Abstract
The aims of the study were to identify a subpopulation more likely to be at greater risk of recurrence in small T1b-c node-negative hormone receptor (HR)-positive breast cancer, and which would benefit from adjuvant chemotherapy. Clinico-pathologic characteristics and clinical outcomes of 538 postoperative HR-positive T1b-cN0 breast cancer patients were retrospectively analyzed. High Ki67 index and a young age (<35 years) were identified as independent risk factors for relapse (p < 0.0001 and 0.015, respectively). A nomogram based on Cox-regression model showed an area under the curve (AUC) of 0.73 in the training set. The validation set showed a good discrimination with an AUC of 0.65. In patients with high nomogram scores (≥100, n = 24, 4.5%) who had high Ki67 index with more than 75%, or young age (<35 years) and a Ki67 index >50%, the relapse-free survival curve of patients who had received anthracycline-containing adjuvant chemotherapy showed a better outcome than those who had not (p = 0.029). Ki67 index and age are valuable surrogate markers to predict recurrence and as indicators of tumors that could benefit from adjuvant chemotherapy in small T1b-c node-negative HR-positive breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the use of screening programs and the awareness of particular concern about early breast cancer grow, small breast tumors are an emerging and increasing problem [1–3]. Small breast cancer represents a subset of favorable prognosis, with relatively low incidence of metastases to axillary lymph node or distant organs, and adjuvant systemic chemotherapy is not routinely recommended in this population [4–7].
Recently, the role of adjuvant chemotherapy for small node-negative breast cancers has been justified in some high-risk patients, which include human epidermal growth factor receptor 2 (HER2)-positive and triple-negative breast cancers (TNBC) [8–12].
In contrast, over the last few years, the added benefits of chemotherapy in hormone receptor (HR)-positive breast cancer patients have been questioned and its role debated, even though HR-positive breast cancer constitutes 60–80% of breast cancer arising in women [13], where endocrine therapy is the mainstay of adjuvant therapy [14–20].
HR-positive breast cancer has been reported to be less sensitive to chemotherapy in several neoadjuvant chemotherapy trials, in which pathologic complete remission was less likely to be achieved in HR-positive patients [21–23]. HR-positive breast cancer is currently defined in terms of estrogen receptor (ER) and/or progesterone receptor (PgR) expression on the basis of the immunohistochemical (IHC) staining. However, HR-positive tumors can be subdivided into subgroups according to intrinsic gene expression profiles [24, 25]. Two biologically distinct ER-positive subtypes of breast cancer have been described: luminal A and luminal B [25–29]. The luminal subtype of breast cancer is characterized by ER-associated gene expression. The major difference between the luminal A and B types is the proliferation signature, where proliferating genes including CCNB1, MKI67, and MYBL2 are more highly expressed in the luminal B type than in the luminal A type [27, 30]. Therefore, a distinction between luminal A and B tumors that is based on proliferation status is important to breast cancer biology and prognosis. Indeed, in HR-positive, HER2-negative disease, patients with high levels of Ki67 show poor prognosis and benefit more from stronger chemotherapy [31, 32].
Reflecting these complexities, according to the current 2011 NCCN guideline, the 21-gene reverse transcription-polymerase chain reaction (RT-PCR) assay can be considered for tumors >0.5 cm in size in HR-positive, HER2-negative cancers. In cases of high recurrence score (≥31), adjuvant chemotherapy in addition to endocrine therapy is recommended as category 2B. Because of the high cost and low feasibility of the gene array, especially outside the United States, it cannot routinely be used in clinical practice and its usefulness still needs to be defined in prospective randomized trials. It would be better if there were valuable markers to determine risk for relapse in this particular setting.
We hypothesized that there could be a subpopulation that might derive clinical benefit from adjuvant chemotherapy, even in this small node-negative HR-positive tumors. Accordingly, the aims of this study were to identify a subpopulation likely to be at greater risk of recurrence, including systemic failure, than others in small T1b-c node-negative, HR-positive breast cancers, and to assess the role of adjuvant chemotherapy for this subpopulation by developing a nomogram to predict recurrence.
Patients and methods
Patients
We retrospectively identified patients who were diagnosed with histologically confirmed invasive breast cancer and received curative surgery at Samsung Medical Center (SMC) from 2004 to 2007, and 505 patients as a validation set from Seoul National University Hospital (SNUH) during the same period. The pathologic characteristics of the tumors including tumor size, nuclear and histologic grade, presence of lymphovascular invasion (LVI), multiplicity, and the result of IHC staining were reviewed and determined by two experienced pathologists. ER and PgR status was defined as positive when the IHC determined Allred score was 3–8 using antibodies to the ER (Immunotech, France) and PgR (Novocastra, UK). In validation set, ER assays were considered positive if there are at least 1% positive tumor nuclei in the sample on testing in the presence of expected reactivity of internal and external controls according to ASCO/COA pathologists guideline recommendations [33]. HER2 status was evaluated using HER2 antibody (DAKO, USA) and/or fluorescence in situ hybridization (FISH). IHC grades 0 and 1 for HER2 were defined as negative, and grade 3 was defined as positive. Amplification of HER2 was confirmed by FISH if HER2 was rated 2+ by IHC. Triple negativity was defined as a lack of ER, PgR, and HER2 expression. IHC Ki67 analyses were evaluated by both independent semiquantitative and quantitative methods (DAKO). For quantitative analysis, the percentage of nuclei staining positive for Ki67 was calculated for each section based on a value of approximately 1,000 carcinoma cell nuclei/section using an image analyzer (I-solution delta, Korea). For semiquantitative analysis, positive signals were graded by two experienced pathologists as follows: <5% staining, 0; 5–25%, 1+; 26–50%, 2+; 51–75%, 3+; >75%, 4+. Our study was approved by the institutional review board of the two institutions.
Statistical analyses
The receiver operating characteristic (ROC) curve was drawn for all 538 T1b-cN0 patients for the nomogram. The power of the Ki67 kinetics was evaluated using the area under the curve (AUC) with a 95% confidence interval (CI). Recurrence free survival (RFS) was defined from the date of curative surgery to the date of breast cancer recurrence. RFS was estimated by the Kaplan–Meier method, and compared using the log-rank test. A p value <0.05 was considered significant. A Cox proportional hazards regression model was used to assess the effect of each potential prognostic variable on RFS. All potential prognostic variables were included and analyzed by a backward selection process using the likelihood ratio test and a significance level of 0.05. Based on the Cox-regression model, a nomogram for recurrence in HR-positive breast cancers with T1b-cN0To was developed. The nomogram performance was quantified with respect to discrimination and calibration. To assess the model’s performance, the bootstrapping method (1,000 repetitions) was used. Discrimination was quantified with the AUC. We performed the calibration using graphic representations of the relationship between the observed outcome frequencies and the predicted probabilities.
REMARK guidelines
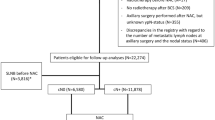
In reporting our study, we have adhered to the guidelines of an important methodological paper from 2005 entitled “Reporting recommendations for tumor marker prognostic studies (REMARK guidelines)” [34, 35]. We included “Patient Cohort” to fulfill these criteria (Fig. 1) to decrease potential bias arising in review of the medical records.
Results
Patient cohort (Fig. 1)
We searched the electronic database at Samsung Medical Center and retrospectively reviewed records for 1,996 patients who were diagnosed with breast cancer and received curative surgery at the center from January 2004 to June 2007. Of these patients, we excluded 113 patients with ductal carcinoma in situ (DCIS) or lobular carcinoma in situ (LCIS), 45 patients with micro-invasive cancer, and 122 patients who did not have available Ki67 index data from the analysis. An additional 152 patients who received neoadjuvant chemotherapy were excluded, leaving a cohort of 1,564 patients. Of these, 1,032 patients (66.0%) were HR-positive (defined as ER-positive and/or PgR-positive and HER2-negative), 532 (34.0%) were HER2-positive (HER2-positive regardless of ER and PgR status), or were TNBC (ER-negative, PgR-negative, and HER2-negative). We excluded 457 patients whose tumor size was ≥2 cm or whose axillary lymph node was positive. Finally, after excluding another 37 patients whose tumor size was ≤0.5 cm, the final patient cohort was 538 patients. A second cohort that included 505 patients who received curative surgery at Seoul National University Hospital (Seoul, Korea) during the same period was used for external validation of the model.
Clinicopathological characteristics in patients with T1b-c node-negative HR-positive breast cancers
The median age at diagnosis was 47 years (range, 22–79 years). Both high nuclear and histologic grades were found in 19.1%. Invasive ductal carcinoma was found in 73.4% of the patients. Mucinous, lobular, and tubular carcionoma were found in 13.8, 5.2, and 3.3% of patients, respectively (Table 1). Twelve patients (2.2%) were ER-negative/PgR-positive, 52 patients (9.7%) were ER-positive/PgR-negative, and the remaining 474 patients (88.1%) were both ER- and PgR-positive. The Ki67 index scores by the semiquantitative method were 0 for five patients (0.9%), 1+ for 330 patients (61.3%), 2+ for 124 patients (23.0%), 3+ for 60 patients (11.2%), and 4+ for 19 patients (3.5%). Adjuvant chemotherapy was administered to 53.3% of patients. Anthracycline-based adjuvant chemotherapy was administered to 44.8% of patients. Adjuvant endocrine and radiation treatment were administered to 94.5 and 63.7% of patients after curative surgery, respectively (Table 1).
Clinical outcomes of 538 T1b-cN0M0 HR-positive breast cancer patients
During the median 60.5 months of follow-up, the 5-year recurrence rate was 5.2%. The 5-year overall survival rate was 99.6%. To identify predictive factors for recurrence in T1b-cN0, we analyzed RFS in 538 patients. There were significant differences regarding RFS by log-rank test according to histologic grade (p = 0.003), nuclear grade (p = 0.032), Ki67 index (p < 0.0001), and age <35 years (p = 0.003) in univariate analyses. There was no significant difference according to tumor size of sub centimeter (<1 cm; p = 0.826), ER status (p = 0.354), and PgR status (p = 0.905). We also analyzed the impact of ER scores on outcomes. However, there is no significant difference between low (3–4) and high (5–8) ER Allred scores in terms of RFS and chemotherapy benefit (data not shown). In Cox-regression multivariate analysis, high Ki67 index and young age (<35 years) were identified as independent risk factors for relapse (p < 0.0001 for Ki67 index and 0.015 for young age; Fig. 2; Table 1).
Recurrence-free survival (RFS) Kaplan–Meier curves in T1b-cN0M0 HR-positive breast cancer patients. a RFS of T1b-cN0M0 HR-positive patients according to Ki67 proliferative index; blue line represents RFS for Ki67 index 0, green line represents RFS for Ki67 index 1+, orange line represents RFS for Ki67 index 2+, purple line represents RFS for Ki67 index 3+, and green line represents of RFS for Ki67 index 4+ (p < 0.0001 by log-rank test). b RFS of T1b-cN0M0 HR-positive patients according to age; blue line represents RFS for patients with age of 35 years old or more and green line represents RFS for patients with age of less than 35 years (p = 0.003 by log-rank test)
Nomogram to predict recurrence in patients with T1b-cN0 HR-positive breast cancer patients and external validation
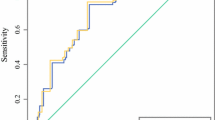
According to the Cox-regression multivariate model, we constructed a nomogram (Fig. 3a). The prediction model had an AUC of 0.75 in the training set (95% CI 0.71–0.78; p < 0.0001) (Fig. 3b). The discrimination in validation set was good, with an AUC of 0.65 (95% CI 0.61–0.70; p = 0.0008) (Fig. 3c), which confirmed the exportability of our nomogram model. According to our nomogram score, we defined high-risk patients as a group of patients who had high nomogram score ≥100 (Ki67 4+ with all ages or age <35 years with Ki67 3+ or more). RFS between high- and low-risk patients were significantly different (Fig. 4a). RFS of high-risk patients was markedly shorter than that of low-risk patients (p < 0.0001).
Nomogram to predict recurrence in T1b-cN0 HR-positive breast cancers and ROC curve on nomogram a is nomogram. Points are translated to probability of recurrence. Predictor points are found on the upper-most point scale that corresponds with each patient variable. The reader then manually adds up the points, and the predicted values can be read at the bottom of the nomogram. Linear predictor is defined according to total points. More than 1 of linear predictor indicates decreased 36 or 60 months of DFS probability. The total projected on the bottom scale indicates the probability of recurrence at 36- and 60-month. b showed ROC curve on nomogram to predict recurrence in T1b-cN0 HR-positive breast cancers. AUC = 0.75, p < 0.0001, 95% CI; 0.71–0.78. c showed ROC curve on nomogram using external validation patients’ set. AUC = 0.65, p = 0.0008, 95% CI; 0.61–0.70
Recurrence-free survival (RFS) Kaplan–Meier curves in T1b-cN0M0 breast cancer patients. a RFS of T1b-cN0M0 HR-positive patients according to the risk group; blue line represents RFS of low-risk patients and green line represents RFS of high-risk patients (nomogram score ≥100; Ki67 index 4+ or less than 35 years with Ki67 index 3+ or more) (p < 0.0001 by log-rank test). b RFS Kaplan–Meier curve in high-risk patient of T1b-cN0M0 breast cancer patients according to anthracycline-based adjuvant chemotherapy; green line represents RFS of the patients who did receive anthracycline-based adjuvant chemotherapy among high-risk patients and blue line represents RFS of the patients who did not receive anthracycline-based adjuvant chemotherapy among high-risk patients (p = 0.029 by log-rank test)
Adjuvant chemotherapy benefit in high-risk small (T1b-c), node negative HR-positive breast cancers using nomogram
To investigate the benefit from adjuvant chemotherapy in patients identified as high-risk from Cox-regression multivariate analysis, we analyzed RFS according to the chemotherapy. The high-risk patients (n = 24, 4.5%) who had a high nomogram score displayed statistically better RFS for anthracycline-containing adjuvant chemotherapy (p = 0.029, Fig. 4b). In cases of patients who did not receive anthracycline-based adjuvant chemotherapy in the high-risk group, the median RFS was 40.8 months, while the median RFS of the patients who did receive anthracycline-based adjuvant chemotherapy was not reached.
Discussion
The aims of the study were to identify the high-risk group for recurrence in T1b-c node-negative, HR-positive patients, and to determine which patients are at the greatest risk of recurrence and so who may benefit from adjuvant chemotherapy. The tailoring of treatment in the management of HR-positive breast cancer is an area of lively debate. We hypothesized that analyzing small HR-positive breast cancer might reveal a subset of high-risk patients having aggressive tumor biology among HR-positive patients.
ER expression continues to rise beyond menopause in contrast to the normal mammary gland ER content, which increases with each decade of age until plateauing with menopause [36]. In general, breast cancers that occur at a younger age have a worse clinical course associated with higher epidermal growth factor receptor expression, higher ER negativity, and higher proliferative gene expression [37–39]. Furthermore, an age <35 years is a risk factor according to the St. Gallen risk category [40]. Considering this, breast cancers in patients’ <35-years-of-age with a high Ki67 index may have a high risk for recurrence even in patients with T1b-c tumors. However, there is a paucity of evidence concerning age-related differences in estrogen-inducible ER pathways and how it affects clinical behavior in HR-positive breast cancer.
In this regard, the current study implicates young age (<35 years) and the Ki67 proliferative index as being important in HR-positive small T1b-cN0 breast cancers. Using our nomogram score, we could readily estimate the expected RFS and identify high-risk patients who could benefit from adjuvant chemotherapy. The identification of the subset of patients could be done readily in the setting of daily clinical practice without any further gene expression assay. Our nomogram was validated by an external patient cohort.
Yerushalmi et al. [41] conclude that, at present, Ki67 cannot be used as a tool for selecting specific chemotherapy or endocrine treatment, nor for assigning patients to specific risk group because of lack of standard methods in interpreting IHC specimens. A recent guideline was proposed for use Ki67 in terms of pre-analytical, analytical, interpretation and scoring, and data handling [42]. The guideline may allow earlier valid applications of Ki67. However, our study has several limitations to interpret. First, more studies for routine clinical use of Ki67 analysis is still needed, even though increasing number of evidences of Ki67 as a prognostic and predictive marker has been shown [41, 43]. Thus, our nomogram for practice use has been limited to the populations which had been validated in terms of analytic techniques and scoring methods. Second, our study population may not be representative and has potential selection bias, which retrospective data usually could have. In spite of these main drawbacks, our results implicate a valuable application of Ki67 for appropriate clinical use.
The high-risk patients comprised a small proportion of HR-positive patients that may experience unexpected recurrence and a pronounced aggressive ER biology. Therefore, our study is important for further translational research as well as clinical impact. Differences in Ki67 index and young age (<35 years) in HR-positive breast cancers are probably the most representative initial key alterations to be defined, because small tumors without nodal involvement reflect tumor biology in early tumorigenesis, which can be the simplest stage before other processes begin as a tumor grows.
In conclusion, the present study indicates the presence of a subpopulation of patients that should be focused on for adjuvant chemotherapy in addition to endocrine therapy even in small (T1b-c) node-negative HR-positive breast cancers. Ki67 index and an age <35 years are two independent risk factors for recurrence. Further prospective study is warranted.
References
Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM et al (2004) Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer 101(1):3–27. doi:10.1002/cncr.20288
Miller BA, Feuer EJ, Hankey BF (1993) Recent incidence trends for breast cancer in women and the relevance of early detection: an update. CA Cancer J Clin 43(1):27–41
Schootman M, Jeffe D, Reschke A, Aft R (2004) The full potential of breast cancer screening use to reduce mortality has not yet been realized in the United States. Breast Cancer Res Treat 85(3):219–222. doi:10.1023/B:BREA.0000025410.41220.67
Rosen PP, Groshen S, Kinne DW, Norton L (1993) Factors influencing prognosis in node-negative breast carcinoma: analysis of 767 T1N0M0/T2N0M0 patients with long-term follow-up. J Clin Oncol 11(11):2090–2100
Joensuu H, Pylkkanen L, Toikkanen S (1999) Late mortality from pT1N0M0 breast carcinoma. Cancer 85(10):2183–2189. doi:10.1002/(SICI)1097-0142(19990515)85:10<2183:AID-CNCR12>3.0.CO;2-K
Chen YY, Schnitt SJ (1998) Prognostic factors for patients with breast cancers 1 cm and smaller. Breast Cancer Res Treat 51(3):209–225
Leitner SP, Swern AS, Weinberger D, Duncan LJ, Hutter RV (1995) Predictors of recurrence for patients with small (one centimeter or less) localized breast cancer (T1a, b N0 M0). Cancer 76(11):2266–2274
Curigliano G, Viale G, Bagnardi V, Fumagalli L, Locatelli M, Rotmensz N et al (2009) Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J Clin Oncol 27(34):5693–5699. doi:10.1200/JCO.2009.22.0962
Park YH, Kim ST, Cho EY, Choi YL, Ok ON, Baek HJ et al (2010) A risk stratification by hormonal receptors (ER, PgR) and HER-2 status in small (< or =1 cm) invasive breast cancer: who might be possible candidates for adjuvant treatment? Breast Cancer Res Treat 119(3):653–661. doi:10.1007/s10549-009-0665-x
Banerjee S, Smith IE (2010) Management of small HER2-positive breast cancers. Lancet Oncol 11(12):1193–1199. doi:10.1016/S1470-2045(10)70119-4
Pagani O, Price KN, Gelber RD, Castiglione-Gertsch M, Holmberg SB, Lindtner J et al (2009) Patterns of recurrence of early breast cancer according to estrogen receptor status: a therapeutic target for a quarter of a century. Breast Cancer Res Treat 117(2):319–324. doi:10.1007/s10549-008-0282-0
Chia S, Norris B, Speers C, Cheang M, Gilks B, Gown AM et al (2008) Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol 26(35):5697–5704. doi:10.1200/JCO.2007.15.8659
Clark GM, Osborne CK, McGuire WL (1984) Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. J Clin Oncol 2(10):1102–1109
Boccardo F, Rubagotti A, Puntoni M, Guglielmini P, Amoroso D, Fini A et al (2005) Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian Tamoxifen Anastrozole Trial. J Clin Oncol 23(22):5138–5147. doi:10.1200/JCO.2005.04.120
Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ et al (2005) Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 97(17):1262–1271. doi:10.1093/jnci/dji250
Thurlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF et al (2005) A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353(26):2747–2757. doi:10.1056/NEJMoa052258
Jonat W, Gnant M, Boccardo F, Kaufmann M, Rubagotti A, Zuna I et al (2006) Effectiveness of switching from adjuvant tamoxifen to anastrozole in postmenopausal women with hormone-sensitive early-stage breast cancer: a meta-analysis. Lancet Oncol 7(12):991–996. doi:10.1016/S1470-2045(06)70948-2
Coombes RC, Kilburn LS, Snowdon CF, Paridaens R, Coleman RE, Jones SE et al (2007) Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (intergroup exemestane study): a randomised controlled trial. Lancet 369(9561):559–570. doi:10.1016/S0140-6736(07)60200-1
Cuzick J, Ambroisine L, Davidson N, Jakesz R, Kaufmann M, Regan M et al (2007) Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. Lancet 369(9574):1711–1723. doi:10.1016/S0140-6736(07)60778-8
Jakesz R, Greil R, Gnant M, Schmid M, Kwasny W, Kubista E et al (2007) Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J Natl Cancer Inst 99(24):1845–1853. doi:10.1093/jnci/djm246
Guarneri V, Broglio K, Kau SW, Cristofanilli M, Buzdar AU, Valero V et al (2006) Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol 24(7):1037–1044. doi:10.1200/JCO.2005.02.6914
Mazouni C, Kau SW, Frye D, Andre F, Kuerer HM, Buchholz TA et al (2007) Inclusion of taxanes, particularly weekly paclitaxel, in preoperative chemotherapy improves pathologic complete response rate in estrogen receptor-positive breast cancers. Ann Oncol 18(5):874–880. doi:10.1093/annonc/mdm008
Colleoni M, Bagnardi V, Rotmensz N, Gelber RD, Viale G, Pruneri G et al (2009) Increasing steroid hormone receptors expression defines breast cancer subtypes non responsive to preoperative chemotherapy. Breast Cancer Res Treat 116(2):359–369. doi:10.1007/s10549-008-0223-y
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA et al (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752. doi:10.1038/35021093
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98(19):10869–10874. doi:10.1073/pnas.191367098
Oh DS, Troester MA, Usary J, Hu Z, He X, Fan C et al (2006) Estrogen-regulated genes predict survival in hormone receptor-positive breast cancers. J Clin Oncol 24(11):1656–1664. doi:10.1200/JCO.2005.03.2755
Hu Z, Fan C, Oh DS, Marron JS, He X, Qaqish BF et al (2006) The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics 7:96. doi:10.1186/1471-2164-7-96
Rouzier R, Pusztai L, Delaloge S, Gonzalez-Angulo AM, Andre F, Hess KR et al (2005) Nomograms to predict pathologic complete response and metastasis-free survival after preoperative chemotherapy for breast cancer. J Clin Oncol 23(33):8331–8339. doi:10.1200/JCO.2005.01.2898
Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K et al (2005) Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 11(16):5678–5685. doi:10.1158/1078-0432.CCR-04-2421
Perou CM, Jeffrey SS, van de Rijn M, Rees CA, Eisen MB, Ross DT et al (1999) Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc Natl Acad Sci USA 96(16):9212–9217
Hugh J, Hanson J, Cheang MC, Nielsen TO, Perou CM, Dumontet C et al (2009) Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol 27(8):1168–1176. doi:10.1200/JCO.2008.18.1024
Penault-Llorca F, Andre F, Sagan C, Lacroix-Triki M, Denoux Y, Verriele V et al (2009) Ki67 expression and docetaxel efficacy in patients with estrogen receptor-positive breast cancer. J Clin Oncol 27(17):2809–2815. doi:10.1200/JCO.2008.18.2808
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S et al (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28(16):2784–2795. doi:10.1200/JCO.2009.25.6529
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2005) Reporting recommendations for tumor marker prognostic studies. J Clin Oncol 23(36):9067–9072. doi:10.1200/JCO.2004.01.0454
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2006) Reporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 100(2):229–235. doi:10.1007/s10549-006-9242-8
Quong J, Eppenberger-Castori S, Moore D 3rd, Scott GK, Birrer MJ, Kueng W et al (2002) Age-dependent changes in breast cancer hormone receptors and oxidant stress markers. Breast Cancer Res Treat 76(3):221–236
El Saghir NS, Seoud M, Khalil MK, Charafeddine M, Salem ZK, Geara FB et al (2006) Effects of young age at presentation on survival in breast cancer. BMC Cancer 6:194. doi:10.1186/1471-2407-6-194
Figueiredo JC, Ennis M, Knight JA, McLaughlin JR, Hood N, O’Malley F et al (2007) Influence of young age at diagnosis and family history of breast or ovarian cancer on breast cancer outcomes in a population-based cohort study. Breast Cancer Res Treat 105(1):69–80. doi:10.1007/s10549-006-9433-3
Rapiti E, Fioretta G, Verkooijen HM, Vlastos G, Schafer P, Sappino AP et al (2005) Survival of young and older breast cancer patients in Geneva from 1990 to 2001. Eur J Cancer 41(10):1446–1452. doi:10.1016/j.ejca.2005.02.029
Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thurlimann B, Senn HJ (2007) Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol 18(7):1133–1144. doi:10.1093/annonc/mdm271
Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA (2010) Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol 11(2):174–183. doi:10.1016/S1470-2045(09)70262-1
Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J et al (2011) Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 103(22):1656–1664. doi:10.1093/jnci/djr393
Luporsi E, Andre F, Spyratos F, Martin PM, Jacquemier J, Penault-Llorca F et al (2011) Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat. doi:10.1007/s10549-011-1837-z
Disclosures
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, Y.H., Im, SA., Cho, E.Y. et al. Small node-negative (T1b-cN0) invasive hormone receptor-positive breast cancers: Is there a subpopulation that might have benefit from adjuvant chemotherapy?. Breast Cancer Res Treat 133, 247–255 (2012). https://doi.org/10.1007/s10549-012-1956-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-1956-1