasching PA, Loibl S, Eidtmann H, Tesch H, Untch M, Hilfrich J, Schem C, Rezai M, Gerber B, Costa SD et al. (2016) BRCA mutations, therapy response and prognosis in the neoadjuvant GeparQuinto study. AACR Cancer Res 76 (4 Suppl): S5-06
Lux MP, Janni W, Hartkopf AD, Nabieva N, Taran FA, Overkamp F, Kolberg HC, Hadji P, Tesch H, Ettl J et al (2017) Update breast cancer 2017—implementation of novel therapies. Geburtshilfe Frauenheilkd 77(12):1281–1290
Fasching PA, Blohmer JU, Burchardi N, Costa SD, Denkert C, Hanusch C, Huober JB, Von Minckwitz G, Paepke S, Schneeweiss A et al. (2016) A randomized phase II trial to assess the efficacy of paclitaxel and olaparib in comparison to paclitaxel/carboplatin followed by epirubicin/cyclophosphamide as neoadjuvant chemotherapy in patients with HER2-negative early breast cancer and homologous recombination deficiency (HRD). GeparOLA. J Clin Oncol 34(15 Suppl):TPS1096
Schneeweiss A, Jackisch C, Schmatloch S, Aktas B, Denkert C, Schem C, Wiebringhaus H, Kümmel S, Rhiem K, Warm M et al. (2018) Survival analysis of the prospectively randomized phase III GeparSepto trial comparing neoadjuvant chemotherapy with weekly nab-paclitaxel with solvent-based paclitaxel followed by anthracycline–cyclophosphamide for patients with early breast cancer—GBG69. In: Proceedings of the 2017 San Antonio Breast Cancer Symposium, AACR, Dec 5–9 2017, San Antonio, TX
von Minckwitz G, Blohmer JU, Costa SD, Denkert C, Eidtmann H, Eiermann W, Gerber B, Hanusch C, Hilfrich J, Huober J et al (2013) Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol 31(29):3623–3630
von Minckwitz G, Hahnen E, Fasching PA, Hauke J, Schneeweiss A, Salat C, Rezai M, Blohmer JU, Zahm DM, Jackisch C (2014) Pathological complete response (pCR) rates after carboplatin-containing neoadjuvant chemotherapy in patients with germline BRCA (g BRCA) mutation and triple-negative breast cancer (TNBC): results from GeparSixto. Int J Clin Oncol 32:5s
Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C et al (2005) Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434(7035):917–921
Mavaddat N, Barrowdale D, Andrulis IL, Domchek SM, Eccles D, Nevanlinna H, Ramus SJ, Spurdle A, Robson M, Sherman M et al (2012) Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev 21(1):134–147
Timms KM, Abkevich V, Hughes E, Neff C, Reid J, Morris B, Kalva S, Potter J, Tran TV, Chen J et al (2014) Association of BRCA1/2 defects with genomic scores predictive of DNA damage repair deficiency among breast cancer subtypes. Breast Cancer Res 16(6):475
Zhang J, Powell SN (2005) The role of the BRCA1 tumor suppressor in DNA double-strand break repair. Mol Cancer Res 3(10):531–539
Narod SA, Huzarski T, Gronwald J, Byrski T, Marczyk E, Cybulski C, Szwiec M, Wisniowski R, Birkenfeld B, Kilar E et al. (2017) Predictors of survival for breast cancer patients with a BRCA1 mutation. Breast Cancer Res Treat 168(2), 513–521
Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q, Juul N, Leong CO, Calogrias D, Buraimoh A et al (2010) Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol 28(7):1145–1153
Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, Kuzma CS, Pluard TJ, Somlo G, Port ER et al (2015) Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 33(1):13–21
von Minckwitz G, Timms K, Untch M, Elkin EP, Hahnen E, Fasching PA, Schneeweiss A, Salat CT, Rezai M, Blohmer J-U et al (2017) Homologous repair deficiency (HRD) as measure to predict the effect of carboplatin on survival in the neoadjuvant phase II trial GeparSixto in triple-negative early breast cancer. AACR Cancer Res 77 (4 Suppl):P1-09-02
Gluz O, Nitz U, Liedtke C, Christgen M, Grischke EM, Forstbauer H, Braun M, Warm M, Hackmann J, Uleer C et al (2017) Comparison of neoadjuvant Nab-Paclitaxel + carboplatin vs nab-paclitaxel + gemcitabine in triple-negative breast cancer: randomized WSG-ADAPT-TN trial results. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djx258
von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, Blohmer JU, Jackisch C, Paepke S, Gerber B et al (2014) Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 15(7):747–756
Von Minckwitz G, Loibl S, Schneeweiss A (2015) Early survival analysis of the randomized phase II trial investigating the addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer (GeparSixto). In: San Antonio Breast Cancer Symposium, San Antonio, Dec 9 2015
Curigliano G, Burstein HJ, E PW, Gnant M, Dubsky P, Loibl S, Colleoni M, Regan MM, Piccart-Gebhart M, Senn HJ et al. (2017) De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the primary therapy of early breast cancer 2017. Ann Oncol 28(8):1700–1712
Hurvitz SA, Martin M, Symmans WF, Jung KH, Huang CS, Thompson AM, Harbeck N, Valero V, Stroyakovskiy D, Wildiers H et al (2018) Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 19(1):115–126
Acknowledgements
The authors are grateful to Michael Robertson for professional medical editing services.
Author information
Authors and Affiliations
Contributions
MW, AH, PG, and PAF contributed substantially to the acquisition and interpretation of data, to the conception and drafting of the manuscript, and to critical revision. LH performed statistical analyses and contributed to the conception, drafting, and critical revision of the manuscript. The contribution of VMF to this publication was made in partial fulfillment of the requirements for obtaining the degree of Doctor of Medicine; parts of the research published here were used for her doctoral thesis at the Medical Faculty of Friedrich Alexander University Erlangen–Nuremberg (FAU). CR, MRB, CCH, MGS, SMJ, JE, RE, ABE, JH, GV, CK, AR, AH, MPL, MWB, and AH were involved in the acquisition of patient and tumor data and genetic information. All authors have read the manuscript and have given their final approval for publication of this study.
Corresponding author
Ethics declarations
Conflict of interest
PAF has received honoraria from Amgen, Celgene, Roche, Pfizer, and Novartis. MPL has received honoraria from MSD and AstraZeneca. PG has received honoraria from Novartis and financial support for symposia from Roche, Novartis, and PharmaMar. All other authors declare that they do not have any conflicts of interest.
Ethical approval
This retrospective study and the anonymized scientific use of the data were approved by the Ethics Committee of the Medical Faculty of Friedrich Alexander University Erlangen–Nuremberg.
Informed consent
Informed consent was obtained from each individual participant included in the study.
Rights and permissions
About this article
Cite this article
Wunderle, M., Gass, P., Häberle, L. et al. BRCA mutations and their influence on pathological complete response and prognosis in a clinical cohort of neoadjuvantly treated breast cancer patients. Breast Cancer Res Treat 171, 85–94 (2018). https://doi.org/10.1007/s10549-018-4797-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4797-8