Abstract
Selenium (Se) is an essential trace element for the body. Various organs of the body, including the intestine, are affected by its deficiency. Se deficiency can induce oxidative stress and inflammatory responses in the intestine. It can also increase intestinal permeability and decrease intestinal immune function in mammals. However, the detailed studies, conducted on the intestinal molecular mechanisms of Se deficiency-induced injury in poultry, are limited. This study explored the adverse effects of Se deficiency on intestinal permeability and its mechanism. A Se-deficient chicken model was established, and the morphological changes in the chicken duodenum tissues were observed using a light microscope and transmission electron microscope (TEM). Western blotting, qRT-PCR, and other methods were used to detect the expression levels of selenoproteins, oxidative stress indicators, inflammatory factors, tight junction (TJ) proteins, antimicrobial peptides, and other related indicators in intestinal tissues. The results showed that Se deficiency could decrease the expression levels of selenoproteins and antioxidant capacity, activate the nuclear factor kappa-B (NF-κB) pathway, cause inflammation, and decrease the expression levels of TJ proteins and antimicrobial peptides in the duodenum tissues. The study also demonstrated that Se deficiency could increase intestinal permeability and decrease antimicrobial peptides via reactive oxygen species (ROS)/NF-κB. This study provided a theoretical basis for the scientific prevention and control of Se deficiency in poultry.
Graphical abstract
Se deficiency decreased the expression levels of selenoproteins and increased ROS levels to activate the NF-κB pathway, resulting in the production of pro-inflammatory cytokines, reducing the expression levels of TJ protein, and weakening the expression of antimicrobial peptides, which contributed to the higher intestinal permeability. Oxidative stress weakened the expression of antimicrobial peptides.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se), an essential trace element for an organism, has antioxidant, immunomodulatory, and anti-inflammatory effects (Zhang et al. 2021a; Jia et al. 2021), thereby playing an important role in various physiological functions, such as metabolism and immunity. Se deficiency can cause various cardiovascular diseases in humans, including Keshan’s disease (KD), heart failure, and myocardial infarction (Shimada et al. 2021). Se deficiency can also cause oxidative and endoplasmic reticulum stress in the liver, which in turn leads to the apoptosis of chicken hepatocytes (Yang et al. 2017). Since the intestine is the major organ for Se absorption, its deficiency can also cause intestinal autophagy, apoptosis, and inflammation, ultimately damaging the morphological structure and immune function of the intestine. Zheng et al. reported that Se deficiency could cause intestinal apoptosis in swine through endoplasmic reticulum (ER) stress (Zheng et al. 2021a), induce the accumulation of reactive oxygen species (ROS) to promote autophagy (Zheng et al. 2021b), and cause the necroptosis of the small intestine, imbalance of T helper cell 1/T helper cell 2 (Th1/Th2), and inflammatory responses in swine (Zhang et al. 2021b). The role of Se is usually associated with selenoproteins; therefore, its deficiency can downregulate the expression levels of selenoproteins, particularly its antioxidant functions. The selenoproteins are mostly redox-active selenoenzymes and play an important role in antioxidative stress (Rocca et al. 2019). The reduced expression levels of selenoproteins could cause oxidative stress, activate the nuclear factor kappa-B (NF-κB) inflammatory pathway, and trigger the renal inflammatory response, thereby deteriorating renal function (Zhang et al. 2016). Previous studies showed that Se deficiency could cause oxidative stress in the human colon by reducing the expression levels of selenoproteins, leading to bowel cancer (Papp et al. 2010). Studies reported that the Se deficiency could reduce the levels of intestinal glutathione peroxidase (GSH-Px) and induce oxidative stress, ultimately causing oxidative damage to intestinal tissues (Yu et al. 2015).
The imbalance between the production and accumulation of ROS in the biological system is called oxidative stress, which can cause damage to organisms by playing a pathogenic role in chronic inflammatory diseases (Popa-Wagner et al. 2013). Superoxide dismutase (SOD), catalase (CAT), GSH-Px, and total antioxidant capacity (T-AOC) play important roles in scavenging ROS (Echeverría et al. 2016; Verma et al. 2013), thereby protecting the biofilm from ROS-induced damages and maintaining the normal functioning of cells (Tsikas 2017). Se is majorly involved in antioxidant defense and in maintaining the dynamic redox balance. The function of Se is associated with the expression levels of selenoproteins; a decrease in its expression levels can reduce the antioxidant capacity of the body, leading to oxidative stress. Oxidative stress can increase the expression levels of pro-inflammatory factors, including tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), thereby causing inflammation (Prabhakar 2013). Numerous studies showed that intestinal oxidative stress could produce pro-inflammatory cytokines, reduce the expression levels of tight-junction (TJ) proteins, and increase intestinal permeability (Hasegawa et al. 2021; Elbadawi et al. 2021; Lan et al. 2021). Innate effector molecules, known as antimicrobial peptides, are one of the important barriers to intestinal immunity due to their bactericidal and anti-inflammatory effects (Rengaraj et al. 2018). The decreased levels of antimicrobial peptides could impair the intestinal immune barrier and cause an imbalance of microbiota, thereby affecting intestinal barrier function and aggravating intestinal injury (Berkowitz et al. 2019). Previous studies showed that the deficiency of threonine-activated juvenile grass could inhibit the intestinal NF-κB signaling pathway and upregulate the mRNA expression levels of intestinal pro-inflammatory cytokines, including TNF-α, IL-1β, IL-6, and IL-8. This could also decrease the mRNA expression levels of the intestinal anti-inflammatory cytokine, including transforming growth factor-β1 (TGF-β1), TGF-β2, and IL-10, and downregulate the antimicrobial peptides, leading to a decrease in the intestinal immunity and aggravating inflammation (Dong et al. 2017). It was observed that intestinal inflammation could not only affect the permeability of the intestine but could also immensely affect the expression levels of intestinal antimicrobial peptides and aggravate intestinal damage. To sum up, Se deficiency in humans and mammals can cause oxidative stress and inflammatory responses, increase intestinal permeability, and reduce intestinal immune function (Zhang et al. 2021b; He et al. 2020).
Se-deficient poultry diets result in slow growth and development, reduced egg production, decreased hatchability, and pancreatic degeneration (Li et al. 2018; Cantor and Scott 1974). Se deficiency targets and affects the intestine, a major organ for the absorption of Se (Gao et al. 2016). In addition to the biological effects of Se on other organs, the disorder of intestinal function also affects the normal functioning of other organs (Chopyk and Grakoui 2020). Therefore, the effects of Se deficiency on the intestine should be explored. Based on previous studies and literature reports, it was speculated that the Se deficiency might alter the intestinal permeability and antimicrobial peptides of broiler through ROS/NF-κB signaling pathway. Therefore, the current study replicated the Se-deficient chicken model and observed the histopathology and cellular ultrastructure of the chicken duodenum under a light microscope and transmission electron microscope (TEM). The expression levels of selenoproteins, oxidative stress indicators, inflammatory factors, TJ proteins, antimicrobial peptides, and other related indicators in intestinal tissues were studied using Western blot, quantitative real-time PCR (qRT-PCR), and other methods. The results indicated that Se deficiency could increase the activation of the NF-κB pathway by ROS, leading to the production of pro-inflammatory cytokines, increased intestinal permeability, and decreased expression of antimicrobial peptides. This study provided a basis for using Se as a feed additive to protect broiler intestinal health.
Materials and methods
Animals and treatment
All the animal experiments were conducted following the Animal Care and Use Committee of Northeast Agricultural University (SRM-11) Harbin, China. A total of 60 one-day-old Arbor Acres (AA) male broiler chickens were purchased from Harbin Yinong Poultry and were raised in cages. They were randomly divided into two groups including control and Se-deficient groups (n = 30 per group). According to the nutrients required for the growth and development of broilers, a full-price diet was formulated. Corn, soybean meal, and wheat bran in the formulated diet were purchased from Longjiang County, a Se-deficient area in Heilongjiang Province. The Se content in the diet of broilers for the control group was 0.2 mg/kg diet, while that for the Se- deficient group was only 0.03 mg/kg diet (Qing et al. 2022). Food and water were provided ad libitum. In the initial days of the experiment, all broilers were fed freely 3 times a day under the same conditions. After 35 days of feeding, the duodenum tissues were quickly removed and rinsed with ice-cold sterile phosphate-buffered saline (PBS) (pH = 7.2). These duodenum tissues were then used for subsequent experiments.
Histological analysis
After their fixation with a 10% formalin phosphoric acid solution, the duodenum tissues were rinsed with water for 12 h. The tissues were then dehydrated using gradient alcohol, made transparent, paraffinized, and then cut into 5 μm thick sections (Chen et al. 2022). The tissue sections were then spread in warm water, fixed on a glass slide, and dried at 37 °C for 12 h. Then, hematoxylin and eosin (H&E) staining (Leagene Biotechnology, Beijing, China) was performed for dewaxing and hydration. The stained tissues were dehydrated with ethanol, made transparent with xylene (Leagene Biotechnology, Beijing, China), and fixed with neutral gum for inspection. After drying, the morphology of cells and damaged tissues was observed under Leica DME100 light microscope.
TEM observation
Duodenum tissues were soaked in 2.5% glutaraldehyde (Leagene Biotechnology, Beijing, China), and then rinsed twice with 0.2 M phosphate buffer (pH = 7.2) for 15 min. The tissues were then fixed in 1% buffered osmium tetroxide for 1 h followed by dehydration with gradient alcohol and embedding in epoxy resin. Finally, the tissue sections were subjected to negative staining and observed using TEM (Hitachi, Japan) following the standard operation.
Oxidative stress marker assay
Briefly, duodenum tissues were homogenized with physiological saline (1:9) on ice and centrifuged at 2500 g for 10 min at 4 °C. The supernatant was collected for further experiments. The levels of total protein (TP) contents (Cat. No. A045-2-2), GSH-Px (Cat. No. A005-1-2), SOD (Cat. No. A001-3-2), CAT (Cat. No. A007-1–1), GSH (Cat. No. A006-2-1), and malondialdehyde (MDA) (Cat. No. A003-2-2) were detected using their respective commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturers’ instructions.
RNA extraction and qRT-PCR
The total RNA was extracted from the duodenum tissues using TRIzol reagent (Beijing Solarbio Technology, Beijing, China) and reverse-transcribed into cDNA using the cDNA First-Strand synthesis kit (Bioer Technology, China) as described previously (Xu et al. 2021). The cDNA was stored at −20 °C for qRT-PCR analysis. All the PCR reactions were performed in a 10 µL reaction volume, containing 5 μL of 2 × SYBR Green PCR Master Mix (Roche, Basel, Switzerland), 1 μL of diluted cDNA, 0.3 μL of each primer (10 μM), and 3.4 μL of deionized water. The qRT-PCR reaction was performed using a Light Cycler® 480 System (Roche, Basel, Switzerland). The mRNA primers used for the qRT-PCR analysis are listed in Table 1. The relative mRNA expression levels were determined using the 2−△△Ct method with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the internal reference control for normalization.
Western blot analysis
Western blot analysis was performed to detect the expression levels of TJ and inflammation-related proteins. The total protein contents in duodenum tissues were extracted using phenylmethyl sulfonyl fluoride (PMSF) and immunoprecipitation (IP) lysate (Biyuntian Biotechnology Co., Ltd., China) as described previously (Miao et al. 2022). The concentration and purity of the extracted proteins were determined. Briefly, the protein samples were separated on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels and then transferred to polyvinylidene fluoride (PVDF) membranes, which were blocked using 5% skim milk at 37 °C for 2 h. The membranes loaded with protein samples were incubated with primary antibodies at 4 °C for 12 h, followed by incubation with the respective secondary antibodies [horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG] (1:5000-1: 8000, Santa Cruz Biotechnology, USA) at 37 °C for 1.5 h. The dilution ratio and source of primary antibodies are listed in Table 2. Image acquisition was performed using the SH-523 system. The images were then analyzed using ImageJ software.
Statistical analyses
All the experiments were performed in three independent replicates for data collection. GraphPad Prism version 8.0 software was used for statistical analysis. Unpaired Student’s t-test was used to identify the statistical significance. All the results were expressed as means ± standard deviation (SD). The P-values of < 0.05 and < 0.01 are represented by single and double asterisks (*), respectively.
Results
Pathological lesion of duodenal inflammation induced by Se deficiency
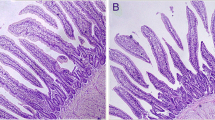
According to a previous study (Gao et al. 2012), the Se level in the duodenum of chickens fed with a normal diet was 0.367 mg/kg, while in the duodenum of chickens fed with a Se-deficient diet, the Se level was 0.241 mg/kg. This showed that the Se-deficient diet could reduce the Se level in the duodenum of chicken. In this study, the body weights of broilers at different time points were measured (Fig. 1A). The results showed that in the Se-deficient group, the body weights of the broilers were lower than those in the control group. The difference in the body weights of broilers between the two groups on the 28th day was extremely significant (P < 0.01) as shown in Fig. 1A. This showed that Se deficiency could impair the growth of broilers. The histopathological studies of the duodenum tissues, obtained from both the control and experimental animals, were performed (Figs. 1B and 1C). In the control group, the duodenum tissues were normal with neatly arranged intestinal villi, clear structure, and normal crypts (Fig. 1B). On the other hand, the architectural details of intestinal epithelium cells and the organization and form of villi were completely lost in the Se-deficient group. Moreover, the upward movement of the crypt and inflammatory cell infiltrations were also observed as indicated by the yellow and red arrows in Fig. 1C, respectively. The histopathological changes are summarized in Table 3.
Pathological damage of duodenum induced by Se deficiency in broilers. A Changes in the weight of broilers. n = 6, *: significant difference (P < 0.05); **: significant difference (P < 0.01). B and C H&E staining of the duodenum in each group. Red arrows indicate the inflammatory cell infiltration, and yellow arrows indicate the upward movement of the crypt. Scale bars are shown in the figures. D and E TEM images of the duodenum in each group. Scale bar: 1 μm. (Color figure online)
The effects of Se deficiency on the ultrastructural changes in cells and organelles of duodenum tissue were further explored and detected using TEM analysis. The results are presented schematically in Fig. 1D and E. In the control group, the TJs between the adjoining cells and epithelial cell surface microvilli were arranged in neat rows (Fig. 1D). In the Se-deficient group, the intestinal villi were sparse with abnormal TJ, damaged structures, and voids in between, resulting in the rupturing of the goblet cell membrane (Fig. 1E).
Effects of Se deficiency on the expression of selenoproteins
As shown in Fig. 2, the results showed that the mRNA expression levels of selenoproteins significantly decreased in the Se-deficient group as compared to the control group (P < 0.05). It was worth noting that among all the selenoproteins, the expression levels of the DIO3 selenoproteins significantly decreased in the Se-deficient group. As compared to the control, the expression levels of selenoproteins in order from high to low in the Se-deficient group were as follows: SELS, SELT, SELI, GPX2, TXNRD3, SELM, SELO, GPX3, TXNRD1, SELPB, SELN, TXNRD2, SELU, SELK, SEPP1, SEP15, SEPX1, SPS2, SELW, DIO1, GPX1, GPX4, DIO2, SELH, and DIO3. Moreover, the selenoproteins GPXs can scavenge ROS, thereby preventing oxidative stress in the organism (Echeverría et al. 2016). Therefore, it was speculated that the Se deficiency-induced decrease in the expression levels of selenoprotein GPXs might cause oxidative stress in the duodenum.
Detection results of oxidative stress index
The oxidative stress in the duodenum caused by the reduced expression levels of selenoproteins was further confirmed by measuring the levels of the oxidative stress markers, including MDA and GSH contents and SOD, GSH-Px, and CAT activities. MDA content was significantly elevated by 2.187 folds as compared to the control group (P < 0.05) (Fig. 3). Correspondingly, the GSH contents were reduced by 18% (P < 0.01), and the activities of SOD and CAT decreased by 17% and 23% in the Se-deficient group (P < 0.05) (Fig. 3A). As compared to the control group, the GSH-Px level decreased by 80% in the Se-deficient group (Fig. 3A). The expression levels of other antioxidant factors, including SOD1, SOD2, CAT, glutathione S-transferase (GST), heme oxygenase 1 (HO-1), glutamate-cysteine ligase catalytic (GCLC) subunit, glutamate-cysteine ligase modifier (GCLM) subunit, and NAD(P)H quinone dehydrogenase 1 (NQO1), were also analyzed. The results showed that the mRNA expression levels of antioxidant factors decreased in the Se-deficient group (SOD1, P < 0.05; SOD2, P < 0.01; CAT, P < 0.05; GST, P < 0.05; HO-1, P < 0.01; GCLC, P < 0.05; GCLM, P < 0.01; and NQO1 P < 0.01) as shown in Fig. 4A.
Effects of low Se on antioxidation ability of duodenum in broilers. A Detection of oxidative stress index. n = 3, *: significant difference (P < 0.05); **: significant difference (P < 0.01). MDA Malondialdehyde; SOD Superoxide dismutase; CAT Catalase; GSH GSH, Glutathione; GSH-Px, Glutathione peroxidase
Effects of low Se on antioxidation ability of duodenum in broilers. A mRNA expression levels of the antioxidant genes. n = 3, *: significant difference (P < 0.05); **: significant difference (P < 0.01). SOD1 superoxide dismutase 1; SOD2 superoxide dismutase 2; CAT Catalase; GST Glutathione S-transferase; HO-1: heme oxygenase 1; GCLC glutamate-cysteine ligase catalytic subunit; GCLM glutamate-cysteine ligase modifier subunit; NQO1 NAD(P)H quinone dehydrogenase1
Se deficiency could activate the NF-κB signaling pathway in the duodenum
The NF-κB pathway is a major inflammatory signaling pathway, which produces inflammatory cytokines in response. The mRNA and protein expression levels of NF-κB p65, an inhibitor of kappa B kinase α (IKKα), IKKβ, and IL-1β were identified to determine the modulatory effects of Se deficiency on the NF-kB pathway. The results showed that the expression levels of NF-κB p65 (P < 0.01), IKKα (P < 0.01), IKKβ (P < 0.05), and IL-1β (P < 0.01) increased significantly in the Se-deficient group as compared to control group (Fig. 5A and B). The inflammatory cytokines have an important role in the inflammatory response. Therefore, the mRNA and protein expression levels of IL-6, IL-10, TNF-α, and interferon-γ (IFN-γ) were determined in the duodenum tissue of chicken in the Se-deficient and control groups. As compared to the control group, the protein and mRNA expression levels of IL-6, IFN-γ, and TNF-α increased, while those of IL-10 significantly decreased (P < 0.05) (Fig. 5A and B).
Effects of Se deficiency on the expression of inflammatory-related factors in the duodenum of broilers. A mRNA expression levels of NF-κB-p65, IKKα, IKKβ, IL-1β, TNFα, IFN-γ, IL-6, and IL-10 were evaluated using qRT-PCR. B Western blot analysis of p-NF-κB-p65, NF-κB-p65, IKKα, IKKβ, IL-1β, TNFα, IFN-γ, IL-6, and IL-10. n = 3, *: significant difference (P < 0.05); **: significant difference (P < 0.01)
Effects of Se deficiency on avian beta-defensins (AvBDS) and TJ
The effects of Se deficiency on AvBDs in duodenum tissues were analyzed by detecting the mRNA expression levels of AvBD1, AvBD2, AvBD8, AvBD9, AvBD10, and AvBD13. The results showed that their expression levels decreased in the Se-deficient group as compared to the control group (Fig. 6A). Among them, the mRNA expression levels of AvBD1 AvBD8, and AvBD10 decreased significantly (P < 0.05), showing a 34%, 85%, and 84% decrease in their expression levels, respectively. TJ proteins are critical for the normal functioning of the intestinal epithelial barrier. Therefore, their expression levels were determined (Fig. 6A and B). The results showed that Se deficiency could significantly decrease the mRNA and protein expression levels of Claudin 1, Occludin, and zonula occludens-1 (ZO-1) (P < 0.05); their mRNA expression levels decreased by 68%, 37%, and 31%, respectively (P < 0.05).
Effects of Se deficiency on AvBDS and TJ. A mRNA expression levels of AvBD1, AvBD2, AvBD8, AvBD9, AvBD10, AvBD13, Claudin1, ZO-1, and Occludin were evaluated using qRT-PCR. B Western blot analysis of Claudin1, ZO-1, and Occludin. n = 3, *: significant difference (P < 0.05); **: significant difference (P < 0.01)
Discussion
Se, a microelement, is an essential element for the normal growth and development of animals (Guo et al. 2018). Numerous epidemiological surveys showed that the deficiency of Se was related to the high incidence of many diseases, such as cancer, reproductive diseases, cardiovascular diseases, and infectious diseases (Rayman 2012). Se deficiency can cause oxidative stress, thereby inducing apoptosis, inflammation, necrosis, immune disorders, diabetes, and energy metabolism disorders (Steinbrenner 2013; Gao et al. 2019). Intestinal inflammation can deteriorate the function of the intestinal epithelial barrier and increase intestinal permeability (Kohan et al. 2016). Numerous studies reported that antimicrobial peptides could regulate TJ proteins in intestinal epithelial cells and enhance intestinal epithelial barrier function. These studies were mainly focused on pigs and mice, and the studies on avian antimicrobial peptides and intestinal TJ proteins are limited (Lijun et al. 2021; Feng et al. 2020; Alessandra et al. 2021; Yu et al. 2018). The current study established the Se-deficient broiler models. They exhibited a large number of inflammatory cell infiltrations in their duodenal epithelial cells, altered intestinal TJ, a significant decrease in the expression levels of selenoproteins, oxidative stress, increased secretion of pro-inflammatory factors, and decreased expression levels of TJ proteins and antimicrobial peptides.
Se is majorly involved in antioxidant defense and functions through selenoproteins with antioxidant properties. The downregulation of these selenoproteins can induce oxidative stress in organisms. Se can also inhibit the formation of oxides by increasing GSH-Px activity, thereby maintaining the dynamic balance of redox (Tang et al. 2022). The levels of SOD, GSH-Px, CAT, GSH, and MDA are used to evaluate the antioxidant capacity of tissues (Tang et al. 2022). The current study indicated that the Se deficiency could decrease the expression levels of 25 selenoproteins and the activities of endogenous antioxidant enzymes, including SOD, CAT, GSH, and GSH-Px in the duodenum of broilers while increasing the contents of MDA, which eventually led to oxidative stress. Qiu et al. detected the expression levels of antioxidant genes, including GCLC, GCLM, HO-1, SOD1, and NQO-1, to reflect the intestinal antioxidant capacity of piglets (Qiu et al. 2021). Similarly, the current study showed that Se deficiency could alter the mRNA expression levels of GCLC, GCLM, HO-1, SOD1, and NQO-1. These results indicated that Se deficiency might decrease the expression levels of selenoproteins in the broilers' duodenum, thereby causing oxidative stress.
In the intestine, the decrease in the antioxidant capacity can cause the excessive accumulation of oxygen free radicals, thereby activating the inflammatory signaling pathway NF-κB. Subsequently, it makes the key transcription factors enter the nucleus and initiate the transcription of inflammatory factors, causing an increase in the secretion of TNF-α, IL-1β, and other pro-inflammatory factors, which ultimately leads to inducing inflammatory injury (Tang et al. 2022; He et al. 2022). TNF-α is a key regulator of intestinal inflammation and can increase the permeability of the intestine (Parveen et al. 2020). Increased gut permeability can promote inflammatory responses, whereas systemic inflammation can increase the permeability of the gut (Rexidamu et al. 2019). Moreover, TNF-α plays a role in the pathology of inflammatory bowel disease (IBD) by decreasing epithelial barrier function and TJ complexity of a human intestinal epithelial cell line. TJ proteins, including Occludin, ZO-1, and Claudins, are often used as an indicator of the barrier function and permeability in the intestine, (Hu et al. 2017). The current study showed that Se deficiency could activate the NF-κB pathway in the duodenum of broilers and decrease the expression levels of TJ proteins, which led to the disruption of the intestinal barrier and an increase in gut permeability. Besides its role in digestion and absorption, the intestine is the largest immune organ of broilers (Yan et al. 2021). In addition to lymphocytes, antimicrobial peptides also play a role in sterilization, anti-inflammation, and promoting body repair of the intestinal barrier. A reduction in the expression levels of antimicrobial peptides in the intestine might increase the expression levels of inflammatory cytokines and intestinal permeability (Vornhagen et al. 2021). The present study showed that Se deficiency could induce oxidative stress in the duodenum to activate NF-κB inflammatory pathway, decrease the expression levels of TJ protein and antimicrobial peptide, affect the intestinal barrier function and permeability, and aggravate intestinal injury.
Conclusions
The current study found that Se deficiency could increase the activation of the NF-κB pathway via oxidative stress, resulting in an increased production of pro-inflammatory cytokines and decreased expression levels of TJ proteins, which contributed to higher intestinal permeability. However, oxidative stress weakened the expression levels of antimicrobial peptides. This study demonstrated the harmful effects of Se deficiency in the intestine of broilers and might provide a theoretical basis and data support for the prevention of disorders, occurring in broilers due to Se deficiency.
Abbreviations
- qRT-PCR :
-
Quantitative real-time PCR
- NF-κB :
-
Nuclear factor kappa-B
- ROS :
-
Reactive oxygen species
- ER :
-
Endoplasmic reticulum
- T-AOC :
-
Total antioxidant capacity
- TNF-α :
-
Tumor necrosis factor-α
- IL-6 :
-
Interleukin 6
- IL-1β :
-
Interleukin 1β
- IL-8 :
-
Interleukin 8
- IL-10 :
-
Interleukin 10
- TGF-β1 :
-
Transforming growth factor-β1
- TGF-β2 :
-
Transforming growth factor-β2
- MDA :
-
Malondialdehyde
- SOD :
-
Superoxide dismutase
- CAT :
-
Catalase
- GSH :
-
Glutathione
- GSH-Px :
-
Glutathione peroxidase
- SOD1 :
-
Superoxide dismutase 1
- SOD2 :
-
Superoxide dismutase 2
- GST :
-
Glutathione S-transferase
- HO-1 :
-
Heme oxygenase 1
- GCLC :
-
Glutamate-cysteine ligase catalytic subunit
- GCLM :
-
Glutamate-cysteine ligase modifier subunit
- NQO1 :
-
NAD(P)H quinone dehydrogenase 1
- Th1/Th2 :
-
T helper cell 1/T helper cell 2
- TEM :
-
Transmission electron microscopy
- GAPDH :
-
Glyceraldehyde-3-phosphate dehydrogenase
- PMSF :
-
Phenylmethyl sulfonyl fluoride
- IP :
-
Immunoprecipitation
- SDS-PAGE :
-
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- PVDF :
-
Polyvinylidene fluoride
- SD :
-
Standard deviation
- IKKα :
-
Inhibitor of kappa B kinase α
- AvBDS :
-
Avian beta-defensins
- ZO-1 :
-
Zonula occludens-1
References
Alessandra F, Vittoria S, Maria D, Brunella P, Giovanna D (2021) Antimicrobial peptides human beta-defensin-2 and -3 protect the gut during candida albicans infections enhancing the intestinal barrier integrity. In Vitro Study Front Cell Infect Microbiol 11:666900
Berkowitz L, Pardo-Roa C, Salazar GA, Salazar-Echegarai F, Miranda JP, Ramírez G, Chávez JL, Kalergis AM, Bueno SM, Álvarez-Lobos M (2019) Mucosal exposure to cigarette components induces intestinal inflammation and alters antimicrobial response in mice. Front Immunol 10:2289
Cantor AH, Scott ML (1974) The effect of selenium in the hen’s diet on egg production, hatchability, performance of progeny and selenium concentration in eggs. Poult Sci 53(5):1870–1880
Chen D, Yao Y, Shi X, Li X, Cui W, Xu S (2022) Cadmium exposure causes mitochondrial fission and fusion disorder in the pig hypothalamus via the PI3K/AKT pathway. Ecotoxicol Environ Saf 242:113880
Chopyk DM, Grakoui A (2020) Contribution of the intestinal microbiome and gut barrier to hepatic disorders. Gastroenterology 159(3):849–863
Dong YW, Jiang WD, Liu Y, Wu P, Jiang J, Kuang SY, Tang L, Tang WN, Zhang YA, Zhou XQ, Feng L (2017) Threonine deficiency decreased intestinal immunity and aggravated inflammation associated with NF-κB and target of rapamycin signalling pathways in juvenile grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Br J Nutr 118(2):92–108
Echeverría C, Romero V, Arancibia R, Klahn H, Montorfano I, Armisen R, Borgna V, Simon F, Ramirez-Tagle R (2016) The characterization of anti-T. cruzi activity relationships between ferrocenyl, cyrhetrenyl complexes and ROS release. Biometals 29(4):743–749
Elbadawi M, Ammar RM, Aziz-Kalbhenn H, Rabini S, Klauck SM, Dawood M, Saeed MEM, Kampf CJ, Efferth T (2021) Anti-inflammatory and tight junction protective activity of the herbal preparation STW 5-II on mouse intestinal organoids. Phytomedicine 88:153589
Feng J, Wang L, Xie Y, Chen Y, Yi H, He D (2020) Effects of antimicrobial peptide cathelicidin-BF on diarrhea controlling, immune responses, intestinal inflammation and intestinal barrier function in piglets with postweaning diarrhea. Int Immunopharmacol 85:106658
Gao X, Xing H, Li S, Li J, Ying T, Xu S (2012) Selenium regulates gene expression of selenoprotein W in chicken gastrointestinal tract. Biol Trace Elem Res 145(2):181–188
Gao X, Zhang Z, Xing H, Yu J, Zhang N, Xu S (2016) Selenium deficiency-induced inflammation and increased expression of regulating inflammatory cytokines in the chicken gastrointestinal tract. Biol Trace Elem Res 173(1):210–218
Guo H, Lin W, Hou J, Wang L, Zhang D, Wu X, Li L, Li D (2018) The protective roles of dietary selenium yeast and tea polyphenols on growth performance and ammonia tolerance of juvenile wuchang bream (megalobrama amblycephala). Front Physiol 9:1371
Gao X, Tang B, Liang H, Yi L, Wei Z (2019) Selenium deficiency induced an inflammatory response by the HSP60 - TLR2-MAPKs signalling pathway in the liver of carp. Fish Shellfish Immunol 87:688–694
Hasegawa T, Mizugaki A, Inoue Y, Kato H, Murakami H (2021) Cystine reduces tight junction permeability and intestinal inflammation induced by oxidative stress in Caco-2 cells. Amino Acids 53(7):1021–1032
He X, Lin Y, Lian S, Sun D, Guo D, Wang J, Wu R (2020) Selenium deficiency in chickens induces intestinal mucosal injury by affecting the mucosa morphology SIgA secretion, and GSH-Px activity. Biol Trace Elem Res 197(2):660–666
He Y, Li Z, Xu T, Luo D, Chi Q, Zhang Y, Li S (2022) Polystyrene nanoplastics deteriorate LPS-modulated duodenal permeability and inflammation in mice via ROS drived-NF-κB/NLRP3 pathway. Chemosphere 307(Pt 1):135662
Hu L, Geng S, Li Y, Cheng S, Fu X, Yue X, Han X (2017) Exogenous fecal microbiota transplantation from local adult pigs to crossbred newborn piglets. Front Microbiol 8:2663
Jia Y, Zhang L, Liu X, Zhang S, Dai J, Huang J, Chen J, Wang Y, Zhou J, Zeng Z (2021) Selenium can regulate the differentiation and immune function of human dendritic cells. Biometals 34(6):1365–1379
Kohan AB, Yang Q, Xu M, Lee D, Tso P (2016) Monosodium glutamate inhibits the lymphatic transport of lipids in the rat. Am J Physiol Gastrointest Liver Physiol 311(4):G648–G654
Lan H, Zhang LY, He W, Li WY, Zeng Z, Qian B, Wang C, Song JL (2021) Sinapic acid alleviated inflammation-induced intestinal epithelial barrier dysfunction in lipopolysaccharide- (LPS-) treated Caco-2 cells. Mediators Inflamm 2021:5514075
Li S, Gao F, Huang J, Wu Y, Wu S, Lei XG (2018) Regulation and function of avian selenogenome. Biochim Biophys Acta Gen Subj 1862(11):2473–2479
Lijun S, Haitao Y, Hongbin L, Meixia C, Xiangfang Z, Shiyan Q (2021) Recombinant antimicrobial peptide microcin J25 alleviates DSS-induced colitis via regulating intestinal barrier function and modifying gut microbiota. Biomed Pharmacother 139:111127
Miao Z, Miao Z, Teng X, Xu S (2022) Chlorpyrifos triggers epithelioma papulosum cyprini cell pyroptosis via miR-124-3p/CAPN1 axis. J Hazard Mater 424(Pt A):127318
Papp LV, Holmgren A, Khanna KK (2010) Selenium and selenoproteins in health and disease. Antioxid Redox Signal 12(7):793–795
Parveen A, Choi S, Kang JH, Oh SH, Kim SY (2020) Trifostigmanoside I, an active compound from sweet potato, restores the activity of MUC2 and protects the tight junctions through PKCα/β to maintain intestinal barrier function. Int J Mol Sci 22(1):291
Popa-Wagner A, Mitran S, Sivanesan S, Chang E, Buga AM (2013) ROS and brain diseases: the good, the bad, and the ugly. Oxid Med Cell Longev 2013:963520
Prabhakar O (2013) Cerebroprotective effect of resveratrol through antioxidant and anti-inflammatory effects in diabetic rats. Naunyn Schmiedebergs Arch Pharmacol 386(8):705–710
Qing Z, Dongliu L, Xuedie G, Khoso PA, Xiaodan H, Shu L (2022) MiR-144-3p targets STC1 to activate PI3K/AKT pathway to induce cell apoptosis and cell cycle arrest in selenium deficiency broilers. J Inorg Biochem 226:111665
Qiu Y, Yang J, Wang L, Yang X, Gao K, Zhu C, Jiang Z (2021) Dietary resveratrol attenuation of intestinal inflammation and oxidative damage is linked to the alteration of gut microbiota and butyrate in piglets challenged with deoxynivalenol. J Anim Sci Biotechnol 12(1):71
Rayman MP (2012) Selenium and human health. Lancet 379(9822):1256–1268
Rengaraj D, Truong AD, Lillehoj HS, Han JY, Hong YH (2018) Expression and regulation of avian beta-defensin 8 protein in immune tissues and cell lines of chickens. Asian-Australas J Anim Sci 31(9):1516–1524
Rexidamu M, Li H, Jin H, Huang J (2019) Serum levels of trimethylamine-N-oxide in patients with ischemic stroke. Biosci Rep. https://doi.org/10.1042/BSR20190515
Rocca C, Pasqua T, Boukhzar L, Anouar Y, Angelone T (2019) Progress in the emerging role of selenoproteins in cardiovascular disease: focus on endoplasmic reticulum-resident selenoproteins. Cell Mol Life Sci 76(20):3969–3985
Shimada BK, Alfulaij N, Seale LA (2021) The impact of selenium deficiency on cardiovascular function. Int J Mol Sci 22(19):10713
Steinbrenner H (2013) Interference of selenium and selenoproteins with the insulin-regulated carbohydrate and lipid metabolism. Free Radical Biol Med 65:1538–1547
Tang X, Fan X, Xu T, He Y, Chi Q, Li Z, Li S (2022) Polystyrene nanoplastics exacerbated lipopolysaccharide-induced necroptosis and inflammation via the ROS/MAPK pathway in mice spleen. Environ Toxicol 37(10):2552–2565
Tsikas D (2017) Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem 524:13–30
Verma K, Mehta SK, Shekhawat GS (2013) Nitric oxide (NO) counteracts cadmium induced cytotoxic processes mediated by reactive oxygen species (ROS) in Brassica juncea: cross-talk between ROS. NO and Antioxid Responses Biometals 26(2):255–269
Vornhagen J, Bassis CM, Ramakrishnan S, Hein R, Mason S, Bergman Y, Sunshine N, Fan Y, Holmes CL, Timp W, Schatz MC, Young VB, Simner PJ, Bachman MA (2021) A plasmid locus associated with klebsiella clinical infections encodes a microbiome-dependent gut fitness factor. PLoS Pathog 17(4):e1009537
Xu S, Xiaojing L, Xinyue S, Wei C, Honggui L, Shiwen X (2021) Pig lung fibrosis is active in the subacute CdCl(2) exposure model and exerts cumulative toxicity through the M1/M2 imbalance. Ecotoxicol Environ Saf 225:112757
Yan L, Lv ZZ, An S, Xing K, Wang ZG, Lv MB, Choct M, Guo YM, Zhou GL (2021) Effects of rearing system and narasin on growth performance, gastrointestinal development, and gut microbiota of broilers. Poult Sci 100(3):100840
Yang J, Zhang Y, Hamid S, Cai J, Liu Q, Li H, Zhao R, Wang H, Xu S, Zhang Z (2017) Interplay between autophagy and apoptosis in selenium deficient cardiomyocytes in chicken. J Inorg Biochem 170:17–25
Yu J, Yao H, Gao X, Zhang Z, Wang JF, Xu SW (2015) The role of nitric oxide and oxidative stress in intestinal damage induced by selenium deficiency in chickens. Biol Trace Elem Res 163(1–2):144–153
Yu H, Shang L, Zeng X, Li N, Liu H, Cai S, Huang S, Wang G, Wang Y, Song Q (2018) Risks related to high-dosage recombinant antimicrobial peptide microcin J25 in mice model: intestinal microbiota, intestinal barrier function and immune regulation. J Agric Food Chem 66(43):11301–11310
Zhang JL, Xu B, Huang XD, Gao YH, Chen Y, Shan AS (2016) Selenium deficiency affects the mRNA expression of inflammatory factors and selenoprotein genes in the kidneys of broiler chicks. Biol Trace Elem Res 171(1):201–207
Zhang Y, Qi X, Chen X, Zhang J, Zhang W, Lin H (2021a) Dietary selenomethionine ameliorates lipopolysaccharide-induced renal inflammatory injury in broilers via regulating the PI3K/AKT pathway to inhibit necroptosis. Food Funct 12(10):4392–4401
Zhang Y, Zhang J, Bao J, Tang C, Zhang Z (2021b) Selenium deficiency induced necroptosis, Th1/Th2 imbalance, and inflammatory responses in swine ileum. J Cell Physiol 236(1):222–234
Zheng Y, Zhang B, Guan H, Jiao X, Yang J, Cai J, Liu Q, Zhang Z (2021a) Selenium deficiency causes apoptosis through endoplasmic reticulum stress in swine small intestine. BioFactors 47(5):788–800
Zheng Y, Guan H, Yang J, Cai J, Liu Q, Zhang Z (2021b) Calcium overload and reactive oxygen species accumulation induced by selenium deficiency promote autophagy in swine small intestine. Anim Nutr 7(4):997–1008
Acknowledgements
The authors thank the Key Laboratory of the Provincial Education Department of Heilongjiang for Common Animal Disease Prevention and Treatment, College of Veterinary Medicine, Northeast Agricultural University for providing conditions.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 32072811, 31872437).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. HY, TX: Conceptualization; Methodology Investigation; Formal analysis; Data curation; Writing-original draft. FX: Software; Investigation. LZ, PL: Investigation; Validation. SG, LS: Resources; Project administration; Writing-review & editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
All procedures used in this study were approved by the Institutional Animal Care and Use Committee (SRM-11) of Northeast Agricultural University.
Consent for publication
All authors have read the manuscript and have agreed to submit the manuscript in its current form for consideration for publication in this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yujiao, H., Xinyu, T., Xue, F. et al. Selenium deficiency increased duodenal permeability and decreased expression of antimicrobial peptides by activating ROS/NF-κB signal pathway in chickens. Biometals 36, 137–152 (2023). https://doi.org/10.1007/s10534-022-00468-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-022-00468-4