Abstract
Recently, we faced difficult treatment decisions regarding appropriate adjuvant systemic treatment, especially for patients who show discordance between stage and tumor biology. The aim of this study was to compare the prognostic relevance of the TNM staging system with that of intrinsic subtype in breast cancer. We retrospectively identified women patients who received curative surgery for stage I–III breast cancer with available data on immunohistochemistry profiles including hormone receptor (HR) status, human epidermal growth factor receptor 2 (HER2) status, and Ki 67 staining at the Samsung Medical Center from January 2004 to September 2008. Primary outcomes were recurrence-free survival (RFS) and overall survival (OS). A total of 1145 patients were diagnosed with breast cancer and received curative surgery. Of these, 463 (40.4 %) patients were stage I, and 682 (59.6 %) were stage II or III. In addition, 701 (61.2 %) patients were HR positive, 239 (20.9 %) were HER2 positive, and 205 (20.9 %) had triple-negative breast cancer. The 5-year RFS for the patients who were HR positive and HER2 negative with a low Ki 67 staining score (0–25 %) was 99 %. The 5-year RFS for patients who were HER2-positive or had triple-negative breast cancer were 89 and 83 %, respectively (P value = <0.001). In multivariate analysis, advanced stage (II/III) and unfavorable biology (HER2 positive or triple negative) retained their statistical significance as predictors of decreased RFS and OS. Patients with advanced-stage disease (II or III) but favorable tumor biology (HR positive and HER2 negative and low Ki 67) had better clinical outcomes than those with stage I disease and unfavorable tumor biology in terms of RFS (99 versus 92 %, P value = 0.011) and OS (99 versus 96 %, P value = 0.03) at 5 years. The current results showed that intrinsic subtype has a greater prognostic impact in predicting clinical outcomes in subpopulations of patients with stage I–III breast cancer who show discordance between stage and biologic subtypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer has heterogeneous clinical features and prognoses according to its biologic subtype. Breast cancer is divided into at least five subtypes according to molecular expression profiles, and hormone receptor (HR)-positive breast cancer constitutes 60–80 % of breast cancers [1]. HR-positive breast cancer has a favorable prognosis, which can be further classified into luminal A and luminal B mainly by proliferative rate [2]. Amplification of human epidermal growth factor receptor 2 (HER2) represents the prototype biomarker for tailored biologic treatment. Many studies have suggested that HER2 positivity is an independent predictor of disease recurrence and shortened overall survival [3, 4]. Triple-negative breast cancer [TNBC; negative for estrogen receptor (ER), progesterone receptor (PgR), and HER2] accounts for 15–20 % of newly diagnosed breast cancer cases [3]. TNBC has a tendency for high rates of relapse, visceral and central nervous system metastases, and early death [5]. TNBC is associated with a poor prognosis because it is refractory to chemotherapeutic agents and has no available targeted therapies [6, 7].
Historically, the American Joint Committee on Cancer (AJCC) TNM staging system has been the main determinant of the prognosis and the need for adjuvant treatment in patients with breast cancer; however, TNM staging is purely anatomical-based staging. Recently, with rapid developments in molecular biology [8], different therapeutic strategies based on biologic subtype have come into focus. However, the TNM staging system still regards breast cancer as a single disease entity; thus, it is suboptimal to reflect biologic heterogeneity. The seventh updated AJCC TNM staging system was intended to reflect the underlying biology by adding a “B” category, in which the status of ER, PgR, HER2, and multigene expression profiles would be incorporated and added to the stage grouping; however, these additions ultimately added little value. New appreciation of the biological diversity of breast cancer subtypes has raised questions concerning the relevance of TNM tumor staging in treatment decisions in addition to its clinical relevance. In the era of personalized tailored therapy based on molecular biology [9], different therapeutic strategies based on biologic subtype have gained prominence. This creates a dilemma that has a particular impact on prognosis when there is discordance between stage and biology. Therefore, in this study, we attempted to compare the prognostic relevance of the TNM staging system with that of intrinsic subtype in breast cancer patients who received curative surgery.
Patients and methods
Patients and data collection
We retrospectively analyzed the medical records of patients who received curative surgery for stage I–III breast cancer between January 2004 and September 2008 and had available immunohistochemistry profiles. All patients had histologically confirmed adenocarcinoma of the breast. We excluded the patients who had neoadjuvant chemotherapy. All pathologic specimens were reviewed by two experienced pathologists who determined the primary tumor characteristics based on histologic and nuclear grades, tumor size, presence of lympho-vascular invasion (LVI), axillary nodal status, and the status of immunohistochemical (IHC) staining for receptors (ER, PgR, and HER2), and Ki 67 staining. For quantitative analysis, the percentage of nuclei that were staining positively for Ki 67 was calculated for each section based on a value of approximately 1000 carcinoma cell nuclei/section using an image analyzer (I-solution delta, Korea). For semi-quantitative analysis, positive signals were graded as follows: 0, 0–5 %; 1+, 5–25 %; 2+, 26–50 %; 3+, 51–75 %; and 4+, >75 %. Positivity for ER and PgR was defined as Allred scores [10] within the range of 3–8 by IHC using antibodies to ER (Immunotech) and PgR (Novocastra), respectively. HER2 status was evaluated using an antibody and/or fluorescence in situ hybridization (FISH). Grades 0 and 1 for HER2 by IHC were defined as a negative result and grade 3 as a positive result. Amplification of HER2 was confirmed by FISH if HER2 was rated 2+ by IHC [3]. Triple negativity was defined as a lack of ER, PgR, and HER2 expressions [11]. We retrospectively collected data on the following clinicopathologic variables and treatment outcomes: patient demographics (age <35 and ≥ 35 years); laboratory data on ER, PgR, and HER2 (categorized as +, −, unknown) and Ki 67 index (low Ki 67: Ki 67 index 0–1+); and TNM stage (AJCC sixth edition). The analyzed outcomes were recurrence-free survival (RFS) and overall survival (OS). Overall survival was measured from the first date of diagnosis of breast cancer to the date of death or the last follow-up visit. Recurrence-free survival was defined as the time from the date of curative resection to the date when breast cancer recurred, irrespective of locoregional recurrence including ipsilateral and contralateral breast recurrence or distant metastases. The present study was approved by the Institutional Review Board of Samsung Medical Center.
Statistical analysis
Patient characteristics were compared using chi-square and Fisher’s exact test (categorical variables). Survival probability was estimated using the Kaplan-Meier method and compared for statistical difference using log-rank analysis. Multivariate analysis was performed using stepwise Cox proportional hazards regression modeling to assess the independent prognostic role of each clinicopathologic variable. Statistical analyses were performed using SPSS 19.0 (SPSS Inc. Chicago, IL, USA), and statistical significance was considered to be P ≤ 0.05.
Results
Patient cohorts
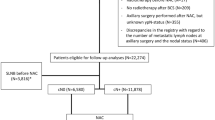
We identified 1556 patients who were diagnosed with breast cancer and received curative surgery for the treatment of stage I–III breast cancer at Samsung Medical Center between January 2004 and September 2008 (Fig. 1). Among the 1556 patients who had curative surgery, 152 patients with ductal carcinoma in situ, lobular carcinoma in situ, or microinvasive cancer were excluded from the analysis. Among the remaining 1404 invasive breast cancer patients, 1145 consecutive patients with available immunohistochemistry profiles were included in this analysis. The median follow-up duration was 95.4 (range 4.6–167.4) months.
Patient characteristics
Patient demographics are displayed in Table 1. We identified 1145 breast cancer patients with complete data. The median age at diagnosis of breast cancer was 46 years old (range 22–83 years). Tumor classification was stage I in 463 patients (40.4 %), stage II in 527 patients (46 %), and stage III in 155 patients (13.5 %). Lympho-vascular invasion was present in 15 % of the patients. Of 1145 total patients, 701 (61.2 %) patients were hormone receptor (HR) positive, 239 (20.9 %) were HER2, and 205 (17.9 %) were TNBC. Adjuvant anti-HER2 treatment was not available under Korean insurance during the study period. None of the patients with HER2-positive breast cancer were treated with anti-HER2 treatment as an adjuvant treatment.
Clinicopathological characteristics according to biologic subtypes
Table 2 shows the relationship between biologic subtypes and stage and histologic grade. Higher histologic grade (2–3) and nuclear grade (intermediate and high grade) were more common in patients with HER2-positive breast cancer and TNBC than in those with HR-positive breast cancer (97.0 and 95.2 versus 65.2 % for histologic grade, P value = <0.004; 96.2 and 93.9 versus 72.4 % for nuclear grade, P value = <0.001). Patients with stage II or III disease were more likely to be in the HER2-positive and TNBC groups than in the HR-positive group (64.0 and 66.8 %, respectively, versus 56.0 %, P <0.004 for stage).
Clinical outcomes
The 5-year RFS probability among HR-positive, HER2-positive, and TNBC groups were 93, 89, and 83 %, respectively, and the 5-year OS probability were 97.0, 92.0, and 87.0 %, respectively (Supplement figure 1 and Table 3). Univariate analysis demonstrated that young age at diagnosis, unfavorable biologic subtype (HER2-positive, TNBC groups), high histologic grade (2–3), nuclear grade (intermediate–high), and the presence of lympho-vascular invasion (LVI) predicted poor RFS and OS with statistical significance (Table 3 and Fig. 2). On multivariate analysis, biologic subtype, TNM stage, and histologic grade retained their statistical significance to predict RFS and OS (Table 4).
Clinical outcomes in subpopulations of patients who showed discordance between stage and biologic subtypes
Among the total patients, 299 patients with discordance between stage and biologic subset (early stage with unfavorable biologic subtypes or advanced stage with favorable biologic subtypes) were included in the subgroup analysis. We compared the clinical outcomes in 97 patients who had stage I disease and HER2-positive or triple-negative status with 202 patients who had stage II or III disease and were HR positive and HER2 negative and had a low proliferative index (Ki 67 index 0–1+) (Fig. 1). Thirty patients with stage I disease and HER2 (+) breast cancer did not receive anti-HER2 targeted therapy. Between two subgroups, age distribution was not difference (Table 5). The patients with advanced-stage disease (II or III) but favorable tumor biology (HR positive and HER2 negative and Ki 67 index 0–1+) had better clinical outcomes than those with stage I disease and unfavorable tumor biology (HER2 positive or triple negative) in terms of RFS (5-year RFS rate 99 versus 92 %, respectively, P value = 0.011) and OS (5-year OS rate 99 versus 96 %, respectively, P value = 0.03) (Fig. 2).
Discussion
The present study has clinical value because it highlights the clinical relevance of discordance between disease stage and tumor biology in breast cancer.
In clinical practice, patients who show discordance between disease stage and tumor biology present a dilemma as to which factor has a greater impact on prognosis. As the use of targeted therapy increases, it becomes more important to classify patients according to the particular biomarker profiles for which specific treatment protocols are available. In this regard, two scenarios exist: the first is a case with an early stage but unfavorable biology and the second is a case with an advanced stage but favorable biology.
Several studies have shown that unfavorable biology has a poor prognostic impact, even in small invasive breast cancers. Appreciation of breast cancer heterogeneity on the molecular level is vital and should be considered in the management of patients with small tumors. For example, adjuvant treatment for small invasive breast cancer shows a survival benefit when tailored according to tumor biology in the subgroup that includes HER2-positive cancer and TNBC [11–16]. These subgroups deserve special consideration because of their aggressive tumor biology irrespective of TNM stage. In previous studies [12], young age (<35 years) and the high Ki 67 proliferative index were valuable surrogate markers to predict recurrence, and these subpopulations with such characteristics might derive clinical benefit from adjuvant chemotherapy for HR-positive small T1b–c N0 breast cancers. Curigliano et al. [13] and Lorenzo et al. [14], reporting on the experience of the European Institute of Oncology of Milan, demonstrated that T1a/T1b N0 tumors with HER2 overexpression were associated with worse distant recurrence-free survival (DRFS). In patients with TNBC, the current TNM staging system might not be adequate for predicting outcomes to make therapeutic decisions [15]. Because TNBC has a relatively poor prognosis irrespective of nodal status, the relevance of the TNM staging system has been questioned and awarded a lower significance in breast cancer [11, 16]. According to the 2013 National Comprehensive Cancer Network (NCCN) Guidelines for HR-positive breast cancer, biologic marker assessment such as 21-gene RT PCR assay can be considered for tumors >0.5 cm, HR-positive cancers, and HER2-negative breast cancers. In cases with a high recurrence score (≥31), adjuvant chemotherapy in addition to endocrine therapy is recommended as category 2A; however, because of the high cost and low feasibility of the gene array, it cannot be routinely used in clinical practice.
In contrast, many patients with advanced-stage disease (II–III) are overtreated with chemotherapy [17] on the basis of the TNM staging system. Albain KS et al. recently described the dilemma in treatment decisions when we face discordance between biology and stage, especially in cases of high-risk disease that have favorable biology (in abstract CS-1 2013 San Antonio Breast Cancer Symposium). With recent advances in our understanding of breast cancer biology and molecular profiles, standard factors that traditionally defined a high recurrence risk (advanced stage) are potentially overruled by other biologic factors such as high estrogen receptor levels, well-differentiated tumors, low proliferative rate (Ki 67), and low 21-gene recurrence score assay [8, 9, 17–21].
Currently, it is standard to consider systemic chemotherapy, endocrine therapy, and/or HER2 targeted treatment for the patients with stage II–III breast cancer if their tumor expresses ER and/or PgR or HER2 [18–20]. The added benefits of adjuvant chemotherapy for HR-positive breast cancer patients have been questioned and its role has been debated, even though HR-positive breast cancer constitutes 60–80 % of breast cancers arising in women, and endocrine therapy is the mainstay of adjuvant therapy in such cases. HR-positive breast cancer has been reported as less sensitive to chemotherapy, and pathologic complete remission is less likely to be achieved in HR-positive patients. Albain et al. demonstrated that the 10-year breast cancer-specific survival was over 90 % in patients with ER-positive, node-positive disease, and a low 21-gene recurrence score assay [21]. These outcomes were the same irrespective of whether the adjuvant treatment was tamoxifen alone or anthracycline-based therapy followed by tamoxifen [21]. Some biologic marker assays are currently in the limelight such as the low 21-gene recurrence score assay and good-risk 70-gene signatures [8].
In our previous studies, we showed that the TNM staging system might not be sufficient for treatment decisions in the subpopulation of patients with TNBC [15]. We also proposed risk stratification strategies by using tumor biology for patients with small invasive breast cancer [12].
In the same vein as previous studies, the current study has clinical value as it provides evidence generating the hypothesis that subgroup analysis by intrinsic biologic subtype overrides tumor staging with respect to prognostic impact. The current results showed that intrinsic subtype had overriding prognostic impact in patients with stage I–III breast cancer who showed discordance between stage and biologic subtype (early stage with unfavorable biologic subtypes and advanced stage with favorable biologic subtype). Patients with advanced-stage disease (II or III) but favorable tumor biology (HR positive and HER2 negative and low Ki 67) had better clinical outcomes than those with stage I disease and unfavorable tumor biology (HER2 positive or triple negative) in terms of RFS and OS. By multivariate analysis, biologic subtype, TNM stage, and histologic grade retained their statistical significance to predict RFS and OS.
Our study has the typical limitations of a retrospective study, and a further prospective study is needed for validation. The present study generates a hypothesis with implications for patients who showed discordance between stage and biology. Among the 1404 invasive breast cancer patients, only 1145 (81.6 %) had available immunohistochemistry profiles and were analyzed. We did not investigate comorbidity and the effect of fasting blood glucose and body mass index [22].
In conclusion, discordance between stage and biology may have great critical clinical relevance for predicting clinical outcomes and establishing the therapeutic strategy, and in such cases, biologic type may override disease stage. Therefore, decision-making with consideration of tumor biology might lead to better outcomes for patients with breast cancer. The current results showed that intrinsic subtype had an overriding prognostic impact on prediction of clinical outcomes in the subpopulations of patients with stage I–III breast cancer who showed discordance between stage and biologic subtype.
References
Hormone dependence and breast cancer. Lancet, 1959. 1(7083): p. 1133–4.
Sun Y et al. Luminal breast cancer classification according to proliferative indices: clinicopathological characteristics and short-term survival analysis. Med Oncol. 2014;31(7):55.
Slamon DJ. Studies of the HER-2/neu proto-oncogene in human breast cancer. Cancer Investig. 1990;8(2):253.
Buzdar AU et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23(16):3676–85.
Dent R et al. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115(2):423–8.
Choi J, Jung WH, Koo JS. Clinicopathologic features of molecular subtypes of triple negative breast cancer based on immunohistochemical markers. Histol Histopathol. 2012;27(11):1481–93.
Choi YL et al. Triple-negative, basal-like, and quintuple-negative breast cancers: better prediction model for survival. BMC Cancer. 2010;10:507.
Torrisi R et al. Potential impact of the 70-gene signature in the choice of adjuvant systemic treatment for ER positive, HER2 negative tumors: a single institution experience. Breast. 2013;22(4):419–24.
Paik S et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–26.
Allred DC et al. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11(2):155–68.
Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007;8(3):235–44.
Park YH et al. Small node-negative (T1b-cN0) invasive hormone receptor-positive breast cancers: is there a subpopulation that might have benefit from adjuvant chemotherapy? Breast Cancer Res Treat. 2012;133(1):247–55.
Curigliano G et al. Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J Clin Oncol. 2009;27(34):5693–9.
Livi L et al. Prognostic value of positive human epidermal growth factor receptor 2 status and negative hormone status in patients with T1a/T1b, lymph node-negative breast cancer. Cancer. 2012;118(13):3236–43.
Park YH et al. Clinical relevance of TNM staging system according to breast cancer subtypes. Ann Oncol. 2011;22(7):1554–60.
Rhee J et al. The clinicopathologic characteristics and prognostic significance of triple-negativity in node-negative breast cancer. BMC Cancer. 2008;8:307.
Paik S et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–34.
Cardoso F et al. The MINDACT trial: the first prospective clinical validation of a genomic tool. Mol Oncol. 2007;1(3):246–51.
Jankowitz RC, McGuire KP, Davidson NE. Optimal systemic therapy for premenopausal women with hormone receptor-positive breast cancer. Breast. 2013;22 Suppl 2:S165–70.
Sparano JA. TAILORx: trial assigning individualized options for treatment (Rx). Clin Breast Cancer. 2006;7(4):347–50.
Albain KS et al. Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374(9707):2055–63.
Minicozzi P et al. High fasting blood glucose and obesity significantly and independently increase risk of breast cancer death in hormone receptor-positive disease. Eur J Cancer. 2013;49(18):3881–8.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Hyun Ae Jung and Yeon Hee Park contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
(DOC 216 kb)
Rights and permissions
About this article
Cite this article
Jung, H.A., Park, Y.H., Kim, M. et al. Prognostic relevance of biological subtype overrides that of TNM staging in breast cancer: discordance between stage and biology. Tumor Biol. 36, 1073–1079 (2015). https://doi.org/10.1007/s13277-014-2730-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2730-2