Abstract
Nitric oxide (NO) is an essential messenger molecule and is associated with inflammation and oxidative stress. Although NO has important biological functions in mammals, its role in the mechanism that occurs after intestinal injuries in chickens remains unknown. The objective of the present study was to investigate the real role of NO and oxidative stress in the intestinal injuries of chickens induced by selenium (Se) deficiency. A total 150 chickens were randomly divided into the following two groups: a low-Se group (L group, fed a Se-deficient diet containing 0.020 mg/kg Se) and a control group (C group, fed a commercial diet containing 0.2 mg/kg Se). The activities and mRNA levels of glutathione peroxidase (GSH-Px), the production of glutathione (GSH) and NO, and the protein and mRNA levels of inducible nitric oxide synthase (iNOS) were examined in the intestinal tissues (duodenum, jejunum, and rectum) at 15, 25, 35, 45, and 55 days. Methane dicarboxylic aldehyde (MDA) levels were also detected by assay kits. Then, the morphologies of the tissues were observed under the microscope after hematoxylin and eosin staining (H&E staining). The results showed that Se deficiency induced higher inflammatory damage and MDA levels (P < 0.05), which were accompanied by higher levels of iNOS and NO but lower levels of GSH and GSH-Px (P < 0.05). Our results indicated that Se deficiency induced oxidative damage in the intestinal tracts of chickens and that low levels of GSH-Px and high contents of NO may exert a major role in the injury of the intestinal tract induced by Se deficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se), an essential trace element, reserves kinds of functions in both human beings and domestic animals [1]. Se mainly incorporates into a selenoprotein that possesses several types of biological functions [2, 3]. In a previous study, an area with soil Se content below 0.5 mg/kg was considered a low-Se region [4]. Generally, the appropriate Se content in feed is 0.1 mg/kg, and when it is less than 0.05 mg/kg, the low Se level could lead to a disease [5]. Se deficiency could induce oxidative stress, inflammation, and apoptosis in many tissues [6]. The intestinal tract, as one main organ absorbing Se, is the tissue targeted by Se deficiency. This disruption of the intestinal tract function may influence the exertion of the biological function of Se and affect the normal function of other organs. Therefore, studying the effects of Se deficiency on the intestinal tract is an important field. It has been indicated that Se deficiency reduced the intestinal dilation of rats [7] and increased the intestinal motility in the chronic phase of parasite infection [8]. Se deficiency influenced the level of intestinal glutathione peroxidase (GSH-Px) and induced inflammation and oxidative stress in the rat intestine. The GSH-Px activity and mRNA level were significantly depressed in the ileum of Se-deficient mice [9]. In addition, it is known that Se deficiency can induce intestinal inflammation in chickens [10]. However, the exact mechanism of the influence of the intestinal mucosal immunization induced by a low-Se diet remains unclear. Moreover, the concentration of previous studies has been on mammals, but the real role of Se in the intestinal mucosal function of chickens has been reported to a lesser extent. As mentioned above, in mammals, GSH-Px is one crucial target of Se in the intestinal tract, but studies that evaluated the effects of Se on the intestinal function of chickens are rare. The change in GSH-Px due to Se deficiency may be the major cause of injury in the intestinal tract. Therefore, it is reasonable to hypothesize that Se deficiency may influence the function of intestinal tracts of chickens by decreasing the activity of GSH-Px.
Nitric oxide (NO), as one type of active messenger molecule, has broad biological functions. NO is produced by nitric oxide syntheses including constitutive NO syntheses (cNOS) and inducible NO syntheses (iNOS) that are mainly expressed in the intestinal tract [11]. It was shown that NO could regulate the intestinal mucosal blood flow and vascular integrity [12]. In research about intestinal injury in mammals due to chronic alcohol use, Bagyanszki et al. confirmed that NO protected the integrity of the intestinal mucosa and enhanced its motivation [13]. In addition, the NO produced by iNOS aggravated gastrointestinal injury by a mechanism of oxidative stress that was induced by NO and ROS in mice [14]. Thus, we can see that NO always exists in conjunction with an intestinal tract injury. However, whether Se deficiency also induces NO production in the intestinal tract is unknown. In the present study, through the detection of the antioxidative selenoprotein, GSH-Px, and the NO level in the intestinal tract of 150 sea blue white laying hens, we discuss the potential mechanism of intestinal damage induced by Se deficiency over 55 days.
Materials and Methods
Animals and Diet
All of the procedures used in the present study were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University. A total of 300 1-day-old sea blue white laying chicks were divided into two groups. Each treatment group consisted of 150 chickens, and each of those groups was randomly divided into groups so that the trials were conducted in triplicate. The chickens were maintained either on a Se-deficient diet (L group, Se-deficient granulated diet including corn, soybean meal, and wheat bran from Longjiang County, a typical Se-deficient region of the Heilongjiang Province in China and blends material that did not add Se (Weiwei Co. Ltd., Harbin, China)) or a normal-Se-content diet (control group, Weiwei Co. Ltd., Harbin, China) for 55 days. According to the different nutritional needs during the growth stages of sea blue white chickens (0–21, 22–42, and 43–55 days), different types of feed were prepared for the control and L groups. Se was added in the form of sodium selenite, and the final content of Se was 0.2 mg/kg in the control group and 0.02 mg/kg in the L group, as determined by fluorescence spectrophotometry using GB/T 13,883-2008 (PONY TEST Co., Beijing, China). Over the entire experimental period, the chickens were allowed ad libitum consumption of feed and water. Clinical symptoms and mortality were also recorded. Then, 15 chickens in each group were killed with sodium pentobarbital at 15, 25, 35, 45, and 55 days old, respectively. The intestinal tissues (duodenum, jejunum, and rectum) were quickly removed, minced, and stored at −80 °C in order to determine the index of oxidative stress and isolate the RNA. In this study, 15 chickens were killed per group at the five sampling events, and five chickens per group were used in the official test (n = 5), which was repeated in triplicate. The remaining tissues were used in the preliminary experiment and served as standby tissues.
Morphologies of the Intestinal Mucosa
The tissues of each segment of the intestine were collected, cut to a size approximately 0.5 cm3, placed in 10 % paraformaldehyde (pH = 7.4) for 24 h at 25 °C, and embedded in paraffin. The fixed well tissues were H&E-stained and mounted after the processes of dehydration, transparent, dipping wax, embedded, sectioned, and affixed. Then, the paraffin sections were placed under a microscope (Leica DM-2500, Germany) with a high-resolution digital camera to observe and record the relevant morphologies.
Gene Expression Analysis
The total RNA was isolated from each of the tissue samples (n = 5/diet group) using TRIzol reagent according to the instructions of the manufacturer (Invitrogen, Carlsbad, CA, USA). The concentrations of the total RNA were determined using GeneQuant 1,300 (GE Healthcare Biosciences, Piscataway, NJ, USA). The reverse transcription reaction (20 μL) consisted of the following: 5 μg of total RNA, 0.5 μL of Moloney Murine Leukemia Virus reverse transcriptase (200 U/μL), 0.5 μL of RNase inhibitor (40 U/μL), 2 μL of deoxynucleoside triphosphate (10 mM), 1 μL of Oligo dT (50 μM), 2 μL of dithiothreitol (0.1 M), and 4 μL of 5× reverse transcriptase buffer. The procedure of the reverse transcription was according to the instructions of the manufacturer (Invitrogen). The reverse transcription products (cDNA) were then stored at −20 °C for PCR.
Primer Premier Software (PREMIER Biosoft International, USA) was used to design specific primers for iNOS (GenBank accession no. NM_204961), GSH-Px (GenBank accession no. NM_001277854.1), and GADPH (GenBank accession no. K01458) based on known chicken sequences. GADPH, a housekeeping gene, was used as an internal reference. Primers were synthesized by Invitrogen Biontechnology Co. Ltd. in Shanghai, China. The following set of primers were used: for iNOS, forward 5′- CCT GGA GGT CCT GGA AGA GT -3′ and reverse 5′- CCT GGG TTT CAG AAG TGG C -3′; for GSH-Px, forward 5′- ATC GCC AAG TCC TTC TAC GA -3′ and reverse 5′- ACG TTC TCG ATG AGG ACC AC-3′; and for GADPH, forward 5′- AGA ACA TCA TCC CAG CGT -3′ and reverse 5′- AGC CTT CAC TAC CCT CTT G -3′. The PCR products were subjected to electrophoresis on 2 % agarose gels, extracted, cloned into the pMD18-T vector (Takara, Ohtsu, Japan), and sequenced.
Real-time PCR was used to detect the expression of the iNOS gene in the intestines by using SYBR Premix Ex Taq (Takara), and real-time PCR was performed on an ABI PRISM 7,500 detection system (Applied Biosystems, USA). The program was 1 cycle at 95 °C for 30 s, 40 cycles at 95 °C for 5 s, and 40 cycles 61 °C for 34 s. The melting curve analysis showed only one peak for each PCR product. Electrophoresis was performed on the PCR products to verify the specificity of the primer and the product purity. The amplification efficiency for each gene was determined using the Data Analysis for Real-Time PCR (DART-PCR) program [15]. The relative abundance of the mRNA was calculated according to the method of Pfaffl [16] and, to account for gene-specific efficiencies, was then normalized to the mean expression of GADPH.
NO and iNOS Activity Assay
The homogenized intestinal tissues of each treatment group were used for the NO and iNOS activity assays. The NO and iNOS activities were determined using NO and iNOS activity assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The method used in the present study was according to the procedure previously published by our group [17] and used an ELX800 Microplate reader (BioTek Instruments, USA) to detect the OD at 550 and 530 nm, respectively.
MDA, GSH, and GSH-Px Activity Assay
According to the manufacturer’s instructions, MDA, GSH, and GSH-Px activity assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were used to determine the MDA and GSH contents as well as the activity of GSH-Px in the intestines using an ELX800 Microplate reader (BioTek Instruments, USA) to detect the OD at 532, 420, and 412 nm, respectively.
Western Blotting Analysis
An equivalent amount of tissue (depending on the tissue examined, between 50 and 150 mg) was homogenized in 800 μL of ice-cold grind buffer (20 mM Tris–HCl, pH = 7.4, 2 mM EDTA, 2 mM EGTA, 1 mM PMSF, 30 mM NaF, 30 mM sodium pyrophosphate, 0.1 % SDS, 1 % Triton X-100, and protease inhibitor cocktail). The sample was then centrifuged for 10 min at 10,000 g and 4 °C, and the supernatant was collected. The protein content was measured according to Bradford’s procedure [18]. Equal amounts of the total protein (40 μg/condition) were subjected to SDS-polyacrylamide gel electrophoresis under reducing conditions on 10 % gels. The separated proteins were then transferred to nitrocellulose membranes using a tank transfer for 2 h at 200 mA in Tris–glycine buffer containing 20 % methanol. Membranes were blocked with 5 % skim milk for 16–24 h and incubated overnight with diluted primary rabbit antibody against iNOS (1:200, Abcam, USA), followed by a horse-radish peroxidase (HRP) conjugated secondary antibody against rabbit IgG (1:1000, Santa Cruz, USA). To verify the equal loading of the samples, the membrane was incubated with a monoclonal β-actin antibody (1:1000, Santa Cruz, USA), followed by incubation with a HRP conjugated goat anti-mouse IgG (1:1000). The protein bands were visualized by enhanced chemiluminescence detection reagents (Applygen Technologies Inc., Beijing, China). The signal was detected by X-ray films (TransGen Biotech Co., Beijing, China). The optical density (OD) of each band was determined by an Image VCD gel imaging system (Beijing Sage Creation Science And Technology Co. Ltd., Beijing, China), and the iNOS expression was expressed as the ratio of the OD of iNOS and the OD of β-actin.
Statistical Analysis
Statistical analysis of all of the data was performed using the SPSS statistical software for Windows (version 13; SPSS Inc., Chicago, IL, USA). The data are expressed as mean ± SD. When a significant value (P < 0.05) was obtained by one-way analysis of variance, further analysis was performed. All of the data showed a normal distribution and passed equal variance testing. Differences between means were assessed by Tukey’s honestly significant difference test for post hoc multiple comparisons. Differences were considered to be significant when P < 0.05.
Results
Mortality Rate of the Chickens
During the experiment, at 25 days of age, disease and death occurred in the chickens (L group). The dead chickens showed poor feathering, pancreatic degeneration, subcutaneous exudation, and necrotic lesions in different tissues. The mortality rate in the L group at 15–35 days was 2.75 %; between 45 and 55 days, no chicken death occurred.
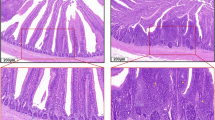
Histopathological Observations of the Intestinal Tissues
During the entire experimental period, no ultrastructural damage was observed in the control group (Fig. 1a–c). Compared to the control group, a large number of inflammatory cells were infiltrated, the mucosa of the duodenum was shed, the structure of the jejunum was loosely arranged, the number of lymphocytes increased, and a large number of macrophages emerged in the L group at each time point (Fig. 1d–f).
Effects of Se Deficiency on the mRNA Level of GSH-Px in the Intestinal Tissues of Chickens
The expression of GSH-Px mRNA in the intestinal tissues of chickens is shown in Fig. 2. Compared with the control group, at each time point there was a significant decrease (P < 0.05) that could be observed in the L group between 15 and 55 days of age. As the experiment progressed, a significant decrease of GSH-Px mRNA expression was observed from 25 to 55 days in all three tissues for chickens that were fed a Se-deficient basal diet.
Effects of Se deficiency on the mRNA expression of the GSH-Px gene in intestinal tissues of chickens. a Effects of Se deficiency on the mRNA level of GSH-Px in the duodenum. b Effects of Se deficiency on the mRNA level of GSH-Px in the jejunum. c Effects of Se deficiency on the mRNA level of GSH-Px in the rectum. Each value is represented as the mean ± SD of five individuals (n = 5). The asterisk indicates that there are significant differences (P < 0.05) between the control group and the L group at the same time point
Effects of Se Deficiency on GSH Production and GSH-Px Activity in the Intestinal Tissues of Chickens
Figures 3 and 4 summarize the changes in GSH production and GSH-Px activity in the intestinal tissues of chickens, and both were significantly lower (P < 0.05) in L the group than in the control group at each time point. Compared to the control group at each of the five time points in the three tissues, the production of GSH was reduced beginning at 15 or 25 days (Fig. 3a–c). GSH-Px activity demonstrated the same behavior and decreased beginning at 15 days (Fig. 4a–c).
Effects of Se deficiency on the GSH production in the intestinal tissues of chickens. a Effects of Se deficiency on the GSH production in duodenum. b Effects of Se deficiency on the GSH production in the jejunum. c Effects of Se deficiency on the GSH production in the rectum. Each value is represented by the mean ± SD of five individuals (n = 5). The asterisk indicates that there are significant differences (P < 0.05) between the control group and the L group at the same time point
Effects of Se deficiency on the GSH-PX activity in the intestinal tissues of chickens. a Effects of Se deficiency on the GSH-PX activity in the duodenum. b Effects of Se deficiency on the GSH-Px activity in the jejunum. c Effects of Se deficiency on the GSH-Px activity in rectum. Each value is represented by the mean ± SD of five individuals (n = 5). The asterisk indicates that there are significant differences (P < 0.05) between the control group and the L group at the same time point
Effects of Se Deficiency on the MDA in the Intestinal Tissues of Chickens
From Fig. 5, compared with the control group at each time point, a significant increase (P < 0.05) was observed in the L group between 15 and 55 days of age. We can observe that the increases in the total MDA began at different time points in each of the three tissues. In both the duodenum and rectum, the MDA increased at 15 days (Fig. 5a, c), but in the jejunum, the increase in the total MDA began at 25 days (Fig. 5b).
Effects of Se deficiency on the MDA production in the intestinal tissues of chickens. a Effects of Se deficiency on the MDA production in the duodenum. b Effects of Se deficiency on the MDA production in the jejunum. c Effects of Se deficiency on the MDA production in the rectum. Each value is represented by the mean ± SD of five individuals (n = 5). The asterisk indicates that there are significant differences (P < 0.05) between the control group and the L group at the same time point
Effects of Se Deficiency on the mRNA and Protein Levels of iNOS in the Intestinal Tissues of Chickens
The expression of iNOS mRNA and protein levels in the intestinal tissues of chickens is shown in Fig. 6. Compared with the control group, a significant increase (P < 0.05) in the expression of the iNOS gene was observed in the L group from 15 to 55 days of age. However, in the rectum, the significant increase (P < 0.05) of the iNOS expression began at 25 days (Fig. 6c). Compared with the control group, a significant increase (P < 0.05) in the expressions of the iNOS protein level in the L group was observed in both the duodenum and jejunum from 15 to 55 days of age (Fig. 6d, e). Interestingly, there was no significant difference at 15 days in the rectum (Fig. 6f) compared with the control group. The conclusion that can be drawn from Fig. 6 is that the protein levels of iNOS were consistent with the mRNA expression levels.
Effects of Se deficiency on the mRNA expression of the iNOS gene in intestinal tissues of chickens. a, d Effects of Se deficiency on the mRNA and protein levels of iNOS in the duodenum. b, e Effects of Se deficiency on the mRNA and protein levels of iNOS in the jejunum. c, f Effects of Se deficiency on the mRNA and protein levels of iNOS in the rectum. The mRNA and protein expressions from the solvent control group were used as the reference values in a–f. Each value is represented by the mean ± SD of five individuals (n = 5). The asterisk indicates that there are significant differences (P < 0.05) between the control group and the L group at the same time point
iNOS Activity and NO Production
The effects of Se deficiency on the iNOS activity in intestinal tissues are shown in Fig. 7. The activity of iNOS was significantly higher (P < 0.05) in the L group than in the control group at each time point. NO production in the intestinal tissues of chickens was measured by calculating the production of nitrite oxide.
Effects of Se deficiency on the iNOS activity in the intestinal tissues of chickens. a Effects of Se deficiency on the iNOS activity in the duodenum. b Effects of Se deficiency on the iNOS activity in the jejunum. c Effects of Se deficiency on the iNOS activity in the rectum. Each value is represented by the mean ± SD of five individuals (n = 5). The asterisk indicates that there are significant differences (P < 0.05) between the control group and the L group at the same time point
The effect of Se deficiency on the production of NO in intestinal tissues is summarized in Fig. 8, and compared with control group, NO production was significantly higher (P < 0.05) in the L group duodenum and jejunum from 15 to 55 days. In the rectum, the significant upward trend (P < 0.05) was begun at 25 days.
Effects of Se deficiency on the NO production in the intestinal tissues of chickens. a Effects of Se deficiency on the NO production in the duodenum. b Effects of Se deficiency on the NO production in the jejunum. c Effects of Se deficiency on the NO production in the rectum. Each value is represented by the mean ± SD of five individuals (n = 5). The asterisk indicates that there are significant differences (P < 0.05) between the control group and the L group at the same time point
Discussion
As one important antioxidant selenoprotein, GSH-Px exists in several types of organs, is especially expressed in intestinal tissues, and has a certain physiological significance on the protection of integrity of the intestinal mucosa [19]. A decrease of GSH-Px or other antioxidative selenoproteins induced by Se deficiency has been shown to cause oxidative damage in some types of cells and intestinal tissues in lambs [20–22]. A low-Se-content diet could induce an ulcer through the down-regulation of the level of GSH-Px in the intestinal tract of rats [23]. The intestinal tissues of chickens were also affected by Se deficiency [3], but whether Se deficiency also induced the change of the GSH-Px level and caused oxidative damage in intestinal tissues of chickens was still unclear. In the present study, we examined the effect of Se deficiency on the levels of GSH-Px and the GSH in intestines of chickens. The relative data may provide us some clues as to the mechanism of Se deficiency-related diseases in intestinal tissues while also revealing the potential role of Se in intestinal damage. As one important antioxidative selenoprotein in the intestinal tract, GSH-Px is one crucial target of Se deficiency. Similarly, a decreased level of GSH-Px induced by Se deficiency has been shown to be the cause of damage to intestinal tissues. Pawłowicz et al. indicated that Se deficiency decreased the level of GSH-Px in the colons of humans and also caused oxidative stress and bowel cancer [24]. Rao reported that Se deficiency reduced GSH-Px and other antioxidant enzymes in rat intestines and led to DNA damage and oxidative stress [25]. Bolkent et al. also confirmed that a concentration of Se below 0.2 mg/kg in the diet caused increased lipid peroxidation levels and decreased glutathione levels, thus exacerbating the intestinal mucosal injury in the intestines of rats [26]. At the same time, previous studies confirmed that not only can the GSH-Px level be reduced by oxidative stress but histological changes, such as lymphocyte infiltration and cell structure disorder in the liver of drake, were induced [27]. An investigation on a model of cerebral ischemia/reperfusion also revealed that acute oxidative injury caused histomorphology changes and even apoptosis in rats [28]. In our study, we found that Se deficiency reduced the GSH-Px activity, the GSH-Px mRNA level, and the GSH content at each time point. As the experiment progressed, the GSH-Px mRNA level decreased continuously with duration of the Se deficiency. The duodenum and jejunum showed sensitive reactions in 15 days, but in the rectum, the mRNA expression began to be reduced at 25 days. Morphological observations also revealed the obvious infiltration of inflammatory cells, intestinal mucosal lesion, and intestinal villi fractures in the duodenum and jejunum. Those results suggested that duodenum and jejunum were the primary organs targeted by Se deficiency, and the rectum has a certain tolerance for the lack of Se. The decrease of these antioxidant enzymes further caused oxidative damage and higher MDA contents, which is consistent with the research of Aw. T. Y. et al. [29]. The reduction of GSH-Px and GSH in the intestine by Se deficiency may decrease the antioxidant capacity of the intestine itself, influencing the normal functions of the intestine and thus causing further damage to the intestinal tract [30]. Because GSH-Px is the main resident selenoprotein in the intestine [31], it was reasonable to speculate that a decrease in GSH-Px may be the main pathway of Se deficiency-related intestinal disease.
NO is a small signaling molecule with important regulatory effects in many tissues. The reaction of NO with molecular oxygen allows it to function as a rapidly reversible, specific, and local signal transduction molecule in tissue damage [32]. Under physiological conditions, the sustainability and low-level expression of NO contribute to maintaining the integrity of the gastrointestinal mucosa. However, a large amount of NO leads to damage of the intestinal barrier and causes cell death [33]. The main mechanism behind this damage is that NO can combine with superoxide anions and form peroxynitrite, which is decomposed into more toxic free radicals, such as the hydroxyl free radical (-OH) and nitrogen dioxide (NO2), causing damage to proteins, nucleic acids, and lipid membranes [34]. Prior studies indicated that Se can influence the generation of NO in intestinal tissues and further regulate the normal function of intestinal tissues. Ozturk showed that Se protected the intestine against free radical damage by inhibiting the expression of iNOS in the ischemia/reperfusion (I/R) model of rats and helped to effectively maintain the integrity of the intestinal mucosal [35]. However, in a study of the small intestines of humans, it was proven that Se deficiency up-regulated the concentration of NO and led to lesions in the mucosa [36]. In addition, Se deficiency was associated with oxidative stress through activating the pathway of the nuclear transcription factor of kappa B (NF-κB), which further stimulated the activity of iNOS, leading to the generation of NO [37, 38]. So, the production of NO may be one important mediator for the Se deficiency-induced intestinal damage in chickens. Consistent with the studies described above, we found that Se deficiency induced high levels of iNOS and NO in all of the samples, and the iNOS mRNA expression levels and protein levels were consistent in the L group. The activity of iNOS was significantly increased at 25 days in all three of the tissues. The study of endothelial dysfunction showed that NO deprivation would reduce the cellular ROS content [39]. Considering the decreased antioxidative selenoproteins and the increased NO level in the intestine, we observed that oxidative damage is the main injury induced by Se deficiency. The results indicated that under the condition of Se deficiency, the antioxidant defense system was undermined in vivo, which led to the accumulation of oxygen free radicals and then the release of a large number of inflammatory mediators while stimulating an increase in the expression of iNOS and the excessive release of NO.
In summary, the present study showed that Se deficiency caused oxidative damage in chicken intestinal tissues by decreasing the GSH-Px production and increasing the NO level, which is released by iNOS. The results suggested that NO and oxidative stress played a major role in the injury of the intestinal tract induced by Se deficiency.
References
Papp LV, Holmgren A, Khanna KK (2010) Selenium and selenoproteins in health and disease. Antioxid Redox Signal 12(7):793–795
Maraldi T, Riccio M, Zambonin L et al (2011) Low levels of selenium compounds are selectively toxic for a human neuron cell line through ROS/RNS increase and apoptotic process activation. Neurotoxicology 32(2):180–187
Saad MB, Gertner LR, Bona TD et al (2009) Selenium influence in the poultry immune response—review. Recent Pat Food Nutr Agric 1(3):243–247
Hartfiel W, Bahners N (1988) Selenium deficiency in the Federal Republic of Germany. Biol Trace Elem Res 15:1–12
Pavlovic Z, Miletic I, Jokic Z et al (2010) The effect of level and source of dietary selenium supplementation on eggshell quality. Biol Trace Elem Res 133(2):197–202
Mishra V, Baines M, Perry SE et al (2007) Effect of selenium supplementation on biochemical markers and outcome in critically ill patients. Clin Nutr 26(1):41–50
Fatmi W, Kechrid Z, Naziroglu M et al (2013) Selenium supplementation modulates zinc levels and antioxidant values in blood and tissues of diabetic rats fed zinc-deficient diet. Biol Trace Elem Res 152(2):243–250
de Souza AP, Sieberg R, Li H et al (2010) The role of selenium in intestinal motility and morphology in a murine model of Typanosoma cruzi infection. Parasitol Res 106(6):1293–1298
Esworthy RS, Yang L, Frankel PH et al (2005) Epithelium-specific glutathione peroxidase, Gpx2, is involved in the prevention of intestinal inflammation in selenium-deficient mice. J Nutr 135(4):740–745
Placha I, Borutova R, Gresakova L et al (2009) Effects of excessive selenium supplementation to diet contaminated with deoxynivalenol on blood phagocytic activity and antioxidative status of broilers. J Anim Physiol Anim Nutr 93(6):695–702
Hofseth LJ (2008) Nitric oxide as a target of complementary and alternative medicines to prevent and treat inflammation and cancer. Cancer Lett 268(1):10–30
Bulbul A, Bulbul T, Sevimli A et al (2013) The effect of dietary supplementation of nitric oxide donor and inhibitor on nNOS expression in and motility of the small intestine of broilers. Biotech Histochem 88(5):258–266
Bagyanszki M, Torfs P, Krecsmarik M et al (2011) Chronic alcohol consumption induces an overproduction of NO by nNOS- and iNOS-expressing myenteric neurons in the murine small intestine. Neurogastroenterol Motil 23(6):e237–248
Barocelli E, Ballabeni V, Ghizzardi P et al (2006) The selective inhibition of inducible nitric oxide synthase prevents intestinal ischemia-reperfusion injury in mice. Nitric Oxide 14(3):212–218
Peirson SN, Butler JN, Foster RG (2003) Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res 31(14):e73
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Zhang ZW, Lv ZH, Li JL et al (2011) Effects of cold stress on nitric oxide in duodenum of chicks. Poult Sci 90(7):1555–1561
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Kipp A, Banning A, van Schothorst EM et al (2009) Four selenoproteins, protein biosynthesis, and Wnt signalling are particularly sensitive to limited selenium intake in mouse colon. Mol Nutr Food Res 53(12):1561–1572
Zhao FQ, Zhang ZW, Yao HD et al (2013) Effects of cold stress on mRNA expression of immunoglobulin and cytokine in the small intestine of broilers. Res Vet Sci 95(1):146–155
Naziroglu M, Cig B, Ozgul C (2013) Neuroprotection induced by N-acetylcysteine against cytosolic glutathione depletion-induced Ca2+ influx in dorsal root ganglion neurons of mice: role of TRPV1 channels. Neuroscience 242:151–160
Neville TL, Redmer DA, Borowicz PP et al (2010) Maternal dietary restriction and selenium supply alters messenger ribonucleic acid expression of angiogenic factors in maternal intestine, mammary gland, and fetal jejunal tissues during late gestation in pregnant ewe lambs. J Anim Sci 88(8):2692–2702
Szymanska E, Siegers CP (1992) The effect of dietary selenium deficiency on the antioxidant enzyme system in the gastrointestinal tract of the rat. Res Commun Chem Pathol Pharmacol 75(3):341–346
Pawłowicz Z, Zachara B, Trafikowska U et al (1991) Blood selenium concentrations and glutathione peroxidase activities in patients with breast cancer and with advanced gastrointestinal cancer. J Trace Elem Electrolyte Health Dis 5(4):275
Rao L, Puschner B, Prolla TA (2001) Gene expression profiling of low selenium status in the mouse intestine: transcriptional activation of genes linked to DNA damage, cell cycle control and oxidative stress. J Nutr 131(12):3175–3181
Bolkent S, Koyuturk M, Bulan OK et al (2007) The effects of combined alpha-tocopherol, ascorbic acid, and selenium against cadmium toxicity in rat intestine. J Environ Pathol Toxicol Oncol 26(1):21–27
Xing H, Wang H, Sun G et al (2013) Antioxidant response, CYP450 system, and histopathological changes in the liver of nitrobenzene-treated drakes. Res Vet Sci 95(3):1088–1093
Uzar E, Acar A, Evliyaoglu O et al (2012) The anti-oxidant and anti-apoptotic effects of nebivolol and zofenopril in a model of cerebral ischemia/reperfusion in rats. Prog Neuropsychopharmacol Biol Psychiatry 36(1):22–28
Aw TY (1999) Molecular and cellular responses to oxidative stress and changes in oxidation-reduction imbalance in the intestine. Am J Clin Nutr 70(4):557–565
Batcioglu K, Ozturk C, Karagozler A et al (2002) Comparison of the selenium level with GSH-Px activity in the liver of mice treated with 7,12 DMBA. Cell Biochem Funct 20(2):115–118
Bartel J, Bartz T, Wolf C et al (2007) Activity of the glutathione peroxidase-2. Differences in the selenium-dependent expression between colon and small intestine. Cancer Genomics Proteomics 4(5):369–372
Velickovic K, Markelic M, Golic I et al (2013) Long-term dietary L-arginine supplementation increases endothelial nitric oxide synthase and vasoactive intestinal peptide immunoexpression in rat small intestine. Eur J Nutr
Robinson EK, Kennison SD, Suliburk JW et al (2005) Rat gastric injury after lipopolysaccharide: role of inducible nitric oxide synthase. Surgery 138(3):523–529
Zimiani K, Guarnier FA, Miranda HC et al (2005) Nitric oxide mediated oxidative stress injury in rat skeletal muscle subjected to ischemia/reperfusion as evaluated by chemiluminescence. Nitric Oxide 13(3):196–203
Ozturk C, Avlan D, Cinel I et al (2002) Selenium pretreatment prevents bacterial translocation in rat intestinal ischemia/reperfusion model. Pharmacol Res 46(2):171–175
Kaushal N, Gandhi UH, Nelson SM et al (2012) Selenium and inflammation. In: Selenium. Springer, pp 443-456
Shimizu M, Ogura K, Mizoguchi I et al (2013) IL-27 promotes nitric oxide production induced by LPS through STAT1, NF-kappaB and MAPKs. Immunobiology 218(4):628–634
Yurdagul A Jr, Chen J, Funk SD et al (2013) Altered nitric oxide production mediates matrix-specific PAK2 and NF-kappaB activation by flow. Mol Biol Cell 24(3):398–408
Cattaneo MG, Cappellini E, Ragni M et al (2013) Chronic nitric oxide deprivation induces an adaptive antioxidant status in human endothelial cells. Cell Signal 25(11):2290–2297
Acknowledgments
This study was supported by the Graduate Innovation Research Fund of Heilongjiang (Grant No.YJSCX2012-027HLJ), the Major Projects of International Cooperation and Exchanges NSFC (31320103920), the National Natural Science Foundation of China (31272626) and the Doctoral Fund of the Ministry of Education of China (20122325110018). The authors thank the members of the veterinary internal medicine laboratory at the College of Veterinary Medicine, Northeast Agricultural University for their help in feeding the chickens and collecting the intestinal tissue samples.
Conflicts of Interest
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yu, J., Yao, H., Gao, X. et al. The Role of Nitric Oxide and Oxidative Stress in Intestinal Damage Induced by Selenium Deficiency in Chickens. Biol Trace Elem Res 163, 144–153 (2015). https://doi.org/10.1007/s12011-014-0164-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0164-8