Abstract
Background
Morbidity of the shoulder after breast cancer is a well-known phenomenon. MRI studies have shown muscle morbidity in cervical cancer and prostate cancer. In breast cancer clinical observations and patient reports include muscle morbidity in a number of muscles acting at the shoulder. Several of these muscles lie in the field of surgery and radiotherapy. Timed interaction between muscles that stabilise the shoulder and those acting as prime movers is essential to achieve a smooth scapulohumeral rthythm during functional elevation of the arm.
Method: Cross-sectional study
Seventy-four women treated for unilateral carcinoma of the breast were included in the study. All patients filled out the Shoulder Pain and Disability Index (SPADI). EMG activity of four muscles was recorded during scaption on the affected and unaffected side. Muscle cross sectional area and signal intensity was determined from MRI scans. The association between EMG and covariates was determined using multiple linear regression techniques.
Results
Three of the 4 muscles on the affected side demonstrated significantly less EMG activity, particularly when lowering the arm. Upper trapezius demonstrated the greatest loss in activity. Decreased activity in both upper trapezius and rhomboid were significantly associated with an increase in SPADI score and increased time since surgery. Pectoralis major and minor were significantly smaller on the affected side.
Conclusion
Muscles affected in the long term are the muscles associated with pain and disability yet are not in the direct field of surgery or radiotherapy. Primary muscle shortening and secondary loss of muscle activity may be producing a movement disorder similar to the ‘Dropped Shoulder Syndrome’. Exercise programmes should aim not only for range of movement but also for posture correction and education of potential long-term effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast screening programmes have allowed more conservative approaches to surgery and radiotherapy for women diagnosed with breast cancer [1]. Despite the use of less extensive surgery and where possible the avoidance of radiotherapy to the axilla, there is still morbidity affecting the shoulder [2–4]. Radiotherapy is standard practice for patients receiving conservative surgery and those at risk of recurrence, and is generally given in 3–5 weekly sessions for up to 6 weeks. The high energy X-rays interact with molecules of the tissues causing ionisation and the release of electrons resulting in secondary damage to adjacent tissues. Radiation injury to normal tissues is believed to be non-specific and generally to produce no pathognomic changes [5]. However, the combined changes in the parenchyma and vascular tissues [6, 7] are thought to characterise the radiation damage to healthy tissues. Changes in the vascular network are thought to cause muscle ischaemia whilst a limited ability to expand, due to connective tissue constraints, is believed to have an effect on the efficacy of muscle contraction [8–10]. Most studies have found axillary radiation to be a prognostic factor for the development of shoulder morbidity [3, 4, 11]. Soft tissue changes have been seen from the onset of radiotherapy (dose dependent) to as late as 3 years after the start of radiotherapy [6, 7]. MRI studies have shown radiation induced muscle morbidity in cervical cancer [9] and prostate cancer [7]. In breast cancer only clinical observations have been reported for muscle morbidity of pectoralis major [6, 10], serratus anterior and lattissimus dorsi [12]. A few studies have highlighted, but not quantified, winging of the scapula in patients demonstrating limited shoulder movements [10, 12]. Conversely, surgery alone does not eradicate arm morbidity, with 19% of patients showing reduced mobility and 39% overall arm morbidity after axillary dissection without radiotherapy [13].
Evaluation of the altered shoulder movement in breast cancer patients has been in the form of clinical observations and goniometric measures of glenohumeral range of movement [3, 13–15]. However, elevation of the arm is a function of both glenohumeral movement and scapulo-thoracic movement [16] which ensures that functional activities can occur without the head of the humerus impacting on the coracoacromial arch and placing the soft tissue structures traversing the shoulder joint, in danger of impingement. The absence of osseous stability at the glenohumeral joint means that the shoulder complex relies on the interaction of both static and dynamic structures to provide joint stability. Muscles of the shoulder form the dynamic structures and can be divided functionally into stabilisers and prime movers. Timed interaction between these two groups of muscles is essential to achieve a smooth scapulohumeral rthythm [16] and movement disorders at the shoulder have been described by several authors [11, 17–20].
The primary aim of this study was to describe shoulder muscle activity (EMG) levels and size (MRI) following treatment for breast cancer and explore the relationship of these findings to the patients report of shoulder pain and function. Secondary aims were to identify the effects of age, handedness, surgical type, adjuvant therapy and duration since surgery on the altered size and activity.
Method
This was a cross sectional study of patients treated for breast cancer. Ethical clearance was granted by the Oxfordshire Local Reseach Ethics Committee (A02,064). The patients included in this study are a subset of a sample from a larger study evaluating shoulder muscle activity, joint kinematics and patients pain and dysfunction.
Participants
A sample size of 25 patients was calculated to determine a difference of 10% of voluntary muscle contraction [21], and a Sd of 0.018 (80% power; α = 0.05,two-tailed test).
Seventy-four women meeting the inclusion and exclusion criteria (Table 1) consented to take part in the study. The time since surgery ranged from 6 months to 6 years. 57 patients consented to a MRI scan.
Glenohumeral elevation—The Polhemus Fastrak™
Glenohumeral elevation in degrees was measured using an electromagnetic position and orientation movement tracking system. This comprises a three axis magnetic dipole source (or transmitter) and a three axis magnetic sensor (or receiver), together with related electronic equipment. The sensors are small and lightweight. Within a 76 cm source- to- sensor separation, the RMS system accuracy is 0.15 degree for orientation and 0.3–0.8 mm for position [21, 22]. The transmitter generates a low frequency magnetic field composed of three sequential excitation states, each of which produces an independent excitation vector.
All patients filled in a Shoulder Pain and Disability Index (SPADI) questionnaire immediately prior to EMG measurements being taken. The SPADI is a valid measure of pain and disability for shoulder dysfunction with high levels of sensitivity and reliability [23, 24]. The scale is a visual analog scale with 13 items (5 for pain and 8 for disability). Scores for pain range from a minimum of 0 mm to a maximum of 500 mm and for disability 0– 800 mm. 0 representing no symptoms of pain or disability.
Measurement of muscle activity
EMG protocol
EMG sensor leads were attached to the skin with the patient in standing. The patient was asked to elevate their arm in the plane of the scapula, taken as 40° anterior to the coronal plane (scaption). Both arms were taken through 3 repeat movements of scaption, each one matched to a metronome at one complete cycle every 8 s and guided to remain in this plane by a flat surface oriented 40° anterior to the coronal plane.
EMG instrumentation
This is a measure of the timing and level of muscle activity during arm movements. EMG data was collected with round pre-gelled silver-silver chloride surface electrodes (Maersk Medical). Signals were amplified with TEL 100 amplifier (Biopac Systems Inc) with a gain of 2000, a maximum input impedance of 10 KΏ was allowed, and a common mode rejection ratio of 110 dB at 60 Hz. Raw EMG signals were collected at a sample rate of 2000 Hz, and monitored throughout data collection to verify signal quality. Data was processed by MotionMonitor™ software.
Pre gelled electrodes were applied to prepared skin sites. The reference electrode was placed on electrically neutral tissue. Surface electrodes were placed parallel with the muscle fibres of pectoralis major (Pmaj), serratus anterior (SA), upper trapezius (UT) and rhomboid (Rhom) muscles as previously described [25]. Pectoralis minor (Pmin) was not included due to tissue depth and the use of surface electrodes. EMG signal quality was verified by having the participant perform a resisted contraction in the manual muscle test position specific to each muscle being investigated.
To minimise collection of ambient noise from the VDU screen and fluorescent lights, subjects were positioned 2 m from the computer and all lights turned off.
MRI measurements
MRI protocol
The patient was scanned in a relaxed supine position with both arms at the sides and palms facing down.
STIR images at thoracic levels T2, T4 and T6 were selected as points of measure as all muscles were present at these 3 levels (pectoralis minor was missing from T2 in many patients but not all). Bilateral cross sectional area (cm3) was measured for pectoralis major, pectoralis minor, rhomboid major and serratus anterior. Upper trapezius was excluded in the clinical MRI protocol and could not be measured.
Analysis of muscle fat and connective tissue content was performed on coronal STIR images by measurement of signal amplitude in the muscle of interest and compared to the signal amplitude of the same muscle on the unaffected side.
MRI instrumentation
Scans were acquired on a Siemens 1.5 tesla Symphony using a combination of spin array and bony array receiver coils. A large field view localiser was obtained followed by (1) Coronal T1 weighted (2) Coronal STIR (3) Axial; T1 weighted and (4) Axial STIR sequences. Sections were sampled with an interslice gap of 6 mm, and 2562 matrix; repetition time/echo (TR/TE) for STIR sequences was 6820/86; inversion time was 150.
Reliability
EMG data collection was carried out by the same two observers (one applied sensors to patient and gave instructions, one operated the computer) blind to the SPADI data. Intrarater reliability was assessed by carrying out repeat measures on a different day for all movements for a random sample of 5 participants. MRI measurements were taken by a single observer blind to the clinical history. Intrarater reliability was assessed by repeated measures of 10 of the 56 scans.
Data reduction and analysis
Descriptive analyses were conducted to assess demographic and clinical characteristics of the sample.
SPADI
Descriptive analysis was performed to determine contributions by individual items score to final score. A one-way ANOVA was conducted to compare the individual items on the pain and disability indices for each of the time scales since surgery (0–2 years, 2–4 years, 4–6 years).
EMG
A normalisation reference was collected for 1 min at rest for each muscle. Following this, average root mean square (RMS) movement values minus the RMS resting value were determined. Maximum Voluntary Contraction was not carried out due to the levels of pain experienced by participants. EMG data was taken at 10° increments of glenohumeral elevation (Fastrak data) and averaged for the three movements.
Owing to the observed variation between participants EMG readings during data collection, scatter plots of EMG data for each muscle for individual participants were first plotted against humeral elevation. The number of data points for the affected side lying above (more active) or below (less active) the unaffected side was expressed as frequencies and an average recorded.
MRI
Three repeat measures for each muscle and each participant were taken and the mean of the three measures used for analysis.
Paired t-test was used to determine the difference between muscle size and signal intensity of affected versus unaffected sides.
Blind bilateral clinical evaluation of scans were documented by SO and analysed independently by DS. Diagnoses relevant to the involved arm only were included in analysis.
Multivariate analyses
Initial exploratory analysis was carried out, plotting EMG values for each muscle and each patient separately. The EMG values for each muscle for affected minus unaffected sides were then analysed using multiple linear regression models. As well as the degree of elevation and direction of movement (“trend” in tables), the following demographic and clinical variables were included in the analysis: age, time in days from surgery, medical treatment protocol, SPADI and handedness. Backwards-stepwise selection was applied to establish the subset of covariates with highest associations with EMG, using a P-value set at 0.005 for inclusion.
Bland–Altman methods were used to determine intra-rater reliability for MRI and EMG measures.
Results
Demographic and medical details are shown in Table 2. The numbers of participants with dominant and non-dominant sides affected were closely represented.
Inter-rater reliability was good for both MRI (r = 0.89) and EMG (r = 0.98) measurements.
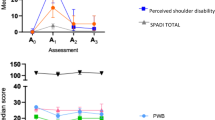
SPADI scores as a function of duration since surgery are shown in Table 3.
No significant difference between years was found but year 4–6 shows a higher score for pain.
In rating pain, 3 items emerged as the main contributors to the total score for pain. These were ‘reaching up to a shelf’ (0–2 years, 23.4%; 2–4 years 27.2%; 4–6 years, 25.57%); ‘lying on involved side’ (0–2 years, 22.66%; 2–4 years, 23.9%) and ‘pushing an object with involved arm’ (4–6 years, 20,22% of total).
Similarly, functional ratings revealed 2 items as contributing towards the majority of the final score. These were ‘placing object on high shelf (0–2 years, 27.6%; 2–4 years, 27.95%; 4–6 years, 27%) and ‘carrying an object of 10pounds or more’ (0–2 years, 20.7%; 2–4 years, 21.09%; 4–6 years, 22.9%). In years 4–6 an additional item emerges, ‘washing back’, which contributes 14.75% towards total score.
MRI data
Only pectoralis major and minor demonstrated a decrease in size on the affected side (Table 4). No significant difference was found in the signal intensity of any of the muscles.
Clinical diagnoses that were limited to the affected side only, were made in only 15.7% (n = 9) of patients (Table 5).
EMG data
Analysis of scatter plots of muscle EMG against humeral elevation for each participant showed a difference between affected and unaffected side for the majority of cases (Table 6). However, a great variation within participants and between participants, with a spread of data, was also observed. Because of the potential for outliers to cancel any mean difference, a linear regression model was fitted to the data (Tables 7 – 10).
Three of the 4 muscles were less active on the affected side, confirming the descriptive statistics shown in Table 6. A much larger difference was found in UT and although a difference was found for PMaj in Table 6 this was not shown to be significant. The covariates degree, trend and duration since surgery were significantly associated with a loss of EMG activity for all muscles. Loss of muscle activity is enhanced on the downward movement (‘Trend’ in all tables and concurs with Table 6), at the highest point of elevation (degree) and the longer the time since surgery. A high SPADI score was only significantly associated with loss of muscle activity in UT and Rhom (Tables 9, 10). Lower PMaj, UT and Rhom muscle activity were significantly associated with treatment protocol 2 (mastectomy and radiotherapy). Loss of activity in UT was significantly associated with all treatment protocols except mastectomy. Treatment protocol 3 (Mastectomy + radiotherapy + axillary radiotherapy) and 6 (WLE + axillary clearance + RT) were significantly associated with a loss in muscle activity in UT and Rhom. SA activity was lower with treatment protocol 6 but higher with protocol 3.
Discussion
This study has shown generalised loss of activity in four key muscles acting on the shoulder complex during elevation of the arm and long term pain and disability.
This concurs with previous studies showing patients who develop pain after treatment for breast cancer experience diminished ability to carry out ADL tasks, reduced health-related QOL, and psycosocial distress [26–28]. Pain scores of >30 mm have a moderate effect on ADL and scores between 30–50 mm have a severe effect on ADL [26]. This study clearly shows pain levels at all time intervals to be above 50 mm, with years 4–6 since surgery recording the highest pain scores. The main activities affected are reaching up or carrying heavy loads, which agree with findings of Karki et al. [29].
The drop in muscle activity found here supports patients reports of weakness as shown by Isaksson and Feuk [30] and Karki et al. [29], where 13% and 17.7% of patients reported weakness respectively. The lower activity in UT concurs with the observation during analysis of the MRI scans of a high number of dropped shoulders (in the presence of a straight thoracic spine) on the affected side. Significant loss of all muscle activity on the downward movement of the arm indicates loss of eccentric muscle control of the shoulder girdle against gravity. Several shoulder movement disorder syndromes have been described by Sahrmann [20] and include the ‘Dropped Shoulder Syndrome’. This syndrome includes decreased UT activity, dropped shoulder, neck-shoulder pain, small pectoralis major and minor, numbness, symptoms aggravated by heavy breasts, heavy arms and carrying heavy objects. Some of the symptoms are believed to be due to pressure in the thoracic outlet space (eg. Numbness, parasthesia) [20].
This study has shown decreased activity of UT, small PMaj and minor, reports of increased pain with carrying objects and lifting the arm. Similarly, the Karki [29] study at 1 year follow up demonstrated that 40.6% of patients reported neck-shoulder pain, 49% reported increased symptoms with carrying objects, and patients with a higher BMI and heavier arms had symptom exacerbation. It would appear that some patients treated for breast cancer are showing many signs and symptoms of a ‘Dropped Shoulder Syndrome’.
Pectoralis muscles and serratus anterior are in the field of surgery and radiotherapy and it is therefore not surprising they are affected. A short pectoralis minor muscle acts to tilt the scapula anteriorly while a weak serratus anterior will cause winging of the medial border of the scapula, confirming clinical observations. Reduction in size of PMaj may affect the patients ability to reach up, particularly as extensibility of this muscle is required to disengage the humeral head from the glenoid cavity at the end of humeral elevation [16]. However, the largest change was in UT and Rhom neither of which are in the line of surgery or radiotherapy. These ‘secondary’ effects noted here for up to 6 years after treatment are corroborated by patients reporting weakness for up to 5 years after treatment [30]. Furthermore, in our study patient levels of pain and functional ability were only associated with reduced UT and Rhom activity. Both UT and Rhom have lower activity associated with most of the treatment protocols. It would appear therefore that secondary muscle changes are occurring and these persist for longer and are associated with the patients ability to perform pain free functional tasks.
However, 25% of patients reported no pain and 20.27% reported no disability. Only 5% of those reporting pain did not report disability, linking pain to the inability to perform functional movements. The question remains as to why some women experience pain and disability and others do not.
Risk factors for chronic pain include more invasive surgery, radiotherapy and acute postoperative pain [29, 30]. Patients experiencing acute postoperative pain may be inclined to adopt protective postures (dropped and rounded shoulder and arm) and reduced use of the arm, resulting in long term changes to muscle length and activity. Acute postoperative pain is most likely to occur in patients experiencing high levels of preoperative anxiety [31] and it is feasible that these women are the ones most likely to move less and protect the arm and treatment site for fear of damaging themselves further. This may be a sub-population of patients needing extra support and guidance through the immediate post-operative period.
Connective tissue changes such as scarring [29] and Axillary Web Syndrome (AWS) [32], or cording, are other known contributory factors to arm morbidity. Large numbers of women are still reporting tightness of the breast scar (29.2%) and axillary scar (36.5%) at 1 year follow up [29]. These effects are likely to be enhanced in more aggressive treatment protocols which have been reported as a risk factor for shoulder morbidity and for acute postoperative pain. In the early stages of recovery the above mentioned postural adjustment may be made to reduce tension on the site, thereby reducing pain. However, these adjustments together with the effects of radiotherapy may lead to the long-term effects reported here.
Very few patients in this study presented with a clinical diagnosis from scans and no pattern could be seen in the SPADI scores for patients with a diagnosed pathology. It would appear therefore that pain and loss of function is not coming from any overt structural pathology. This however, does need further clarification, as this report did not look at the connective tissue, vascular or neural changes in the axilla, clavipectoral and other regions. Vascular and fibrotic changes have been noted and could lead to the observations of weakness and fatigue [33, 34]. In light of the current findings, an in depth analysis of the scans and adjuvant treatment protocols is being undertaken.
Conclusion
Patients treated for breast cancer demonstrate altered muscle activity in three key muscles acting at the shoulder, reduced muscle size in two muscles in the line of surgery and radiotherapy, and persistent pain and functional limitation for up to 6 years after treatment. These results suggest that the normal biomechanics of the shoulder complex is altered. Our laboratory is currently analysing the kinematic data for the glenohumeral joint and the scapula, which should provide further guidance in developing the exercise component of a rehabilitation programme.
References
Mamounas EP (2005) Continuing evolution in breast cancer surgical management. J Clin Onc 23(8):1603–1606
Box RC, Reul-Hirche H, Bullock-Saxton JE, Furnival C (2002) Shoulder movement after breast cancer surgery: results of a randomised controlled study of postoperative physiotherapy. Br Canc Res Treatment 75(1):35–45
Rietman J, Dijkstra P, Hoekstra H, Eisman W, Szabo B, Groothoff J, Geertzen J (2003) Late morbidity after treatment of breast cancer in relation to daily activities and quality of life: a systematic review. Eur J Surg Oncol 29:229–238
Gossenlink R, Rouffaer L, vanhelden P, Piot W, Troosters T, Christiaens M (2003) Recovery of upper limb function after axillary dissection. J Surg Oncol 83:204–211
Rezvani M, Hopewell JW, Robbins MEC (1995) Initiation of on-neoplastic late effects: the role of endothelium and connective tissue. Stem Cells 13(suppl 1):248–256
Wedgewood KR, Benson EA (1992) Non-tumour morbidity and mortality after modified radical mastectomy. Ann Royal College Surg Engl 74:314–317
Soulen RL, Romero JA, Chuba PJ et al (1997) Musculoskeletal complications of neutron therapy for prostate cancer. Radiat Oncol Investig 5(2):81–91
Aitken RJ, Gaze MN, Rodger A et al (1989) Arm morbidity within a trial of mastectomy and either nodal sample with selective radiotherapy or axillary clearance. Br J Surg 76:568–571
Blomlie V, Rofstad EK, Tvera K, Lien HH (1996) Non-critical soft tissues of the female pelvis: Serial MR imaging before, during and after radiation therapy. Radiology 199:461–468
Gutman H, Kersz T, Barzilai T et al (1990) Achievements of physical therapy in patients after modified radical mastectomy compared with quadrantechtomy, axillary dissection and radiation for carcinoma of the breast. Arch Surg 125:389–391
McConnell J (1994) The McConnell approach to the problem shoulder, Chapter 3. Course notes, McConnell Institute, Neutral Bay
Gerber L, Lampert M, Wood C et al (1992) Comparison of pain, motion and oedema after modified radical mastectomy versus local excision with axillary dissection and radiation. Breast Cancer Res Treat 21:139–145
Keramopoulos A, Tsionou C, Minaretzis D et al (1993) Arm morbidity following treatment of breast cancer of total axillary dissection: a multivariated approach. Oncology 50:445–449
Sneeu KCA, Arenson NK, Jaarnold JR (1992) Cosmesis and functional outcomes of breast conserving treatments for early stage breast cancer. Comparisons of patients ratings, observers ratings and objective measurements: a multivariate approach. Radiother Oncol 25:1532–1539
Kuehn T, Klauss W, Darsow M, regele S, Flock F, Maiterth C et al (2000) Long term morbidity following axillary dissection in breast cancer patients – clinical assessment, significance for life quality and the impact of demographic, oncologic and therapeutic factors. Breast Cancer Res Treat 64:275–286
Donatelli RA (2000) Physical therapy of the shoulder, 3rd edn. Churchill Livingstone Inc
Richardson C, Jull G (1995) Muscle control-pain control. What exercises would you prescribe? Manual Therapy 1(1):1–9
Mcquade KJ, Dawson J, Smidt GL (1995) Scapulothoracic muscle fatigue associated with alterations in scapulohumeral rhythm kinematics. J Orthop Sports Phys Ther 28(2):74–84
Babyar SR (1996) Excessive scapular motion in individuals recovering from painful and stiff shoulders: causes and treatment strategies. Phys Ther 76(3):226–238
Sahrmann SA (2002) Diagnosis and treatment of movement impairment syndromes. Mosby Inc
Ludewig PM, Cook TM (2000) Alterations in shoulder kinematics with associated muscle activity in people with symptoms of shoulder impingement. Phys Ther 80(3):276–291
Harryman DJ, Sidles JA, Matsen FA (1992) Laxity of the normal glenohumeral joint: a quantitative in-vivo assessment. J Shoulder Elbow Surg 1:66–76
Roach KE, Budiman-Mak E, Songsriridej N, Lertatanakul Y (1991) Development of a shoulder pain and disability index. Arthritis Care Res 4:143–149
Williams JW, Holleman DR, Simel DL (1995) Measuring shoulder function with the shoulder pain and disability index. J Rheumatol 22(4):727–732
Ludewig PM, Cook TM, Nawocszenski DA (1996) Three-dimensional scapula orientation and muscle activity at selected positions of humeral elevation. JOSPT 24(2):57–65
Tengrup I, Tennvall-Nittby L, Christiansson I, Laurin M (2000) Arm morbidity after breast conserving therapy. Acta Oncologica 39:393–397
Akechi T, Okuyama T, Imoto S, Yamawaki S, Uchitomi Y (2001) Biomedical and psycosocial determinants of psychiatric morbidity among post-operative ambulatory breast cancer patients. Breast Cancer Res Treat 65:195–202
Stevens PE, Dibble SL, Miaskowski C (1995) Prevalence, characteristics and impact of post mastectomy pain syndrome. An investigation of women’s experiences. Pain 61:61–68
Karki A, Simonen R, Malkia E, Selfe J (2005) Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. J Rehabil Med 37:180–188
Isaksson G, Feuk B (2000) Morbidity from axillary treatment in breast cancer. A follow up study in a district hospital. Acta Oncol 39:335–336
Katz J, Poleshuck EL, Katz J, Andrus CH, Hogan LA, Jung BF, Kulick DI, Dworkin RH (2005) Risk factors for acute postoperative pain and its persistence following breast cancer surgery: a prospective study. Pain 119:16–25
Leidenuis M, Leppanen E, krogerus L, Von Smitten K (2003) Motion restriction and axillary web syndrome after sentinel node biopsy and axillary clearance in the breast cancer. Am J Surg 185:127–130
Jung BF, Herrman D, Griggs J, Oaklander AL, Dworkin RH (2005) Neuropathic pain associated with non-surgical treatment of breast cancer. Pain 118:10–14
Johansson S, Svensson H, Denekamp J (2000) Timescale of evolution of late radiation injury after postoperative radiotherapy of breast cancer patients. Int J Radiol Oncol Biol Phy 48(3):745–750
Acknowledgments
We would like to thank the Oxford Hospitals Research Charities for providing the funds for this research. Thank you to Colleen Berrington and Ion Lascurain-Aguiberra for their invaluable technical assistance, and Dr Karen Barker and Jane Moser for their critical reviews.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shamley, D.R., Srinanaganathan, R., Weatherall, R. et al. Changes in shoulder muscle size and activity following treatment for breast cancer. Breast Cancer Res Treat 106, 19–27 (2007). https://doi.org/10.1007/s10549-006-9466-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-006-9466-7