Abstract
Late effects of treatment for breast cancer on shoulder function have been documented by a number of investigators; however, many studies include only prevalence data. When comparisons are provided that assess differences between treatment groups, only P-values without magnitudes of effect are often reported. The purpose of this systematic review was to identify literature that could be used to examine the magnitude of late effects of breast cancer treatments on shoulder function with a particular focus on axillary lymph node dissection (ALND) and on radiotherapy. A comprehensive search of online databases was performed for research papers published between 1980 and 2008 that provided comparison data between treatment groups, between the affected and unaffected side of individuals, or between pre-operative and subsequent assessments 12 months or more after diagnosis of breast cancer. Papers that met inclusion criteria were reviewed using a methodological checklist. Standardized effect sizes were computed for continuous data; odds ratios and 95% confidence intervals were computed for dichotomous data if not already available. Twenty-two papers met the inclusion criteria. With a few exceptions, most analyses showed excess shoulder morbidity with breast cancer treatment, ALND, or radiotherapy. Although effect sizes varied, moderate to large effects predominated across the different outcomes. There is sufficient evidence of late effects of ALND or radiotherapy post-breast cancer to warrant careful attention to shoulder function across time in individuals who have had breast cancer. Implications for future shoulder dysfunction are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Current evidence suggests that upper extremity impairments from treatment for breast cancer can extend beyond the acute stages of recovery and may be considered a component of chronic illness [1, 8, 21, 43]. Investigators have found that a proportion of women treated for breast cancer continue to experience upper extremity functional limitations two or more years after treatment [18, 20, 26, 41]. Of particular concern relative to shoulder morbidity in the breast cancer patient are nodal dissection and radiotherapy.
Upper extremity lymphedema following breast cancer treatments is a well-documented phenomenon that has received considerable attention [7]. However, the magnitude of shoulder impairments as late effects from breast cancer surgery and radiotherapy independent of lymphedema has received considerably less attention. Moreover, most studies on shoulder impairments post-treatment for breast cancer do not account for pretreatment shoulder morbidity or control in some way for the effects of aging. Because both factors are related to long-term shoulder morbidity in the breast cancer population [16, 30, 42], prevalence data on shoulder morbidity may be inflated and potentially dismissed as weak evidence. Studies that attempt to control for selected covariates by conducting comparisons over time, between groups, or between affected and unaffected arms often report only P-values rather than magnitude of effects. The actual impact of breast cancer treatment on shoulder function cannot be ascertained from P-values alone. Further, the acknowledged variability of effects among patients may mask potentially important impairments for many individuals with relatively high P-values.
Both axillary lymph node dissection (ALND) and radiotherapy have been reported to affect long term shoulder function. Studies indicate that sentinel node biopsy (SNB) reduces shoulder morbidity as compared to ALND [19, 21, 31, 40], although both procedures may contribute to shoulder impairments [19]. Shoulder impairments following radiotherapy may occur after “latent periods” of several months to several years, with late reactions continuing beyond that period in some individuals [1, 10, 47]. Patients receiving axillary radiation (as opposed to chest wall radiation alone) are at higher risk for late arm morbidity [3, 35, 41]. Cheville and Tchou noted that failure to recognize lasting sequelae from treatment for breast cancer delays treatment referrals and may lead to greater long-term shoulder morbidity [7]. Inattention to such morbidity may be an issue for both individuals who have been treated for breast cancer and their health care providers. The purpose of this systematic review was to identify literature that reported or would allow assessment of the magnitude of late effects of breast cancer treatments on shoulder function 1 year or more after diagnosis, with a particular focus on lymph node dissection and on radiotherapy. Presentation of such data in one place will assist health care professionals who have periodic contact with individuals treated for breast cancer in understanding the extent to which late effects on the shoulder may affect such an individual.
Methods
Literature search
Eligible papers had to include breast cancer subjects at least 1 year post-diagnosis as the minimum criterion for late effects. Papers had to be published after 1980 and could not include pre-1980 radiotherapy to minimize the likelihood of including subjects receiving older forms of radiotherapy no longer meeting current standards. Outcomes had to include assessment of impairments or functional activities of the shoulder and, to the extent to which they can be separated, were not to include symptoms related to lymphedema. Pain was accepted as an outcome only if it was reported with functional activity. Quality of life outcomes were not considered because the study focus was to isolate the physical effects of shoulder morbidity. Studies required at least two comparison groups or comparisons to the unaffected side. Comparisons across time were acceptable only when baseline measures were performed preoperatively or pre-radiotherapy. Randomized controlled trials, prospective, retrospective and cross-sectional designs were considered acceptable. The available data had to include means and standard deviations (SDs) or 95% confidence intervals (CIs) for both groups, or group data that would permit computation of odds ratios if not reported by the authors.
Search engines used were PubMed, Medline, CINAHL, Cochrane, Health Source Nursing, Google, and Google Scholar. Search terms were limited to the title, abstract, or keywords and included combinations of breast neoplasm, breast cancer, shoulder, arm, scapula, or humerus, along with sentinel node, brachial plexopathy, pectoralis major, pectoralis minor, latissimus dorsi, rotator cuff, teres minor, late effects, ROM, range of motion, radiotherapy, and radiation. Retrieved abstracts were reviewed for possible inclusion. When warranted, full articles were obtained for review. All full articles were reviewed by the two authors. Any differences in opinion on eligibility were resolved by discussion. There was agreement on all papers that were finally accepted as eligible for inclusion in the systematic review. A methodological checklist was adapted from several sources to assess quality of the included papers [27, 33, 44]. Each eligible paper was subjected to methodological review using the checklist (Table 1).
Data analysis
When means and standard deviations were reported for groups, standardized effect sizes were calculated as (M 1 − M 2)/SD, where M is the mean for each group and the mean difference is standardized using the standard deviation of the referent or control group [34]. Although there is no consensus on the interpretation of standardized effect sizes [34], the guidelines proposed by Cohen were used; an effect size of 0.20 is considered to be small, 0.50 to be moderate and 0.80 to be large [9]. When data were dichotomous, odds ratios were computed to estimate effect size using frequency counts. If frequency counts were not available, reported proportions were used and so noted.
Results
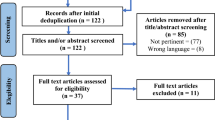
The searches yielded a total of 375 citations from January 1980 through May 2008. After review of all abstracts, 88 papers available in English were retrieved for further examination. Of those, 22 were determined to meet eligibility criteria [3, 6, 12–14, 16, 17, 19, 20, 22, 25, 28–31, 33, 37–40, 42, 45]. The results of the methodological review are shown in Table 1.
Overall breast cancer treatment effects on shoulder function
Four studies were found that permitted comparison of the affected shoulders of subjects treated for breast cancer to the same shoulder pre-treatment [33], to the uninvolved shoulder [30] or to subjects without breast cancer [36, 37]. In these studies, specific treatments were not evaluated; rather, the effects of all treatments were assessed. Effect sizes are reported in Table 2. For two of the studies, standardized effect sizes were computed from available data [30, 33]. For one study, crude odds ratios and 95% CIs were computed for each of the presented age groups and are reported along with the adjusted odds ratios given by the authors [36]. For one paper, the authors reported odds ratios and 95% CIs for each age group using ridit analyses for ordinal data and logistic regression [37]. In Table 2, the effect size represents the additional morbidity associated with treatment for breast cancer compared to the referent of pretreatment condition, the untreated arm, or subjects without breast cancer. All data show increases of varying magnitudes in morbidity with breast cancer with the exception of selected tasks in the 75–84 year old group in the Satariano et al. study [37].
Magnitude of effect on shoulder function from ALND
Twelve studies included shoulder morbidity data for subjects who were randomized to or underwent either ALND or SNB (or no axillary dissection) [6, 12, 19, 20, 22, 25, 29, 31, 33, 39, 40, 45]. As shown in Table 3, crude odds ratios and 95% CIs were estimated from available data from eight studies [6, 12, 19, 20, 22, 39, 40, 45], while standardized effect sizes were computed for two [29, 31]. The SNB group was considered the referent group with the exception of Lash and Sillman [20] and Caban et al. [6] where the referent to ALND was no axillary surgery. Each of these effect sizes represents the excess shoulder morbidity of ALND over the referent. Mansel et al. [25] reported 95% CIs rather than SDs so effect sizes could not be computed. The magnitude of changes after 1 year in the affected arm for each group are reported in Table 3. Reitman et al. [33] reported the means and SDs of change scores for each group after 2 years (Table 3) but did not include the SD for preoperative (baseline) measures. If the SD of the change score for the SNB group is used in lieu of the SD of the SNB group’s preoperative measures, the standardized effect sizes for the difference between groups in reduction from preoperative to 2 year values can be obtained. Using this strategy, the standardized effect size for reduction in ranges of shoulder abduction and abduction/external rotation and for reductions in grip strength are 0.73, 0.46 and 0.50, respectively, with ALND showing higher morbidity.
Most of the data in Table 3 show an increase in morbidity with ALND with at least selected functions. Caban et al. [6] did not demonstrate an effect on flexion range of motion. Lash and Sillman [20] found a paradoxical protective effect in both the crude and adjusted odds ratios of ALND compared to no nodal dissection on self-reported decline in one or more of three tasks compared to preoperative status. Schulze et al. [40] found a slight protective effect of ALND compared to SNB when self-reported loss of mobility was assessed, although objective data showed a strong positive association for reduced abduction range of motion.
Magnitude of chest wall and axillary radiotherapy effect on shoulder function
Ten studies were found that permitted one or more comparisons of shoulder morbidity between radiotherapy and no radiotherapy, or between differing radiotherapy fields [3, 6, 13, 14, 16, 17, 20, 28, 39, 42]. Five of the papers included comparisons of a subject group with any radiotherapy (or unspecified radiotherapy) to a group without radiotherapy [3, 6, 13, 14, 20]. Three papers compared chest wall radiotherapy alone to a group without radiotherapy [17, 39, 42], while four compared a group with chest wall radiotherapy to a group with both chest wall and at least axillary radiotherapy [16, 17, 39, 42]. One paper compared a group with multiple radiation fields including the full axilla to a group with multiple radiation fields but only at the apex of the axilla [28]. Johansson et al. [17] also compared a group with chest wall and axillary radiotherapy to a group with no radiotherapy. For eight of the studies, effect sizes were computed from published data or data provided by the authors (Table 4). Standardized effect sizes were computed when appropriate measurement data were available (two studies); crude odds ratios and 95% CIs were estimated when only frequencies were available (seven studies). Adjusted odds ratios obtained via logistic regression were reported in five studies and are included in Table 4 [6, 13, 16, 28, 39]. For all calculated effect sizes, the referent was the no radiotherapy group (compared to a radiotherapy group), or the chest wall radiotherapy group (compared to a chest wall and axillary radiation group). The effect sizes, therefore, represent the excess shoulder morbidity associated with radiotherapy or more extensive radiotherapy. The only exception to this generalization is the adjusted (logistic) odds ratio reported by Caban et al. [6]. Their odds ratio and 95% CI represents the reduction in morbidity (protective effect) associated with not having radiotherapy because their referent was the radiotherapy group. Unless otherwise noted in Table 4, the extent of radiotherapy (inclusion of the axilla or additional fields) was not specified.
The majority of studies in Table 4 show increased morbidity associated with more extensive radiotherapy in at least some of the assessed functions. The exceptions are Johannson et al. [17] whose data showed some small but protective effects of chest radiation compared to no radiation on selected range and strength measures, and the Lash and Sillman [20] data where the crude odds ratio showed a protective effect of radiotherapy compared to no radiotherapy on decline in upper body function. The adjusted odds ratio reported by Lash and Sillman, however, showed a small excess of morbidity associated with radiotherapy.
Discussion
Review of Tables 2, 3, and 4 show, with the few noted exceptions, that there is an excess morbidity associated with treatment for breast cancer with ALND compared to SNB, and with radiotherapy or more extensive radiotherapy. However, the effect sizes vary dramatically from small standardized effect sizes (0.20 or less or odds ratios near 1.0) to substantial standardized effect sizes well in excess of 0.80 and odds ratios of 2.0–3.0 or more. Moderate to large effects predominate, especially where abduction and flexion ROMs were reported. This review would largely appear to reinforce the conclusion of Blomqvist et al. [3] that shoulder morbidity, while most evident at the individual level, is sufficient to be seen at the group level. The variations in magnitude and in the size of standard deviations and confidence intervals also reflect the variations in morbidity that exist between studied individuals.
Variability in shoulder morbidity effects may be attributed in part to the diversity of outcome measures and the diversity of methods by which even similar outcomes were assessed and reported. In ten studies, patient self-report of loss of strength, range of motion (ROM) or functional ability were used [11, 13, 14, 20, 22, 36, 37, 39, 40, 45]. In these instances, data were either dichotomized or categorized. When categorical data were collected, data were collapsed by the study authors or by the authors of this paper into impairment as present or absent because of small cell numbers. Only the ridit analyses of Satariano et al. [37] maintained categories of effect within a functional limitation. Two studies subjectively assessed observed impairment and dichotomized the outcomes [6, 16]. Of the studies that measured ROM objectively, seven reported actual ranges [3, 17, 25, 29–31, 33], while five dichotomized their findings [13, 19, 22, 40, 42]. Ranges found to be 10°, 20° or 10% less than the contralateral limb or full ROM were considered to be impaired. This is a strategy similar to that used in other studies [5, 15, 43, 46]. Box et al. and Voogd et al. [5, 46] specifically addressed losses of 20° from preoperative or contralateral measures as associated with decreased function. Some variability in effect size can be attributed to estimates of crude as opposed to adjusted odds ratios. In six of the studies, odds ratios computed from logistic regression indicated morbidity associated with radiotherapy after adjusting for other covariates including number of nodes dissected, or ALND adjusted for other covariates including radiotherapy. Another source of variability in effect may be the different follow-up periods. Caban et al. [6] used a 12 month follow-up; however, they suggested that this observation period may be insufficient to see the full impact of radiation-induced fibrosis and recommended a follow-up period of 5 years to show the late effects of radiotherapy. Lastly, self-reported estimates of morbidity may be affected by individual expectations associated, for example, with age and by the individual’s ability to adapt over time to limitations [1, 37, 38].
The methodological checklist was not intended to be a quantitative assessment; however, it included 20 possible checks for randomized controlled trials and 19 for other designs. Of note was that only two studies achieved 16 checks [12, 30]. While thirteen studies obtained fewer than 10 checks (see Table 1). Given the variability in magnitudes of effect for ascertainment of morbidity and in methodological quality among these papers, it was determined that attempts to summarize the data using meta-analytic techniques were inappropriate. Rietman et al. [32] came to a similar conclusion in their systematic review of late morbidity after treatment for breast cancer.
Most of the studies included in this review followed subjects out for 3 years or less. Bentzen et al. [2] found that time to expression of 90% of the ultimately expected damage from radiotherapy was 3.7–4.2 years depending on the clinical covariates in the model (although radiotherapy techniques in their subjects may have varied from current standards). Fathers and Thrush [10] encountered symptoms of brachial plexopathy in the affected limb 8–20 years after radiotherapy. Because many of the studies included in this review assessed subjects only 1–2 years post-diagnosis or treatment, it may be that morbidity will continue to increase over time for some percentage of the subjects who received radiation. No reference to potential time effects of morbidity related to ALND or SNB were found in the literature although increases over time cannot be ruled out.
Current and future shoulder morbidity may be linked to radiation-induced changes. Bentzen et al. [2] hypothesized that damage to the pectoralis major muscle from radiotherapy was a significant factor in shoulder movement impairment even without clinically detectable subcutaneous tissue fibrosis. Shamley et al. [41] found that pectoralis major and minor muscles decreased in size on the affected side in a series of 57 breast cancer patients from 6 months to 6 years post-surgery, most of whom had chest wall radiation. However, surgery in the vicinity of the pectoralis major or minor may also create fibrotic changes with healing. Fibrosis in the pectoralis major and minor may be a factor in range of motion limitations which may also increase the risk for shoulder impingement syndrome or rotator cuff tears [23]. Ludewig and Cook [24] found that subjects with symptoms of impingement showed greater scapular anterior tipping, decreased upward rotation, and increased scapular medial rotation under load conditions. Borstad and Ludewig [4] found that subjects with a clinically determined short pectoralis minor muscle demonstrated scapular kinematics similar to those found for subjects with shoulder impingement. Decreased scapular upward rotation and increased anterior tipping may be critical in limiting adequate clearance for the rotator cuff tendons. Ludewig and Cook [24] hypothesized that even small limitations in scapular motion (4–6°) may be clinically important given the small size of the suprahumeral space, potentially contributing to initiation or progression of shoulder impingement symptoms. These same limitations to scapular motion are likely to reduce available shoulder range of motion given that the scapulothoracic joint contributes 60° to the total shoulder range of flexion and abduction [23]. Consequently, even the small decreases shown in motions like shoulder abduction and flexion among subjects who have had breast cancer may place these individuals at increased risk for shoulder impingement or rotator cuff problems over time. The magnitude of risk may be hypothesized to increase with increases in motion restrictions.
Limitations
Numerous factors prevent precise determination of the contribution of breast cancer treatments to impaired shoulder function. Tables 2, 3 and 4 and the discussion highlight several of these factors, but are not all inclusive. Bentzen and Dische and Langer et al. [1, 19] provide a comprehensive review of confounding and interactive factors that affect study results related to determination of post-treatment shoulder morbidity, including the lack of a uniformly accepted system for recording and grading shoulder morbidity that limits comparisons across treatment types and across studies.
Conclusion
This systematic review demonstrates the magnitude and the variability in effect sizes of shoulder morbidity attributable to treatment for breast cancer, with a focus on ALND and radiotherapy. Although mathematical aggregation of data was not attempted, the large majority of studies indicated increased shoulder impairments as a late effect from these treatments, with effects varying from small to substantial. Given the functional limitations that are described and the hypothesized relation between even minor limitations to shoulder motion and the potential for shoulder impingement or rotator cuff tear, this review supports the importance of health care professionals routinely asking patients about shoulder function, assessing function, and referring patients for remediation when even subtle limitations are present. Chirikos et al. [8] found that a breast cancer group was more likely to experience adverse economic outcomes compared to age-matched controls, with work-related differences narrowly missing statistical significance. This observation suggests that economic benefits of interventions that minimize or prevent problems are potentially high. The authors of this paper also concur with the recommendations of other investigators that use of selected valid and reliable shoulder function measures is required to enable comparisons across studies and to determine meaningful conclusions. Much work remains to be done to confirm the need to attend to late effects of breast cancer treatments on shoulder function.
References
Bentzen SM, Dische S (2000) Morbidity related to axillary irradiation in the treatment of breast cancer. Acta Oncol 39:337–347. doi:10.1080/028418600750013113
Bentzen SM, Overgaard M, Thames HD (1989) Fractionation sensitivity of a functional endpoint: impaired shoulder movement after post-mastectomy radiotherapy. Int J Radiat Oncol Biol Phys 17:531–537
Blomqvist L, Stark B, Engler N, Malm M (2004) Evaluation of arm and shoulder mobility and strength after modified radical mastectomy and radiotherapy. Acta Oncol 43:280–283. doi:10.1080/02841860410026170
Borstad JD, Ludewig PM (2005) The effect of long versus short pectoralis minor resting length on scapular kinematics in healthy individuals. J Orthop Sports Phys Ther 35:227–238
Box RC, Reul-Hirche HM, Bullock-Saxton JE, Furnival CM (2002) Shoulder movement after breast cancer surgery: results of a randomised controlled study of postoperative physiotherapy. Breast Cancer Res Treat 75:35–50. doi:10.1023/A:1016571204924
Caban ME, Freeman JL, Zhang DD, Jansen C, Ostir G, Hatch SS, Goodwin JS (2006) The relationship between depressive symptoms and shoulder mobility among older women: assessment at one year after breast cancer diagnosis. Clin Rehabil 20:513–522. doi:10.1191/0269215506cr966oa
Cheville AL, Tchou J (2007) Barriers to rehabilitation following surgery for primary breast cancer. J Surg Oncol 95:409–418. doi:10.1002/jso.20782
Chirikos TN, Russell-Jacobs A, Jacobsen PB (2002) Functional impairment and the economic consequences of female breast cancer. Women Health 36:1–20. doi:10.1300/J013v36n01_01
Cohen J (1988) Statistical power analysis for the social sciences. Earlbaum, Hillsdale, NJ
Fathers E, Thrush D, Huson SM, Norman A (2002) Radiation-induced brachial plexopathy in women treated for carcinoma of the breast. Clin Rehabil 16:160–165. doi:10.1191/0269215502cr470oa
Fleisch M, Meier B (1997) Images in cardiovascular medicine. Radiation therapy-induced cardiac injury. Circulation 96:2462–2463
Fleissig A, Fallowfield LJ, Langridge CI, Johnson L, Newcombe RG, Dixon JM, Kissin M, Mansel RE (2005) Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat 95:279–293. doi:10.1007/s10549-005-9025-7
Hojris I, Andersen J, Overgaard M, Overgaard J (2000) Late treatment-related morbidity in breast cancer patients randomized to postmastectomy radiotherapy and systemic treatment versus systemic treatment alone. Acta Oncol 39:355–372. doi:10.1080/028418600750013131
Isaksson G, Feuk B (2000) Morbidity from axillary treatment in breast cancer—a follow-up study in a district hospital. Acta Oncol 39:335–336. doi:10.1080/028418600750013104
Ivens D, Hoe AL, Podd TJ, Hamilton CR, Taylor I, Royle GT (1992) Assessment of morbidity from complete axillary dissection. Br J Cancer 66:136–138
Johansen J, Overgaard J, Blichert-Toft M, Overgaard M (2000) Treatment of morbidity associated with the management of the axilla in breast-conserving therapy. Acta Oncol 39:349–354. doi:10.1080/028418600750013122
Johansson K, Ingvar C, Albertsson M, Ekdahl C (2001) Arm lymphoedema, shoulder mobility and muscle strength after breast cancer treatment—a prospective 2-year study. Adv Physiother 3:55–66
Kwan W, Jackson J, Weir LM, Dingee C, McGregor G, Olivotto IA (2002) Chronic arm morbidity after curative breast cancer treatment: prevalence and impact on quality of life. J Clin Oncol 20:4242–4248. doi:10.1200/JCO.2002.09.018
Langer I, Guller U, Berclaz G, Koechli OR, Schaer G, Fehr MK, Hess T, Oertli D, Bronz L, Schnarwyler B, Wight E, Uehlinger U, Infanger E, Burger D, Zuber M (2007) Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery: a prospective Swiss multicenter study on 659 patients. Ann Surg 245:452–461. doi:10.1097/01.sla.0000245472.47748.ec
Lash TL, Silliman RA (2000) Patient characteristics and treatments associated with a decline in upper-body function following breast cancer therapy. J Clin Epidemiol 53:615–622. doi:10.1016/S0895-4356(99)00176-6
Lauridsen MC, Christiansen P, Hessov I (2005) The effect of physiotherapy on shoulder function in patients surgically treated for breast cancer: a randomized study. Acta Oncol 44:449–457. doi:10.1080/02841860510029905
Leidenius M, Leivonen M, Vironen J, von Smitten K (2005) The consequences of long-time arm morbidity in node-negative breast cancer patients with sentinel node biopsy or axillary clearance. J Surg Oncol 92:23–31. doi:10.1002/jso.20373
Ludewig PM, Borstad JD (2005) The shoulder complex. In: Levangie PK, Norkin CC (eds) Joint structure and function: a comprehensive analysis. F.A. Davis, Philadelphia
Ludewig PM, Cook TM (2000) Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys Ther 80:276–291
Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, Yiangou C, Horgan K, Bundred N, Monypenny I, England D, Sibbering M, Abdullah TI, Barr L, Chetty U, Sinnett DH, Fleissig A, Clarke D, Ell PJ (2006) Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC trial. J Natl Cancer Inst 98:599–609
McCredie MR, Dite GS, Porter L, Maskiell J, Giles GG, Phillips KA, Redman S, Hopper JL (2001) Prevalence of self-reported arm morbidity following treatment for breast cancer in the Australian Breast Cancer Family Study. Breast 10:515–522. doi:10.1054/brst.2000.0291
Moher D, Schulz KF, Altman DG (2001) The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 357:1191–1194. doi:10.1016/S0140-6736(00)04337-3
Nesvold IL, Dahl AA, Lokkevik E, Marit Mengshoel A, Fossa SD (2008) Arm and shoulder morbidity in breast cancer patients after breast-conserving therapy versus mastectomy. Acta Oncol 47:835–842. doi:10.1080/02841860801961257
Purushotham AD, Upponi S, Klevesath MB, Bobrow L, Millar K, Myles JP, Duffy SW (2005) Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol 23:4312–4321. doi:10.1200/JCO.2005.03.228
Rietman J, Dijkstra P, Debreczeni R, Geertzen J, Robinson D, De Vries J (2004) Impairments, disabilities and health related quality of life after treatment for breast cancer: a follow-up study 2.7 years after surgery. Disabil Rehabil 26:78–84. doi:10.1080/09638280310001629642
Rietman JS, Dijkstra PU, Geertzen JH, Baas P, de Vries J, Dolsma WV, Groothoff JW, Eisma WH, Hoekstra HJ (2004) Treatment-related upper limb morbidity 1 year after sentinel lymph node biopsy or axillary lymph node dissection for stage I or II breast cancer. Ann Surg Oncol 11:1018–1024. doi:10.1245/ASO.2004.03.512
Rietman JS, Dijkstra PU, Hoekstra HJ, Eisma WH, Szabo BG, Groothoff JW, Geertzen JH (2003) Late morbidity after treatment of breast cancer in relation to daily activities and quality of life: a systematic review. Eur J Surg Oncol 29:229–238. doi:10.1053/ejso.2002.1403
Rietman JS, Geertzen JH, Hoekstra HJ, Baas P, Dolsma WV, de Vries J, Groothoff JW, Eisma WH, Dijkstra PU (2006) Long term treatment related upper limb morbidity and quality of life after sentinel lymph node biopsy for stage I or II breast cancer. Eur J Surg Oncol 32:148–152. doi:10.1016/j.ejso.2005.11.008
Rosenthal R (1994) Parametric measures of effect size. In: Cooper H, Hedges LV (eds) The handbook of research synthesis. Russell Sage Foundation, New York, pp 231–244
Ryttov N, Holm N, Qvist N, Blichert-Toft M (1988) Influence of adjuvant irradiation on the development of late arm lymphedema and impaired shoulder mobility after mastectomy for carcinoma of the breast. Acta Oncol 27:667–670
Satariano WA, DeLorenze GN (1996) The likelihood of returning to work after breast cancer. Public Health Rep 111:236–241
Satariano WA, Ragheb NE, Branch LG, Swanson GM (1990) Difficulties in physical functioning reported by middle-aged and elderly women with breast cancer: a case–control comparison. J Gerontol 45:M3–M11
Satariano WA, Ragland DR (1996) Upper-body strength and breast cancer: a comparison of the effects of age and disease. J Gerontol A Biol Sci Med Sci 51:M215–M219
Schijven MP, Vingerhoets AJ, Rutten HJ, Nieuwenhuijzen GA, Roumen RM, van Bussel ME, Voogd AC (2003) Comparison of morbidity between axillary lymph node dissection and sentinel node biopsy. Eur J Surg Oncol 29:341–350. doi:10.1053/ejso.2002.1385
Schulze T, Mucke J, Markwardt J, Schlag PM, Bembenek A (2006) Long-term morbidity of patients with early breast cancer after sentinel lymph node biopsy compared to axillary lymph node dissection. J Surg Oncol 93:109–119. doi:10.1002/jso.20406
Shamley DR, Srinanaganathan R, Weatherall R, Oskrochi R, Watson M, Ostlere S, Sugden E (2007) Changes in shoulder muscle size and activity following treatment for breast cancer. Breast Cancer Res Treat 106:19–27
Sugden EM, Rezvani M, Harrison JM, Hughes LK (1998) Shoulder movement after the treatment of early stage breast cancer. Clin Oncol (R Coll Radiol) 10:173–181. doi:10.1016/S0936-6555(98)80063-0
Tengrup I, Tennvall-Nittby L, Christiansson I, Laurin M (2000) Arm morbidity after breast-conserving therapy for breast cancer. Acta Oncol 39:393–397. doi:10.1080/028418600750013177
van der Heijden GJ, Beurskens AJ, Koes BW, Assendelft WJ, de Vet HC, Bouter LM (1995) The efficacy of traction for back and neck pain: a systematic, blinded review of randomized clinical trial methods. Phys Ther 75:93–104
Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, Intra M, Veronesi P, Robertson C, Maisonneuve P, Renne G, De Cicco C, De Lucia F, Gennari R (2003) A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med 349:546–553. doi:10.1056/NEJMoa012782
Voogd AC, Ververs JM, Vingerhoets AJ, Roumen RM, Coebergh JW, Crommelin MA (2003) Lymphoedema and reduced shoulder function as indicators of quality of life after axillary lymph node dissection for invasive breast cancer. Br J Surg 90:76–81. doi:10.1002/bjs.4010
Wallgren A (1992) Late effects of radiotherapy in the treatment of breast cancer. Acta Oncol 31:237–242. doi:10.3109/02841869209088909
Acknowledgments
The authors would like to thank Lucinda Pfalzer, PT, PhD of University of Michigan—Flint and Tygre Whittington, BS of Oakland University, Rochester, MI. Dr. Pflazer assisted with conceptualization, literature search and article retrieval. Ms. Whittington assisted with literature search and article retrieval.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Levangie, P.K., Drouin, J. Magnitude of late effects of breast cancer treatments on shoulder function: a systematic review. Breast Cancer Res Treat 116, 1–15 (2009). https://doi.org/10.1007/s10549-008-0246-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-008-0246-4