Abstract
Purpose
To evaluate in a contemporary cohort the impacts of chemotherapy and oophorectomy on survival for breast cancer patients with a BRCA1 mutation.
Experimental design
We reviewed the pathology reports and medical records of 372 women with breast cancer and a BRCA1 mutation, diagnosed from 2005 to 2017, between the ages of 25 and 65 and followed them for death from all causes and death from breast cancer. Death was ascertained through the Poland vital statistics registry. We performed survival analysis to evaluate the impacts of chemotherapy (including neoadjuvant cisplatinum) and of oophorectomy on survival.
Results
After a mean follow-up of 5.6 years (median 5.2), 66 of the 372 women died; 56 of the deaths were from breast cancer and 6 were from ovarian cancer. 127 women received neoadjuvant cisplatinum and 245 women received other chemotherapies. Cisplatinum (versus all other therapies) was associated with a hazard ratio of 0.42 (95%CI 0.20–0.87) on breast cancer-specific survival. The 10-year actuarial all-cause survival for women who had both cisplatinum and an oophorectomy was 94.4%. The 10-year all-cause survival for women who had neither cisplatinum nor an oophorectomy was 65.4% (p < 0.01).
Conclusions
Cisplatinum and oophorectomy are effective therapies for women with breast cancer and a BRCA1 mutation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The potential benefit of precision medicine as applied to the management of breast cancer may be realized by defining combinations of host and tumor factors which predict treatment response. Among the relevant host factors is the presence of a genetic mutation in a susceptibility gene. A mutation may indicate a difference in the performance of a specific prognostic factor, such as ER status and survival in BRCA2 carriers [1] or in outcome, such as poor survival of PALB2 carriers with breast cancer [2]. BRCA1-associated cancers differ from nonhereditary cancers for a range of pathologic factors, including tumor grade and histologic appearance [3, 4]. It is also reported that the BRCA1 host genotype predicts response to treatment; for example, BRCA1 carriers differ from noncarriers in that very small breast cancers appear to benefit from chemotherapy [5]. We see a high rate of pathologic complete response (PCR) with cisplatinum and with conventional chemotherapies [6, 7]. We have shown that ER-negative breast cancer patients with a BRCA1 mutation benefit from oophorectomy [8,9,10]. Recently, a significant prolongation in cancer-free progression with the PARP inhibitor olaparib has been reported [11].
The current study was performed as a component of a large ongoing, multicenter research program conducted in Poland at the Pomeranian Medical University, designed to characterize the hereditary burden of breast cancer in the country and to identify strategies for prevention, screening and treatment for high-risk women. In Poland, there are three BRCA1 founder mutations (5382insC, C61G and 4153delA), which account for the great majority of all BRCA1 mutations in Polish families [12]. The Pomeranian Medical University Hereditary Cancer Center has collected information on family history, on genetic test results and clinical information on a large number of breast cancer patients diagnosed at various centers throughout the country. Since 2005, genetic testing of new breast cancer has been widespread in Poland and oophorectomy has been routinely recommended. Neoadjuvant cisplatinum has become standard therapy in three centers (Szczecin, Krakow, Bielsko-Biala). In an earlier study, we reported that a high rate of complete pathologic response was achieved using cisplatin chemotherapy as a single agent for BRCA1 carriers in the neoadjuvant setting [6], but it has not been shown that the high rates of pathologic complete response translate into better overall survival. In the current report, we evaluate for the first time, the impact of cisplatinum on cancer mortality in breast cancer patients with and without an oophorectomy. The current study includes a contemporary cohort of patients who were diagnosed and treated from 2005 to 2017 all of whom tested positive for a BRCA1 founder mutation.
Materials and methods
Patient eligibility
Female patients age 25 years and above and who were known to carry a BRCA1 mutation were eligible. Patients were diagnosed between 2005 and 2017. Patients were recruited from 17 cancer hospitals in Poland affiliated with the Pomeranian Medical University. Death data were obtained from the Poland vital statistics registry. The protocol was approved by the Ethics Committee of the Pomeranian Medical University.
The patient’s course of treatment was at the physician’s discretion and treatment decisions were not made as a result of participating in this (observational) study. Neoadjuvant chemotherapy was only offered to patients in selected centers. The recommendation for or against adjuvant chemotherapy following neoadjuvant chemotherapy was also at the doctors’ discretion. Of the 86 women who had a PCR, 50 (63.2%) had adjuvant chemotherapy (7 missing data). Of the 101 women who had no response or partial response to neoadjuvant therapy, 75 (78.1%) had adjuvant chemotherapy (5 missing data).
Statistical methods
Actuarial survival rates were calculated using the Kaplan–Meier method. In these analyses, left censoring to the date of genetic testing was done. Oophorectomy was considered as a time-dependent variable. A series of survival analyses were conducted using the Cox proportional hazard model.
Size was assessed in two ways: Clinical size was based on presurgical, pre-chemotherapy evaluation, based on imaging (MRI, ultrasound, mammography) and physical examination. Clinical nodal status was defined in the same way. Pathological size was defined by examination of the surgical specimens (resected breast tissue and axillary lymph nodes). For patients who received neoadjuvant chemotherapy, the absence of cancer in the primary specimen and nodes was considered to be a pathologic complete response.
Subjects were followed from the date of diagnosis until death or July 2017. Covariates included clinical tumor size (3 categories) clinical nodal status (negative/positive) and ER status (±, missing). Chemotherapy was categorized as platinum, other, none. Oophorectomy was categorized as a time-dependent variable. Surgery was coded as mastectomy or lumpectomy. For estimation of breast cancer-specific mortality, women who died of another cause were censored as unaffected at the date of death. Survival analysis was done using left censoring to the date of genetic testing. 142 women had genetic testing prior to breast cancer and 228 women had genetic testing after breast cancer. Of those that had testing after breast cancer, on average, 14.9 months had elapsed from the date of diagnosis to the date of genetic testing.
Results
Patient characteristics
Between November 2005 and December 2016, 447 potentially eligible women were identified. Each of these women had breast cancer and had been tested for the presence of three BRCA1 founder mutations and each had been found to be positive. Patients were excluded if the cancers were noninvasive (DCIS) (n = 4); if they had previously been treated for a contralateral breast cancer (n = 41) or if they were older than age 65 at diagnosis (n = 16). 10 patients were excluded because of a prior history of ovarian cancer; 5 subjects were excluded because of another chemotherapy before cisplatinum for the same cancer.
This patient population was notable for its young age at diagnosis (median age 43.5 years) and the predominance of triple-negative cancers (70.3%). Only 20 patients were HER2-positive. 129 of the patients were (clinically) node-positive at diagnosis.
Eleven women had bilateral cancer at diagnosis, of these six had bilateral mastectomy (for the other 5, surgery data were missing). 321 subjects had unilateral breast cancer, of these four had bilateral mastectomy.
204 of the patients were treated with neoadjuvant chemotherapy, including 127 patients treated with cisplatinum. Of the 127 patients treated with cisplatinum, 90 received additional adjuvant chemotherapy after surgery (27 no; 10 missing). The patients who received neoadjuvant cisplatinum chemotherapy are compared with the other patients in Table 1.
183 of the patients had an oophorectomy (49%). Of these, 54 had the oophorectomy prior to the diagnosis of breast cancer, 84 had the oophorectomy within 1 year of the diagnosis of breast cancer and 41 had the oophorectomy one or more years after the breast cancer (4 missing data of oophorectomy).
66 of the women have died. 56 died of breast cancer, 6 died of ovarian cancer one died of peritoneal cancer and three died of other causes. The 5-year actuarial breast cancer survival was 85.0%. The 10-year actuarial breast cancer survival was 80.0%.
204 of the 372 patients received neoadjuvant chemotherapy. 127 women received neoadjuvant cisplatinum; of these 75 (59.1%) had a PCR. 77 of the women received another form of neoadjuvant chemotherapy; of these, 60 had pathological report, and 11 of the 60 (12.8%) had a PCR.
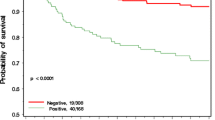
Figure 1 shows the 10-year actuarial breast cancer mortality (Kaplan–Meier) for the 127 women who had neoadjuvant cisplatinum chemotherapy compared with the 77 women who had other types of neoadjuvant chemotherapy.
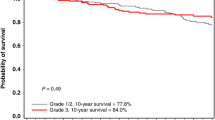
Figure 2 shows the 10-year actuarial breast cancer mortality (Kaplan–Meier) for the 86 women who had a PCR after neoadjuvant chemotherapy compared with the 101 women who had a partial response or no response after neoadjuvant chemotherapy.
Cisplatinum was only given as neoadjuvant therapy, but other forms of chemotherapy were given as adjuvant or neoadjuvant therapy. Figure 3 shows the 10-year actuarial breast cancer mortality (Kaplan–Meier) for the 127 women who had neoadjuvant cisplatinum compared with the 245 women who had other types of chemotherapy or no chemotherapy.
Figure 4 shows the 10-year actuarial mortality from all causes of death (Kaplan–Meir) for the 125 women who had an oophorectomy after the diagnosis of breast cancer and 187 women who had no oophorectomy (neither before nor after diagnosis).
Figure 5 shows the 10-year actuarial mortality from breast cancer (Kaplan-Meir) for the 125 women who had an oophorectomy before or after the diagnosis of breast cancer and the 187 women who had no oophorectomy (neither before nor after diagnosis). The adjusted hazard ratio (all cause mortality) for oophorectomy for cases of all ages was 0.59 (95% CI 0.32–1.07; p = 0.08). After excluding the 55 patients who had an oophorectomy before breast cancer, the adjusted hazard ratio (all cause mortality) for oophorectomy for cases of all ages was 0.49 (95% CI 0.25–0.96 p = 0.04).
We conducted a Cox proportional hazard model to compare the effects of various chemotherapies on breast cancer-specific survival. (Table 2) We included clinical tumor size and clinical nodal status in the model because these values were determined before chemotherapy (whereas pathologic size and nodal status were determined after chemotherapy for the cisplatinum group.) We also include ER status, age of diagnosis and oophorectomy in the model. The survival analysis was left censored to the date of genetic testing. Oophorectomy was considered a time-dependent variable. The first analysis was chemotherapy and breast cancer mortality (platinum, other, none). Compared with women who did not have chemotherapy, those who had neoadjuvant cisplatinum had an adjusted hazard ratio of 0.19 (0.05–0.78; p = 0.02) for breast cancer-specific death. Compared with women who did not have chemotherapy, those who had other forms of chemotherapy (adjuvant or neoadjuvant) had a hazard ratio of 0.40 (0.11–1.44; p = 0.16) for breast cancer-specific death.
Compared with women who had other forms of chemotherapy, those who had neoadjuvant cisplatinum had a hazard ratio of 0.48 (0.23–1.01; p = 0.05) for breast cancer-specific death.
Compared with women who had other forms of chemotherapy or no chemotherapy, those who had neoadjuvant cisplatinum had a hazard ratio of 0.46 (95% CI 0.22–0.96; p = 0.04 for breast cancer-specific death.
Figure 6 shows the ten-year actuarial mortality from breast cancer (Kaplan–Meier) for the 75 women who had an oophorectomy and platinum, for the 106 women who had an oophorectomy only, for the 52 women who had platinum only and for the 135 women who had neither therapy. Figure 7 shows the 10-year actuarial mortality from breast cancer (Kaplan–Meier) for the 233 women who had an oophorectomy or platinum and for the 135 women who had neither therapy.
Discussion
In this study, we report that among newly diagnosed cases of breast cancer in women with a BRCA1 mutation, those who were treated with neoadjuvant cisplatinum experienced a superior ten-year survival rate (90.5%) than women who were treated with other forms of chemotherapy or with no chemotherapy (75.7%) (Fig. 3). Exceptional survival was seen for those patients who experienced a pathologic complete response to neoadjuvant cisplatinum (10 year survival 97%); among women who experienced a partial response or no response the ten-year survival rate was much less (67 and 11%, respectively). The high rate of pathologic complete response reported here among cisplatinum users (59%) is slightly less than that we reported in an earlier analysis of this cohort (61%) [6]. Until now, we were not confident that the high rates of pathologic complete response would translate into a high survival rate and we did not make clinical recommendations based on PCR rates alone. However, in the present study, of the 86 women who experienced a pathologic complete response, only two died of breast cancer after a mean of 5.5 years of follow-up. The combination of cisplatinum and oophorectomy appears to be an efficacious approach to treatment, and we encourage further research in this area.
The principal strength of our study is that the primary outcome of interest was death from all causes, and this was determined systematically and in an identical fashion for all patients by linkage to the Polish vital statistics registry. That is, the evaluation of the benefit of cisplatinum was not reliant on the judgment of physicians, nor did it require the review of medical records and images (as is the case for treatment response and for tumor-free progression). Further, the validity of mortality as a relevant clinical endpoint is not in doubt. The determination of vital status per se was not subjective, although there may be occasional issues related to assigning the underlying cause of death.
We also observed a survival benefit associated with bilateral oophorectomy, confirming our earlier reports [8,9,10]. The principal indication for oophorectomy during the period of this study was for the prevention of ovarian cancer, and, to our knowledge, the timing of oophorectomy was not chosen with the view to enhance breast cancer therapy. The hazard ratio associated with oophorectomy done after diagnosis was 0.49 (95% 0.25–0.96). We suggest that the oophorectomy be performed as soon as possible after breast cancer diagnosis, but data in this study are insufficient to allow us to distinguish between patients with oophorectomies done in the various time frames. Of the women who did not have an oophorectomy, 41 died of breast cancer and six died of ovarian cancer—supporting the premise that the benefit of oophorectomy is based on a combination of outcomes.
Recently, enthusiasm has been expressed for the conduct of fallopian tubes-only surgery for cancer prevention in women with BRCA1 mutations [13]. The rationale behind this approach is that the majority of serous cancers in BRCA1 carriers originate in the fallopian tubes [14], and these can be prevented through salpingectomy, even if the ovaries are left intact. Further, two recent cohort studies did not support the hypothesis that oophorectomy prevents breast cancer in BRCA1 carriers [15, 16], and these studies dampened enthusiasm for preventive oophorectomy. However, we emphasize that preventive oophorectomy has been associated with a large risk reduction in mortality after breast cancer surgery in several studies and the substantial benefit is not expected to be realized with tubes-only surgery. Therefore, we recommend that candidacy for tubes-only surgery be limited to women who have not been diagnosed with breast cancer.
We believe that our data support the rationale to offer neoadjuvant cisplatinum as first-line treatment to women with breast cancer and a BRCA mutation. However, several important questions remain. In this study, 90 of the 127 women with cisplatinum also received adjuvant chemotherapy and it is not clear if adjuvant chemotherapy needs to be recommended to carriers who experience a PCR after single agent cisplatinum. We did not include BRCA2 carriers in this study because these are rare in Poland and it is important that these studies be replicated in countries where BRCA2 mutations are prevalent.
Our study has several strengths. The identification of 372 mutation carriers with breast cancer was the result of a comprehensive genetic testing program coordinated throughout the country, and the mutation-positive cohort represented here is the product of our testing of 14,050 breast cancer patients in a single laboratory between 2005 and 2016. Also, we were able to enroll all the tested patients in our central repository in our clinical research studies. In order to achieve the maximum benefit from personalized therapy, it is important that genetic testing be offered widely to breast cancer patients in a timely fashion at the time of diagnosis.
There are also several weakness to our study. This is a nonrandomized study, and treatment was at the discretion of the individual physician. The choice of neoadjuvant platinum chemotherapy was based on BRCA1-status alone, but for other forms of neoadjuvant chemotherapy, treatment was given preferentially to those with locally advanced disease. Thus, in the nonplatinum group, those who were given neoadjuvant chemotherapy had more advanced disease than those who were given conventional adjuvant chemotherapy, and the comparison of platinum-based versus other forms of neoadjuvant chemotherapy is not robust. Although this is the largest study of its type, the subgroups were relatively small, and the conclusions were based only on 56 deaths from breast cancer. The principal outcome is ten-year survival, and ideally, we would have 10 years of follow-up on all patients. Cisplatinum was introduced in 2006 and the average follow-up time was 5 years. It is important that we expand this cohort and continue to follow it for new events.
It is inherently difficult to compare women treated with neoadjuvant chemotherapy and those treated with adjuvant chemotherapy in an observational cohort study, because of the complexity in adjusting for tumor size and nodal status. Different sources of information are used; in the patients treated with neoadjuvant therapy who experience a PCR, pathologic size is recorded as ‘no tumor detected’ and nodes are ‘clear’ and therefore for neoadjuvant patients, stage must be determined prior to treatment using clinical variables (examination and imaging). Lymph nodes which are reported as negative on clinical examination may be reported as positive on pathologic examination. We sought to be comprehensive in obtaining complete stage information from both clinical sources and from pathologic reports and to be consistent in how stage was evaluated when the two groups were compared, but there were many patients with missing values and the adjusted analyses are not robust because of missing information. Nevertheless, we believe that the women who got cisplatinum and those who got other forms of chemotherapy were comparable in terms of inherent prognosis. Patients were not selected to receive neoadjuvant chemotherapy based on tumor size, on nodal status or on other prognostic factors, but rather on where they received their treatment. 101 of the 127 of the women who received neoadjuvant cisplatinum chemotherapy were treated in Szczecin and in this single center 101 of 127 (80%) of all patients received neoadjuvant cisplatinum. We recognize that in ideal circumstances preferences regarding the choice of chemotherapy should be determined by randomized trial but randomized trials are difficult to conduct and in general, in a given trial, comparisons are restricted to two of many possible treatments. To our knowledge, no randomized trials are being conducted using neoadjuvant or adjuvant cisplatinum in BRCA1 carriers and we are not aware of other large clinical research cohorts where this drug is now being administered. In the realm of ‘precision medicine’ for other genetically defined subgroups of cancer patients, benefit is often measured in far less rigorous terms, such as the anecdotal ‘response to treatment’ (tumor shrinkage) in one or a few patients or ‘progression-free survival’ and we are reluctant to offer advice to our patients in the absence of a demonstrated mortality difference.
References
Jonasson JG, Stefansson OA, Johannsson OT, Sigurdsson H, Agnarsson BA, Olafsdottir GH, Alexiusdottir KK, Stefansdottir H, Munoz Mitev R, Olafsdottir K, Olafsdottir K, Arason A, Stefansdottir V, Olafsdottir EJ, Barkardottir RB, Eyfjord JE, Narod SA, Tryggvadóttir L (2016) Oestrogen receptor status, treatment and breast cancer prognosis in Icelandic BRCA2 mutation carriers. Br J Cancer 115(7):776–783
Cybulski C, Kluźniak W, Huzarski T, Wokołorczyk D, Kashyap A, Jakubowska A, Szwiec M, Byrski T, Dębniak T, Górski B, Sopik V, Akbari MR, Sun P, Gronwald J, Narod SA (2015) Lubiński J; Polish Hereditary Breast Cancer Consortium. Clinical outcomes in women with breast cancer and a PALB2 mutation: a prospective cohort analysis. Lancet Oncol 16(6):638–644
Marcus JN, Watson P, Page DL, Narod SA, Tonin P, Lenoir GM, Serova O, Lynch HT (1997) BRCA2 hereditary breast cancer pathophenotype. Breast Cancer Res Treat 44:275–277
Lakhani SR, Jacquemier J, Sloane JP, Gusterson BA, Anderson TJ, van de Vijver MJ, Farid LM, Venter D, Antoniou A, Storger-Isser A, Smyth E, Steel CM, Haites N, Scott RJ, Goldgar D, Neuhausen S, Daly PA, Ormiston W, McManus R, Scherneck S, Ponder BA, Ford D, Peto J, Stoppa-Lyonnet D, Bignon YJ, Struewing JP, Spurr NK, Bishop DT, Klijn JG, Devilee P et al (1998) Multifactorial analysis of differences between sporadic breast cancers and cancers involving BRCA1 and BRCA2 mutations. J Natl Cancer Inst 90:1138–1145
Narod SA, Metcalfe K, Lynch HT, Ghadirian P, Robidoux A, Tung N, Gaughan E, Kim-Sing C, Olopade OI, Foulkes WD, Robson M, Offit K, Jakubowska A, Byrski T, Huzarski T, Sun P, Lubinski J (2013) Should all BRCA1 mutation carriers with stage I breast cancer receive chemotherapy? Breast Cancer Res Treat 138(1):273–279
Byrski T, Huzarski T, Dent R, Marczyk E, Jasiowka M, Gronwald J, Jakubowicz J, Cybulski C, Wisniowski R, Godlewski D, Lubinski J, Narod SA (2014) Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat 147(2):401–405
Arun B, Bayraktar S, Liu DD, Gutierrez Barrera AM, Atchley D, Pusztai L, Litton JK, Valero V, Meric-Bernstam F, Hortobagyi GN, Albarracin C (2011) Response to neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers: a single-institution experience. J Clin Oncol 29(28):3739–3746
Finch AP, Lubinski J, Møller P, Singer CF, Karlan B, Senter L, Rosen B, Maehle L, Ghadirian P, Cybulski C, Huzarski T, Eisen A, Foulkes WD, Kim-Sing C, Ainsworth P, Tung N, Lynch HT, Neuhausen S, Metcalfe KA, Thompson I, Murphy J, Sun P, Narod SA (2014) Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol 32(15):1547–1553
Metcalfe K, Lynch HT, Foulkes WD, Tung N, Kim-Sing C, Olopade OI, Eisen A, Rosen B, Snyder C, Gershman S, Sun P, Narod SA (2015) Effect of oophorectomy on survival after breast cancer in BRCA1 and BRCA2 mutation carriers. JAMA Oncol. 1(3):306–313
Huzarski T, Byrski T, Gronwald J, Cybulski C, Oszurek O, Szwiec M, Gugała K, Stawicka M, Morawiec Z, Mierzwa T, Falco M, Janiszewska H, Kilar E, Marczyk E, Kozak-Klonowska B, Siołek M, Surdyka D, Wiśniowski R, Posmyk M, Domagała P, Sun P, Lubiński J (2016) Narod SA; Polish Breast Cancer Consortium. The impact of oophorectomy on survival after breast cancer in BRCA1-positive breast cancer patients. Breast Cancer Res Treat 156(2):371–378
Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A, Wu W, Goessl C, Runswick S, Conte P (2017) Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med 377(6):523–533
Górski B, Byrski T, Huzarski T, Jakubowska A, Menkiszak J, Gronwald J, Pluzanski A, Bebenek M, Fischer-Maliszewska L, Grzybowska E, Narod SA, Lubinski J (2000) Founder mutations in BRCA1 gene in Polish families with breast-ovarian cancer. Am J Hum Genet 66:1963–1968
Long Roche KC, Abu-Rustum NR, Nourmoussavi M, Zivanovic O (2017) Risk-reducing salpingectomy: let us be opportunistic. Cancer 123(10):1714–1720
Labidi-Galy SI, Papp E, Hallberg D, Niknafs N, Adleff V, Noe M, Bhattacharya R, Novak M, Jones S, Phallen J, Hruban CA, Hirsch MS, Lin DI, Schwartz L, Maire CL, Tille JC, Bowden M, Ayhan A, Wood LD, Scharpf RB, Kurman R, Wang TL, Shih IM, Karchin R, Drapkin R, Velculescu VE (2017) High grade serous ovarian carcinomas originate in the fallopian tube. Nat Commun 8(1):1093
Heemskerk-Gerritsen BA, Seynaeve C, van Asperen CJ, Ausems MG, Collée JM, van Doorn HC, Gomez Garcia EB, Kets CM, van Leeuwen FE, Meijers-Heijboer HE, Mourits MJ, van Os TA, Vasen HF, Verhoef S, Rookus MA, Hooning MJ; Hereditary Breast and Ovarian Cancer Research Group Netherlands (2015) Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djv033
Kotsopoulos J, Huzarski T, Gronwald J, Singer CF, Moller P, Lynch HT, Armel S, Karlan B, Foulkes WD, Neuhausen SL, Senter L, Tung N, Weitzel JN, Eisen A, Metcalfe K, Eng C, Pal T, Evans G, Sun P, Lubinski J, Narod SA, Hereditary Breast Cancer Clinical Study Group (2016) Bilateral oophorectomy and breast cancer risk in BRCA1 and BRCA2 Mutation Carriers. J Natl Cancer. https://doi.org/10.1093/jnci/djw177
Acknowledgements
We thank Ewa Putresza for excellent technical support. We thank the Peter Gilgan Foundation Tour de Bleu and Estée Lauder Companies for their generous support of this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Narod, S.A., Huzarski, T., Gronwald, J. et al. Predictors of survival for breast cancer patients with a BRCA1 mutation. Breast Cancer Res Treat 168, 513–521 (2018). https://doi.org/10.1007/s10549-017-4605-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4605-x