Abstract
Selenium (Se) is an essential trace element for humans and animals and is associated with many physiological functions. Previous studies have shown that low-Se diet may affect inflammatory cytokine productions and histology in the digestive system and that sulfide hydrogen (H2S) may contribute to the protection against tissue injury and the inhibition of inflammation in the gastrointestinal tract. In this study, we investigated the relationship between Se deficiency-induced inflammation and H2S production in the small intestine in chickens. One hundred twenty 1-day-old chickens were fed with diets with different Se concentrations (0.15 mg/kg in the control and 0.028 mg/kg in the low-Se-diet group). Chickens were euthanized and small intestinal tissues were extracted. We observed histology, measured H2S concentration, and evaluated the mRNA expression of H2S-producing enzymes cystathionine γ-lyase (CSE), cystathionine β-synthase (CBS), and 3-mercaptopyruvate sulfurtransferase (3-MST), and inflammatory factors TNF-α, NF-κB p50, COX-2, and PTGES. Our results showed that chickens fed with low-Se diet exhibited histological changes, lower H2S production, and lower mRNA expression of H2S-producing enzymes (CSE, CBS, and 3-MST) whereas higher mRNA expression of intestinal inflammatory factors (TNF-α, NF-κB p50, COX-2, and PTGES) compared to controls. Our results indicate that low-Se diet could impact H2S, H2S-producing enzymes, and inflammatory factors in the small intestine, implying that Se is important in maintaining intestinal functions and H2S production is downregulated in Se deficiency-induced inflammation. The downregulation exacerbates the inflammation and impacts H2S-mediated intestinal functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an essential trace element and is associated with various biological activities in both humans and animals. Se was first regarded as a toxic substance and later was recognized as a necessary micronutrient with anti-oxidant, immune-regulation, and anti-inflammatory effects [1, 2]. Se plays an important role in the major metabolic pathways and the immune functions [3–5]. Nutritional deficiency of Se could cause high blood pressure or even hypertension [6], pancreatic atrophy [7, 8], cardiovascular disease [3], and cancer [9]. For instance, muscle diseases were found to be associated with low-Se diet in calves, lambs, and chickens and can be prevented by Se supplementation [10–12]. Keshan disease, prevalent in a wide belt extending from northeast to southwest China, was found to be associated with Se-deficient soil, and Se supplementation has been adopted to reduce the affliction [13].
The gastrointestinal tract is responsible for transporting and digesting food, and absorbing nutrients. Mucosal immune responses are subtle and many factors may cause digestive disorders including bacterial or viral infection, alteration of intestinal flora, and immune dysfunctions. Dietary Se is absorbed mainly in the small intestine, which is also believed to be the target organ of Se deficiency. In chickens, Se deficiency-induced significantly higher inflammatory damage and methane dicarboxylic aldehyde (MDA) levels, accompanied with significantly higher levels of inducible nitric oxide synthase (iNOS) and nitric oxide (NO) but significantly lower levels of glutathione (GSH) and glutathione peroxidase (GSH-Px) in the small intestinal tissues [14]. In chickens, Se deficiency may cause functional disorders or even histological changes and may induce inflammatory factor production in the gastrointestinal tract including the small intestine [15, 16].
On the other hand, the use of Se supplementation is considered to improve host immune responses because of its anti-oxidant, anti-inflammatory functions. It was found that Se-enriched diet could increase intestinal motility in Trypanosoma cruzi (T. cruzi) infected mice and that Se could be used to modulate inflammatory responses involved in intestinal disturbances caused by T. cruzi infection [17].
Hydrogen sulfide (H2S) is an endogenous gaseous transmitter and is considered to be the third endogenously produced gaseous signaling molecule [18]. H2S has been involved in multiple regulations of physiologic and pathologic functions. H2S plays an important role in the cardiovascular, central nervous, and gastrointestinal systems. H2S is generated from L-cysteine, cysteine, and homocysteine by three enzymes: cystathionine γ-lyase (CSE), cystathionine β-synthase (CBS), and 3-mercaptopyruvate sulfurtransferase (3-MST) [19, 20]. There have been multiple studies on the effects of H2S in the gastrointestinal tract [21], including the stimulation of intestinal secretion [22], the reduction in colorectal distension-induced visceral pain [23], and the relaxation of ileal smooth muscle [24]. In addition, studies have demonstrated that H2S exhibits anti-inflammatory actions, including the inhibition of leukocyte endothelial adherence, the reduction in edema formation, and the enhancement of gastric ulcer healing [25]. It was also found that H2S could attenuate the gastric injury caused by anti-inflammatory non-steroidal drugs [20].
The relationship between Se and the gastrointestinal tract has been studied in two directions. In one direction, low absorption caused by gastrointestinal damage could lead to Se deficiency. In the other direction, low-Se diet could impact gastrointestinal inflammatory cytokine productions and histology. However, more studies are still in need on Se deficiency-induced inflammation as well as the impacts of Se on gastrointestinal functions. The current study focused on H2S production considering the following two observations: (1) H2S is closely involved in many gastrointestinal functions; (2) endogenous H2S production could improve inflammation in the gastrointestinal tract and the inhibition of H2S production will exacerbate the inflammation [26]. By studying H2S production, we intended to have more understanding of Se deficiency-induced inflammation, as well as the impact of low-Se diet on H2S-mediated gastrointestinal functions.
Materials and Methods
Animal and Experimental Design
Animal use protocols were approved by the Institutional Animal Care and Use Committee at Nanjing Agricultural University in accordance with the Guidelines for Experimental Animals of the Ministry of Science and Technology (2006, Beijing, China).
One hundred twenty 1-day-old chickens were obtained from Bada Livestock and Poultry Co., Ltd. Jiangsu (Changzhou, Jiangsu, China), and were randomly divided into 2 groups with 60 in each group. These chickens were fed either with commercial granulated diet containing 0.15 mg Se/kg (control group), or the Se-deficient diet containing 0.028 mg Se/kg (low-Se-diet group). Food and water were provided with free access. In each group, at days 10, 20, and 30, respectively, 15 chickens were randomly selected from the remaining live chickens and then were euthanized. In this experiment, we raised more chickens than chickens euthanized (120 chickens were raised and 90 chickens were euthanized) to ensure there were enough chickens even when some non-euthanized chickens were dead. Small intestines were harvested and frozen immediately in liquid nitrogen and then stored at −80 °C.
Histological Analysis
Small intestinal tissues were collected when chickens were sacrificed at day 30. Tissues were fixed in 10 % neutral buffered formalin, embedded in paraffin, deparaffinized with xylene, and rehydrated with graded alcohol. The sections were stained with hematoxylin and eosin (H&E) and visualized with a microscope (Olympus, Japan).
Measurement of H2S Production in Small Intestinal Tissue
The procedure was described in a previous study [27]. Briefly, small intestinal tissues were homogenized into 10 % (w/v) homogenates in 4 °C 50 mM potassium phosphate buffer (pH 6.8). Reactions were performed in 5-mL Erlenmeyer flasks and the following were added: 0.5 ml of 1 % zinc acetate, 2.5 ml of distilled water, 0.1 ml of homogenates in potassium phosphate buffer, 0.5 ml of 20 mM N,N-dimethyl-p-phenylenediamine sulfate salt in 7.2 M hydrochloric acid (HCl), and 0.4 ml of 30 mM FeCl3 in 1.2 M HCl, incubated 20 min at room temperature. After incubation, 1 ml of 10 % trichloroacetic acid was added with distilled water to total volume 5 ml. Centrifuged 4000g for 5 min, supernatant was collected and measured by a spectrometer (Shimadzu UV 2100, Japan) at 670 nm. Standard curve was plotted based on NaHS at different concentrations and the linear regression function was y = 0.00020571 x + 0.00138095 with R 2 = 0.98962774. The H2S concentration in homogenates was determined by this standard curve. The result was expressed in micromoles per liter.
Quantitative Real-Time Polymerase Chain Reaction
Small intestinal samples were harvested and stored at −80 °C at the time of sacrifice. Total RNA was extracted using TRIzol reagent (Invitrogen, Shanghai, China) according to the manufacturer’s instructions. The RNA pellet was dissolved in diethyl-pyrocarbonate-treated water, and the concentration and purity of the total RNA were determined by spectrophotometrically at 260/280 nm. First-strand cDNA was synthesized from 10 μg of total RNA using Oligo dT primers, M-MLV reverse transcriptase, and RNase inhibitor (TaKaRa, Dalian, Liaoning, China) according to the manufacturer’s protocol.
PCR primers were designed using Primer Premier 5.0 (PREMIER Biosoft International, USA) and synthesized by Invitrogen on the basis of known chicken sequences (Table 1). General PCRs were first performed to confirm the specificity of the primers. The PCR products were electrophoresed on 2 % agarose gels, extracted, cloned into the pMD18-T vector (TaKaRa, China), and sequenced. Quantitative RT-PCR reactions were carried out using the ABI Prism Step One Plus detection system (Applied Biosystems, Foster City, CA, USA). Reactions were performed in a 20-μl reaction mixture containing 10 μl of 2 × SYBR Green I PCR Master Mix (TaKaRa), 2 μl of cDNA, 1 μl of each primer (10 μM), and 6 μl of PCR-grade water. The PCR procedure consisted of a 95 °C step for 30 s followed by 40 cycles consisting of 95 °C for 15 s and 60 °C for 30 s. A dissociation curve was run for each plate to confirm the production of a single product. GAPDH was used as an internal control. Results (fold changes) were determined using the 2−ΔΔ Ct method.
Data Analysis
All statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad, San Diego, CA, USA). Our study has two groups (control group and low-Se-diet group) and three time points (days 10, 20, and 30). Because we are interested both in group effect and time effect, and want to test jointly for group effect (comparison between groups) and time effect (comparison across time), we used two-way ANOVA with the Bonferroni multiple-test adjustment. Results were presented as the mean ± standard deviation (S.D.). P values less than 0.05 were considered to be statistically significant.
Results
Histological Changes
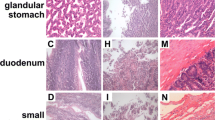
To evaluate the effect of Se on the intestinal tract, we fed 1-day-old chickens with low-Se diet (low-Se-diet group) or normal diet (control group). Small intestinal tissues were collected when chickens were sacrificed at day 30. As shown in Fig. 1, there was no pathological changes observed in the control group (Fig. 1a), whereas in the low-Se-diet group, we observed that inflammatory cells were involved in submucosal, that some epithelial cells were destroyed, that local mucosa showed edema and hyperemia, and that there was architectural distortion (Fig. 1b). These results indicated low-Se diet may cause inflammatory lesion in the small intestine.
Effect of Se on H2S Concentration
It has been reported that H2S could protect the integrity of gastrointestinal mucosa. To evaluate whether Se may impact the H2S production in the small intestine, small intestinal tissues were harvested when chickens were euthanized at days 10, 20, and 30. H2S concentration was evaluated. As shown in Fig. 2, we found that there were no significant changes in H2S concentration over time (days 10, 20, and 30) in the control group whereas H2S concentration decreased with time (days 10, 20, and 30) in the low-Se-diet group. Compared with the control group, the low-Se-diet group showed a significantly higher H2S concentration at day 10 and a significantly lower H2S concentration at day 20 and 30. The largest difference between the control group and low-Se-diet group was observed at day 30. These results indicated low-Se diet may decrease H2S concentration over the 30-day period, which could impact many H2S-mediated intestinal functions.
Effect of Se on H2S production in small intestinal tissues. H2S concentration in small intestinal tissues harvested from chickens fed with low-Se diet and normal diet at days 10, 20, and 30 were determined. Data were presented as the mean ± standard deviation (S.D.), n = 15. An asterisk symbol indicates statistically significant difference in each time point between the low-Se-diet group and control group (p < 0.05). A number sign indicates statistically significant difference in the low-se-diet group at day 20 or 30 compared to that in the low-se-diet group at day 10 (p < 0.05)
Effect of Se on mRNA Expression of H2S-Producing Enzymes
We next investigated whether low-Se diet reduced the expression of H2S-producing enzymes. CSE, CBS, and 3-MST have been found to be closely associated with H2S production, and they are crucial in influencing gastrointestinal functions especially via H2S production. The H2S-producing enzymes CSE, CBS, and 3-MST mRNA expression in small intestinal tissues from the low-Se-diet group and control group were measured by quantitative real-time polymerase chain reaction (qPCR).
As shown in Fig. 3, the mRNA expression of CSE and CBS in the low-Se-diet group was significantly higher than that in the control group at day 10, whereas significantly lower than that in the control group at days 20 and 30. The mRNA expression of 3-MST also shows the similar pattern in that significantly higher at days 20 and 30, though failed to show significant difference from that in the control group at day 10. Over time, the mRNA expression of CSE, CBS, and 3-MST in the control group did not show significant changes, whereas there were significant changes and patterns on the dynamics of the expression in the low-Se-diet group over time. All the expression of CSE, CBS, and 3-MST in the low-Se-diet group decreased with time. Moreover, H2S concentration (Fig. 2) and CSE and CBS mRNA expression (Fig. 3) showed similar patterns in the dynamics.
Effect of Se on mRNA expression of CSE, CBS and 3-MST in small intestinal tissues. a CSE, b CBS, and c 3-MST mRNA expression in small intestinal tissues of low-Se-diet and normal-diet chickens at days 10, 20, and 30 were measured by qPCR. Copy number was normalized to GAPDH and was expressed as fold change relative to normal-diet controls at day 10. Data were presented as the mean ± standard deviation (S.D.), n = 15. An asterisk symbol indicates statistically significant difference in each time point between the low-Se-diet group and control group (p < 0.05). A number sign indicates statistically significant difference in the low-se-diet group at day 20 or 30 compared to that in the low-se-diet group at day 10 (p < 0.05)
These results indicated that low-Se diet may reduce the mRNA expression of H2S-producing enzymes CSE, CBS, and 3-MST. They also indicated that the change of CSE is very similar to the change of H2S concentration in terms of both between-group difference and across-time dynamics. Thus, Se could impact both H2S concentration and H2S-producing enzymes CSE, CBS, and 3-MST and are very closely associated to intestinal functions.
Effect of Se on mRNA Expression of Crucial Inflammatory Factors
We next measured the mRNA expression of inflammatory factors. Crucial inflammatory factors considered were TNF-α, NF-κB p50, COX-2, and PTGES, playing important roles in gastrointestinal functions, especially gastrointestinal immune responses. As shown in Fig. 4, the mRNA expression of all the four inflammatory factors TNF-α, NF-κB p50, COX-2, and PTGES in the low-Se-diet group was significantly higher than that in the control group at all the three time points (days 10, 20, and 30). Regarding across-time difference, the mRNA expression of all the 4 inflammatory factors TNF-α, NF-κB p50, COX-2, and PTGES in the low-Se-diet group increased with time whereas the mRNA expression of these cytokines in the control group did not show significant difference over time.
Effect of Se on mRNA expression of TNF-α, NF-κB p50, COX-2, and PTGES in small intestinal tissues. a TNF-α, b NF-κB p50, c COX-2, and d PTGES mRNA expression in small intestinal tissues of low-Se-diet and normal-diet chickens at days 10, 20, and 30 were measured by qPCR. Copy number was normalized to GAPDH and was expressed as fold change relative to normal-diet controls at day 10. Data were presented as the mean ± standard deviation (S.D.), n = 15. An asterisk symbol indicates statistically significant difference in each time point between the low-Se-diet group and control group (p < 0.05). A number sign indicates statistically significant difference in the low-se-diet group at day 20 or 30 compared to that in the low-se-diet group at day 10 (p < 0.05)
These results indicated that low-Se diet may increase the mRNA expression of inflammatory factors TNF-α, NF-κB p50, COX-2, and PTGES, implying the upregulation of these inflammatory factors and Se deficiency-induced inflammation.
Discussion
Se plays an important role in a variety of physiological processes in both humans and animals. Se deficiency could induce intestinal disorders and inflammation. H2S exhibits anti-inflammatory functions and has many effects on the digestive system. In this study, we found that Se deficiency could cause inflammation, decrease H2S concentration and the mRNA expression of H2S-producing enzymes and increase the mRNA expression of inflammatory factors in the small intestinal tissue, which implied that H2S is downregulated in the Se deficiency-induced inflammation and that Se could impact many H2S-mediated intestinal functions.
In the gastrointestinal tract, H2S is involved in many physiological and pathophysiological processes and could protect gastric mucosal integrity [28]. The inhibition of H2S synthesis in colon with inflammation exacerbated colitis and the inhibition of H2S synthesis in healthy rats caused small intestinal inflammation and mucosal injury [26]. On the other hand, exogenous H2S donors could increase the resistance of the mucosa to injury [20, 29, 30]. H2S-producing enzymes CBS and CSE are widely expressed in the gastrointestinal tract [21]. More than 70–95 % of colonic H2S synthesis has been inhibited if the colonic tissue was co-incubated with inhibitors of CSE and CBS [26]. It was reported that mice completely deficient in CBS could not survive because of the H2S-altered leukocyte interactions with the endothelium [31]. A study was conducted by treating rats with a number of inhibitors of H2S synthesis, and results indicated that H2S produced in the context of colitis could exert a predominantly beneficial effect, i.e., inhibition of its synthesis could lead to exacerbated colitis [26]. In the present study, we observed reduced H2S concentration as well as reduced mRNA expression of H2S-producing enzymes CSE, CBS, and 3-MST in the small intestinal tissues from the low-Se-diet group. The downregulation of H2S and H2S-producing enzymes could impact many gastrointestinal functions as above discussed. To our best knowledge, there is no other study on the impact of low-Se diet on H2S and H2S-producing enzymes though this issue is very important.
Pro-inflammatory cytokines were increased in the inflammatory response [32]. Se deficiency was found to slow down the growth of immune organs and decrease immune functions, which causes many inflammatory diseases [33]. In the acute phase of immune responses, TNF-α is produced mainly by activated macrophages and is involved in systematic inflammation. TNF-α secretion is important in maintaining immune homeostasis. However, over-expression of TNF-α could cause severe inflammation. NF-κB plays a crucial role in inflammatory responses by the regulation of genes encoding pro-inflammatory cytokines including the expression of TNF-α [34]. Our results are consistent with Gao et al.’s (2016) observation in that low-Se diet increased mRNA expression of TNF-α and NF-κB, implying Se deficiency-induced inflammation in the small intestine [15].
H2S is closely associated with gastrointestinal functions and has anti-inflammatory functions. The inhibition of NF-κB by H2S could downregulate many pro-inflammatory cytokine productions. For example, the H2S donor GYY4137 inhibited TNF-α production in an endotoxic shock model [35, 36]. Allyl disulfide significantly inhibited NF-κB activation and production of TNF-α in patients with ulcerative colitis [37]. H2S-releasing drugs significantly reduced the expression of TNF-α and IFN-γ in rodent models of colitis [26, 38]. Researchers have found that both endogenous and exogenous H2S promotes resolution of colitis while the inhibition of H2S production could exacerbate colitis [26]. In the current study, we are the first to observe the downregulation of H2S and H2S-producing enzymes in Se deficiency-induced inflammation in chickens. The downregulation of H2S could exacerbate the inflammation and impact many intestinal functions. Our observation of up-regulated NF-κB and TNF-α, together with the down-regulated H2S and H2S-producing enzymes, is consistent with the studies discussed above on their interactions [26, 35, 36, 38]. All indicate that H2S production is closely associated with gastrointestinal inflammation and is believed as a crucial mediator.
There are also many studies on the interaction of H2S and inflammatory factors COX-2 and PTGES, as well as their intestinal functions. It was found that H2S could reduce COX-2 expression in the circumstance of inflammation, partially due to the inhibition of NF-κB [39, 40]. COX-2 could catalyze the production of PG with PTGES in an inflammation response [41]. Increased expression of COX-2 was found to facilitate the inflammation in the gastrointestinal tract [42]. In the inflammation of gastrointestinal tract, both COX-2 and PTGES were increased [43]. In the current study, we found an increase in COX-2 and PTGES mRNA expression in mice fed with low-Se diet, implying Se deficiency-induced inflammation. We also found a downregulation of H2S and H2S-producing enzymes in mice fed with low-Se diet, implying inflammation is exacerbated and many H2S-mediated intestinal functions are impacted in Se deficiency-induced inflammation.
In summary, Se is important in regulating H2S and inflammation in the digestive system. Low-Se diet could reduce H2S concentration, down-regulate H2S-producing enzymes CSE, CBS and 3-MST, and up-regulate inflammatory factors TNF-α, NF-κB, COX-2 and PTGES. This H2S down-regulation could exacerbate Se deficiency-induced inflammation and impact many H2S-mediated intestinal functions. There are interactions between Se, H2S, H2S-producing enzymes, and inflammatory factors in the gastrointestinal tract. Further studies will be conducted for more understanding of the impact of Se on the gastrointestinal tract.
References
Yao HD, Wu Q, Zhang ZW, Li S, Wang XL, Lei XG, Xu SW (2013) Selenoprotein W serves as an antioxidant in chicken myoblasts. Bba-Gen Subj 1830:3112–3120. doi:10.1016/j.bbagen.2013.01.007
Gan F, Chen XX, Liao SFF, Lv CH, Ren F, Ye GP, Pan CL, Huang D, Shi J, Shi XL, Zhou H, Huang KH (2014) Selenium-enriched probiotics improve antioxidant status, immune function, and selenoprotein gene expression of piglets raised under high ambient temperature. J Agr Food Chem 62:4502–4508. doi:10.1021/jf501065d
Brown KM, Arthur JR (2001) Selenium, selenoproteins and human health: a review. Public Health Nutr 4:593–599
Stadtman TC (1991) Biosynthesis and function of selenocysteine-containing enzymes. J Biol Chem 266:16257–16260
Chen XX, Ren F, Hesketh J, Shi XL, Li JX, Gan F, Huang KH (2012) Selenium blocks porcine circovirus type 2 replication promotion induced by oxidative stress by improving GPx1 expression. Free Radic Biol Med 53:395–405. doi:10.1016/j.freeradbiomed.2012.04.035
Nawrot TS, Staessen JA, Roels HA, Den Hond E, Thijs L, Fagard RH, Dominiczak AF, Struijker-Boudier HA (2007) Blood pressure and blood selenium: a cross-sectional and longitudinal population study. Eur Heart J 28:628–633. doi:10.1093/eurheartj/ehl479
Thompson JN, Scott ML (1970) Impaired lipid and vitamin E absorption related to atrophy of the pancreas in selenium-deficient chicks. J Nutr 100:797–809
Whitacre ME, Combs GF Jr, Combs SB, Parker RS (1987) Influence of dietary vitamin E on nutritional pancreatic atrophy in selenium-deficient chicks. J Nutr 117:460–467
Clark LC, Combs GF Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Krongrad A, Lesher JL Jr, Park HK, Sanders BB Jr, Smith CL, Taylor JR (1996) Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA 276:1957–1963
Westermarck HW (1987) Selenium in long-term feeding and the frequency of white muscle disease in cattle in Finland during the years 1978-1985. J Agr Sci Finland 59:47–50
Yao HD, Zhao WC, Zhao X, Fan RF, Khoso PA, Zhang ZW, Liu W, Xu SW (2014) Selenium deficiency mainly influences the gene expressions of antioxidative selenoproteins in chicken muscles. Biol Trace Elem Res 161:318–327. doi:10.1007/s12011-014-0125-2
Zhang ZW, Zhang JL, Zhang YH, Wang QH, Li S, Wang XL, Xu SW (2013) Effect of oxygen free radicals and nitric oxide on apoptosis of immune organ induced by selenium deficiency in chickens. Biometals 26:355–365. doi:10.1007/s10534-013-9612-8
Chen JS (2012) An original discovery: selenium deficiency and Keshan disease (an endemic heart disease). Asia Pac J Clin Nutr 21:320–326
Yu J, Yao H, Gao X, Zhang Z, Wang JF, Xu SW (2015) The role of nitric oxide and oxidative stress in intestinal damage induced by selenium deficiency in chickens. Biol Trace Elem Res 163:144–153. doi:10.1007/s12011-014-0164-8
Gao XJ, Zhang ZW, Xing HJ, Yu J, Zhang NS, Xu SW (2016) Selenium deficiency-induced inflammation and increased expression of regulating inflammatory cytokines in the chicken gastrointestinal tract. Biol Trace Elem Res. doi:10.1007/s12011-016-0651-1
Gao XJ, Xing HJ, Li S, Li JL, Ying T, Xu SW (2012) Selenium regulates gene expression of selenoprotein W in chicken gastrointestinal tract. Biol Trace Elem Res 145:181–188. doi:10.1007/s12011-011-9175-x
de Souza AP, Sieberg R, Li H, Cahill HR, Zhao D, Araujo-Jorge TC, Tanowitz HB, Jelicks LA (2010) The role of selenium in intestinal motility and morphology in a murine model of Typanosoma cruzi infection. Parasitol Res 106:1293–1298. doi:10.1007/s00436-010-1794-1
Wang R (2002) Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J: Off Publ Fed Am Soc Exp Biol 16:1792–1798. doi:10.1096/fj.02-0211hyp
Szabo C (2007) Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 6:917–935
Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi S, Zanardo R, Renga B, Di Sante M, Morelli A, Cirino G, Wallace JL (2005) Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology 129:1210–1224. doi:10.1053/j.gastro.2005.07.060
Fiorucci S, Distrutti E, Cirino G, Wallace JL (2006) The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology 131:259–271. doi:10.1053/j.gastro.2006.02.033
Schicho R, Krueger D, Zeller F, Von Weyhern CW, Frieling T, Kimura H, Ishii I, De Giorgio R, Campi B, Schemann M (2006) Hydrogen sulfide is a novel prosecretory neuromodulator in the Guinea-pig and human colon. Gastroenterology 131:1542–1552. doi:10.1053/j.gastro.2006.08.035
Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Antonelli E, Roviezzo F, Morelli A, Cirino G, Wallace JL, Fiorucci S (2006) Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J Pharmacol Exp Ther 316:325–335. doi:10.1124/jpet.105.091595
Teague B, Asiedu S, Moore PK (2002) The smooth muscle relaxant effect of hydrogen sulphide in vitro: evidence for a physiological role to control intestinal contractility. Br J Pharmacol 137:139–145. doi:10.1038/sj.bjp.0704858
Wallace JL, Dicay M, McKnight W, Martin GR (2007) Hydrogen sulfide enhances ulcer healing in rats. FASEB J: Off Publ Fed Am Soc Exp Biol 21:4070–4076. doi:10.1096/fj.07-8669com
Wallace JL, Vong L, McKnight W, Dicay M, Martin GR (2009) Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology 137:569–578 . doi:10.1053/j.gastro.2009.04.012578.e561
Zhang CY, Du JB, Bu DF, Yan H, Tang XY, Tang CS (2003) The regulatory effect of hydrogen sulfide on hypoxic pulmonary hypertension in rats. Biochem Biophys Res Commun 302:810–816. doi:10.1016/S0006-291x(03)00256-0
Wallace JL (2012) Hydrogen sulfide: a rescue molecule for mucosal defence and repair. Dig Dis Sci 57:1432–1434. doi:10.1007/s10620-012-2119-2
Wallace JL (2010) Physiological and pathophysiological roles of hydrogen sulfide in the gastrointestinal tract. Antioxid Redox Signal 12:1125–1133. doi:10.1089/ars.2009.2900
Wallace JL, Caliendo G, Santagada V, Cirino G (2010) Markedly reduced toxicity of a hydrogen sulphide-releasing derivative of naproxen (ATB-346). Br J Pharmacol 159:1236–1246. doi:10.1111/j.1476-5381.2009.00611.x
Kamath AF, Chauhan AK, Kisucka J, Dole VS, Loscalzo J, Handy DE, Wagner DD (2006) Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood 107:591–593. doi:10.1182/blood-2005-06-2506
Miller LS, Pietras EM, Uricchio LH, Hirano K, Rao S, Lin HP, O’Connell RM, Iwakura Y, Cheung AL, Cheng GH, Modlin RL (2007) Inflammasome-mediated production of IL-1 beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J Immunol 179:6933–6942
Zhang W, Zhang RX, Wang TC, Jiang HC, Guo MY, Zhou ES, Sun Y, Yang ZT, Xu SW, Cao YG, Zhang NS (2014) Selenium inhibits LPS-induced pro-inflammatory gene expression by modulating MAPK and NF-kappa B signaling pathways in mouse mammary epithelial cells in primary culture. Inflammation 37:478–485. doi:10.1007/s10753-013-9761-5
Liang Y, Zhou Y, Shen PP (2004) NF-kappa B and its regulation on the immune system. Cell Mol Immunol 1:343–350
Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK (2008) Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation 117:2351–2360. doi:10.1161/Circulationaha.107.753467
Whiteman M, Haigh R, Tarr JM, Gooding KM, Shore AC, Winyard PG (2010) Detection of hydrogen sulfide in plasma and knee-joint synovial fluid from rheumatoid arthritis patients: relation to clinical and laboratory measures of inflammation. Ann N Y Acad Sci 1203:146–150. doi:10.1111/j.1749-6632.2010.05556.x
Bai A-P, Ouyang Q, Hu R-W (2005) Diallyl trisulfide inhibits tumor necrosis factor-α expression in inflammed mucosa of ulcerative colitis. Dig Dis Sci 50:1426–1431. doi:10.1007/s10620-005-2857-5
Fiorucci S, Orlandi S, Mencarelli A, Caliendo G, Santagada V, Distrutti E, Santucci L, Cirino G, Wallace JL (2007) Enhanced activity of a hydrogen sulphide-releasing derivative of mesalamine (ATB-429) in a mouse model of colitis. Br J Pharmacol 150:996–1002. doi:10.1038/sj.bjp.0707193
Fan H, Guo Y, Liang X, Yuan Y, Qi X, Wang M, Ma J, Zhou H (2013) Hydrogen sulfide protects against amyloid beta-peptide induced neuronal injury via attenuating inflammatory responses in a rat model. J Biomed Res 27:296–304. doi:10.7555/JBR.27.20120100
Lee HJ, Lee HG, Choi KS, Surh YJ, Na HK (2013) Diallyl trisulfide suppresses dextran sodium sulfate-induced mouse colitis: NF-kappaB and STAT3 as potential targets. Biochem Biophys Res Commun 437:267–273. doi:10.1016/j.bbrc.2013.06.064
Lugo B, Ford HR, Grishin A (2007) Molecular signaling in necrotizing enterocolitis: regulation of intestinal COX-2 expression. J Pediatr Surg 42:1165–1171. doi:10.1016/j.jpedsurg.2007.02.006
Mifflin RC, Saada JI, Di Mari JF, Adegboyega PA, Valentich JD, Powell DW (2002) Regulation of COX-2 expression in human intestinal myofibroblasts: mechanisms of IL-1-mediated induction. Am J Physiol-Cell Ph 282:C824–C834. doi:10.1152/ajpcell.00388.2001
Keita AV, Soderholm JD, Ericson AC (2010) Stress-induced barrier disruption of rat follicle-associated epithelium involves corticotropin-releasing hormone, acetylcholine, substance P, and mast cells. Neurogastroent Motil 22:770–e222. doi:10.1111/j.1365-2982.2010.01471.x
Acknowledgments
This work was funded by the National Natural Science Foundation of China (31272627, 31472252) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (Jiangsu, China).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wu, C., Xu, Z. & Huang, K. Effects of Dietary Selenium on Inflammation and Hydrogen Sulfide in the Gastrointestinal Tract in Chickens. Biol Trace Elem Res 174, 428–435 (2016). https://doi.org/10.1007/s12011-016-0735-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0735-y