Abstract
The stimulation of plant growth and biomass accumulation by elevated CO2 may be limited by soil nitrogen (N) availability. However, our understanding of the response of soil N cycling to elevated CO2 and when progressive N limitation occurs remains limited. Here, we used an open top chamber experiment to examine the effects of 10 years of elevated CO2 on ecosystem carbon (C) and N dynamics in a Quercus mongolica (oak) dominated system in northeastern China. Elevated CO2 increased oak biomass, C and N stocks and C/N by 26.4, 26.2, 16.5 and 8.6% respectively, which suggests increased plant N demand. Soil gross N mineralization, re-mineralization of microbial N and nitrification were accelerated likely due to increased photosynthesis (by 34.9%) and microbial biomass (by 24.2%) under elevated CO2. Thus, the supply of soil available N can sustain the tree growth stimulated by elevated CO2, and to date progressive N limitation has not happened. Nevertheless, both the annual increase of oak biomass, C and N stocks and C/N ratio and the seasonal variations of soil available N and microbial N concentrations, and net N transformation rates indicated that gradual N deficiency may be occurring and the CO2 fertilization effect has weakened with increasing treatment duration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbon dioxide (CO2) concentration in the atmosphere has been rising since the industrial revolution and has reached about 400 ppm in 2013, 40% above preindustrial levels (IPCC 2013). Higher CO2 typically increases plant photosynthesis, biomass and ecosystem net primary productivity (Ainsworth and Long 2005; Finzi et al. 2007), and may increase ecosystem carbon (C) storage (Luo et al. 2006), thereby slowing the rate of further increases in atmospheric CO2 concentration. However, any CO2 fertilization effect could be tempered by increased nitrogen (N) limitation with time (Luo et al. 2004; Reich et al. 2006). Increased ecosystem C storage may lead to sequestration of available N in longer-lived plant and soil pools and reduce available N in the soil, causing progressive N limitation (Luo et al. 2004). When and if progressive N limitation occurs is still under debate (Luo et al. 2004; Rütting et al. 2010; Leuzinger et al. 2011; Feng et al. 2015; Rütting and Andresen 2015; Liang et al. 2016). On one hand, increasing N deposition has enhanced N supply to terrestrial ecosystems, attenuating N limitation (Xia and Wan 2008). On the other hand, elevated CO2 may stimulate soil organic matter (SOM) decomposition (Schneider et al. 2004; Finzi et al. 2007; Müller et al. 2009), accelerate soil N turnover (Williams et al. 2000; Finzi et al. 2007; McKinley et al. 2009), increase biological N fixation (Hu et al. 2006; Liang et al. 2016), or allow plants to mine deeper soil N by increasing fine root production (McKinley et al. 2009; Iversen et al. 2011). These changes under elevated CO2 may attenuate N limitation to plants by increasing soil N availability. In this context, it is important to study the responses of soil N cycling to elevated CO2 in order to better understand when progressive N limitation happens and better predict future ecosystem trajectories in the twenty-first century.
To date, there have been a variety of observed responses of the production (e.g. mineralization and nitrification) and consumption (e.g. immobilization) processes of soil available N (inorganic N) to elevated CO2. This begs the question of how predictable those N cycle responses may be. For example, elevated CO2 increased N mineralization and ammonium immobilization in a temperate heathland dominated by Calluna vulgaris and Deschampsia flexuosa, but also decreased gross nitrification (Björsne et al. 2014). In a Florida scrub oak ecosystem dominated by an N2 fixing plant, Galactia elliottii, Hungate et al. (1999) also found NH4+ immobilization was stimulated and gross nitrification was reduced by elevated CO2, but they did find reduced gross N mineralization. In contrast, in two different annual grasslands, gross N mineralization increased under elevated CO2, while NH4+ immobilization did not change, leading to increased nitrification due to higher NH4+ availability (Hungate et al. 1997a). These studies indicated that the effect of elevated CO2 on soil internal N cycling depends on ecosystem nutrient status (de Graaff et al. 2006; Rütting and Andresen 2015). It is likely that a better understanding of the responses of microbial biomass C and N, and isotopic characteristics may help elucidate soil N cycle responses to elevated CO2, because microbes are the main driver for soil C and N cycles, and their biomass C and N and isotopic characteristics may indicate these changes (Booth et al. 2005; Dijkstra et al. 2008a; Björsne et al. 2014; Rütting and Andresen 2015). Moreover, the re-mineralization of microbial N as an important process for replenishing available N for plants and microbes should be considered, especially in systems with high microbial N immobilization (Zak et al. 1993; Hungate et al. 1999; Thornley and Cannell 2000; Rütting et al. 2010). However, information on how elevated CO2 influences microbial N turnover is scarce (Zak et al. 1993; Rütting et al. 2010).
The conditions of N limitation of plant growth usually vary with time due to changing plant N demand throughout the growing season in temperate forest ecosystems (Chapin III et al. 2002). During the first half of the growing season, plants need more N to support rapid growth (Jaeger III et al. 1999), and elevated CO2 may enhance plant and microbial N demands and intensify the competition between plants and microbes for available N (Williams et al. 2000; Barnard et al. 2004, 2006). In the late growing season, N may not be limiting due to reduced plant growth and N demand (Jaeger III et al. 1999; Williams et al. 2000; Zhou et al. 2010). Therefore, it is important to assess the temporal variations of plant and soil N cycles to better understand when progressive N limitation occurs under elevated CO2.
In this study, we investigated the effects of 10 years of elevated CO2 on temporal dynamics of coupled C and N cycling processes and N availability in a Quercus mongolica dominated system in open top chambers. Our objective was to understand if progressive N limitation has diminished the CO2 fertilization effect in this system. Because previous studies at this site suggested that belowground C inputs increased under elevated CO2 (Li et al. 2010; Zheng et al. 2010; Zhou et al. 2010), we hypothesized that (1) elevated CO2 would accelerate soil N cycling due to increased microbial biomass and activity caused by increased C inputs; and (2) accelerated soil N cycling may sustain plant N demand and progressive N limitation to date may not have occurred under 10 years of elevated CO2 treatment.
Materials and methods
Site description
The experimental open top chamber (OTC) facility was established in 2004 in the Changbai Forest Ecosystem Research Station (42°24′N, 128°06′E) in Jilin Province, northeastern China. The study area is characterized by typical temperate climate, with a cold, long winter, and a warm, rainy summer. Mean annual precipitation is 745 mm, with rains mainly between June and September. Mean annual temperature is 3.6 °C, and the warmest month, July, averages 17.5 °C. Quercus mongolica (oak) is a widely distributed dominant tree species from approximately 700 to 1100 m asl (Li et al. 2010). The soil is dark brown soil developed from volcanic ash (Albic Luvisol), with well-drained, loamy sand texture. Basic soil characteristics were measured as part of a series of studies at this site in April 2014 (Table 1).
OTC description
The facility consists of six hexagonal OTC experimental plots, 4.4 m in diameter by 4 m high, and the area of each OTC is 12.6 m2. As the oak is continually growing, the height was increased to 6 m in 2010. Three plots were treated with pure CO2 to elevate the CO2 concentration by 180 μmol mol−1 above ambient, while the other three chambers were maintained at ambient CO2 concentration. Fans equipped in OTCs were used to increase air circulation. Infrared gas analyzers (A-SENSE-D, SenseAir, Delsbo, Sweden) placed in OTCs were used to monitor the CO2 concentration. A computerized control system recorded 10-s averages of CO2 concentration every 3 min, and periodically adjusted the flow of pure CO2 into the OTCs to maintain the elevated CO2 concentration.
Two years old oak seedlings were transplanted into these OTCs in the autumn of 2004. Twenty-two oak seedlings were planted in each OTC, and the seedlings were from the same nursery and had the same genotype (Li et al. 2010). CO2 fumigation started in 2005 and had been carried out for 10 years until 2014 (turned off). Plants were exposed to elevated CO2 during daytime from May to October in the growing season every year. Mean plant height and diameter at breast height significantly increased respectively under elevated CO2 (4.53 ± 0.07 m, 2.45 ± 0.04 cm) compared with that under ambient CO2 (4.15 ± 0.09 m, 2.20 ± 0.06 cm).
Oak biomass and C and N stocks measurement
The dry biomass of oak leaf, branch, stem and root were calculated according to the regression equation from Huo et al. (2011). The amount of leaf C or N of each OTC was calculated by multiplying the total leaf dry biomass of 22 oaks by the mean leaf C or N concentration which was measured during the growing season (May–September) in 2014. Similarly, the amount of C or N of branch, stem and root was calculated by multiplying total dry biomass by the corresponding mean C or N concentration which was based on the concentration ratios of leaf to branch, stem or root in Lu et al. (2015). C or N stock of different oak components in each OTC was calculated by dividing the amount of C or N of corresponding components by the area of OTC respectively. Total C or N stock was the sum of leaf, branch, stem and root C or N stock respectively.
Leaf sampling and plant photosynthesis measurement
Plant photosynthetic rate was measured from June to September in 2014, three times in each month using Li-6400 Portable Photosynthesis System (LI-COR Biosciences, Lincoln, NE, USA) by the artificial light source (LED-6400-02B) at approximate light saturation point (1300 μmol m−2 s−1). The fixed photosynthetically active radiation (PAR) was set to eliminate the effect of different PAR on photosynthesis. Undamaged growing leaves (six in each OTC) were selected and measured from 9:00 to 12:00 on sunny days. Measurements were consecutively repeated ten times for each leaf and the time interval was 10 s. Because the growing leaves are more sensitive to the change of soil available N than old leaves (Hikosaka et al. 1994), we chose to sample the growing leaves to explore the seasonal responses of plant to elevated CO2. After the photosynthesis measurement, three growing leaves were sampled, dried, mixed and ground for C and N concentrations and isotopic compositions analysis.
Soil NH4 +and NO3 − concentrations and isotopic compositions
Soil samples were taken six times each month from June to October in 2014. Two soil subsamples (0–10 cm) were randomly taken in each OTC, and fully mixed and sieved (< 2 mm) for analysis of soil properties. Soil (20 g) was extracted with 100 ml 2 M KCl shaking for 60 min at 200 rpm at 25 °C. The extracts were filtered with ash-less filter papers (Qualitative Filter Paper, BH92410262) and 10 ml of the extracts were used to determine the concentrations of NH4+ and NO3− with a Continuous Flow Analyzer (Bran-Luebbe Inc., Germany). The 15N natural abundance of NH4+ and NO3− were measured using the modified “ammonium diffusion” method according to Sebilo et al. (2004) with the details described by Sun et al. (2016), and were analyzed by an Elemental Analyzer (Thermo-Element Flash EA 1112, USA) coupled with an Isotope Ratio Mass Spectrometer (Thermo Fisher MAT 253, USA).
Microbial biomass C and N and isotopic compositions
The rest sieved soil samples were used to analyze microbial C, N, DOC and DON concentrations and isotopic compositions. Chloroform fumigation method was used for microbial C and N analysis (Brookes et al. 1985). The method to measure microbial C and N isotopic compositions was described in Dijkstra et al. (2006). Briefly, soil (20 g) was extracted with 100 ml 0.05 M K2SO4 and shaken for 60 min at 200 rpm at 25 °C, while the other 20 g soil was fumigated with chloroform followed by K2SO4 extraction. The extracted solution was dried at 60 °C and ground to fine powder for analysis of C and N concentrations and isotopic compositions.
The C and N concentrations and isotopic compositions of the soil, leaf, and K2SO4 extracts were analyzed at the Stable Isotope Faculty of University of California, Davis. About 5 mg leaf, 60 mg soil and 70 mg K2SO4 extracts were separately loaded into capsules and measured using an Elementar Vario EL Cube (Elementar Analysis system GmbH, Hanau, Germany) interfaced to a PDZ Europa 20-20 isotope ratio mass spectrometer (Sercon Ltd., Cheshire, UK), with an overall precision better than 0.2‰. Carbon or nitrogen isotope ratios are presented in δ notation:
where RSAMPLE is the 13C/12C or 15N/14N of a sample and RSTD is the 13C/12C of Pee Dee Belemnite or 15N /14N of atmospheric N2. Microbial C and N were calculated as:
where M(C) and M(N) are the microbial C and N respectively; C and N are the dissolved organic C and N in K2SO4 extracts respectively; subscript e and f represent before and after chloroform fumigation respectively. The extraction efficiency factors were 0.45 for microbial C (Vance et al. 1987) and 0.54 for microbial N (Brookes et al. 1985). Microbial N isotopic composition was calculated according to Dijkstra et al. (2008a):
where δ15NMic is the isotopic composition of microbial N; δ15Ne and δ15Nf are the isotopic compositions of N in the K2SO4 extracts before and after chloroform fumigation respectively. Microbial δ13C was calculated based on the same principle.
Net N transformation rates
Net N mineralization, nitrification, and ammonification rates were measured in situ with the intact-core incubation method (Raison et al. 1987) from April to October in 2014. On the 17th of each month, three pairs of soil cores (0–10 cm) were randomly collected from each OTC using PVC soil corers (5 cm diameter by 12 cm deep). Three soil cores (one core from each pair) were immediately brought to the lab to measure NH4+, NO3−, and soil gravimetric water content. The other three PVC tubes were taken out and packed with absorbent gauze at the bottom of the tubes respectively, preventing the roots from penetration. Then, the PVC tubes were covered with lids to allow air circulation but prevent water exchange, and immediately placed back in the soil. After 30 days, the incubated samples were collected and lab analysis was repeated. Net N mineralization rate was determined from changes in the sum of NH4+ and NO3− concentrations; net nitrification rate was determined from changes in NO3− concentration; net ammonification rate was determined from changes in NH4+ concentration over 30 days.
Gross N transformation rates
Gross N transformation rates were determined by the FLUAZ 15N tracing model (Mary et al. 1998). Five soil cores (0–10 cm) were collected in each OTC and mixed to one sample at the end of the growing season (17th October). The soil samples were sieved (< 2 mm), then air dried for the 15N tracing incubation experiment.
Each soil sample was divided into 16 subsamples (32.5 g air dried soil per subsample) and each subsample was put into a 200 ml plastic bottle. Half of the 16 bottles were used for 15NH4NO3 treatment and the other half were used for NH 154 NO3 treatment. Deionized water (4.5 ml) was added to each bottle, and then sealed with parafilm which prevented water evaporation but still allowed airflow. The bottles were pre-incubated for 24 h at 20 °C prior to 15N tracer addition. After the pre-incubation, 2.5 ml 15NH4NO3 or NH 154 NO3 solution (99.2 at.% 15N, 32.4 mg l−1) was added to each bottle. In order to reduce the effect of N addition on N cycling processes, the added N was minimized to 0.89 μg N g−1 soil. The soil was adjusted to 60% field capacity and incubated for 16 days at 20 °C. Soils were retrieved at 0 h (instantly extracted after 15N application), 1, 2, 4, 6, 8, 13 and 16 d after 15N application to determine NH4+ and NO3− concentrations and atomic percent excess (at.% excess). The residual soil after the KCl extraction was subsequently washed with 100 ml deionized water for 3 times, oven-dried at 60 °C and ground for soil organic 15N analysis. The 15N at.% and at.% excess are defined as:
where 0.3663% is the at.% of the standard (atmospheric N2).
Soil moisture, temperature, texture and pH
Standard oven-drying method was carried out to measure soil gravimetric water content. The temperatures of air and soil at 10 cm depth were recorded in situ at each sampling time using a digital thermometer. Soil texture was determined by the pipette sedimentation method (Gee and Bauder 1986). Soil pH was measured in a 1:5 (w/v) soil to water (CO2-free) ratio using a pH detector.
Statistical analyses
The paired-samples t test was used to compare the differences of mean gross N transformation rates between treatments. Traditional statistical tests were not appropriate for the results of the FLUAZ model due to the large number of iterations (Yoccoz 1991). The statistical significance of gross N transformation rates under both treatments was indicated by comparing the standard deviations (68%) and 95% confidence intervals to distinguish three cases: (a) if the 95% confidence intervals did not overlap, the parameters were deemed significantly different; (b) if the standard deviations did not overlap, but the 95% confidence intervals overlapped, the parameters were not considered significantly different, but presented a clear tendency of difference; (c) if the standard deviations overlapped, the parameters were not considered different (Müller et al. 2009; Sun et al. 2016). Linear correlation was used to analyze the correlations between microbial C and N and isotopic compositions and plant and soil variables. Independent-samples t test was used to compare the differences of plant, soil and microbial properties between control and elevated CO2 treatment for each sampling time separately. Repeated measures ANOVA was used to examine the effects of elevated CO2 on plant photosynthetic rate, leaf C and N, soil NH4+, NO3−, DON, DOC, microbial C and N concentrations and isotopic compositions, and net N transformation rates using CO2 fumigation treatment as the between subject factor and sampling time as the within subject factor. Data were transformed to normalize variance across treatments before analysis when necessary. Repeated measures ANOVA was carried out with R 3.2.2 software, and other analyses were carried out with SPSS 17.0 software (SPSS, Chicago, IL, USA).
Results
Elevated CO2 for 10 years did not change soil organic C, total N, pH or soil texture, but significantly increased soil C/N by 3.4% (p < 0.05, Table 1). Air and soil (10 cm depth) temperatures and soil moisture were almost identical under the two treatments (Fig. S1).
Oak biomass and C and N stocks
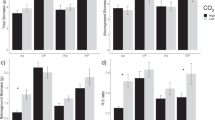
Elevated CO2 significantly increased the mean biomass of oak leaf, branch, stem, root and total biomass by 22.1, 43.1, 28.5, 23.6 and 26.4% respectively compared with the increase under ambient CO2 (p < 0.05, Table 2). Elevated CO2 significantly increased the mean oak leaf, branch, stem, root and total C stocks by 21.8, 43.0, 28.3, 23.3 and 26.2% respectively, and increased N stocks by 13.6, 25.0, 17.5, 15.6 and 16.1% respectively throughout the growing season in 2014 (p < 0.05, Table 3). The mean annual increases in oak biomass and C and N stocks after elevated CO2 treatment for 3 years (7.6, 10.2 and 7.1% respectively) were higher than those after elevated CO2 treatment for 10 years (2.6, 2.6 and 1.6% respectively) (p < 0.05, Fig. 1a). Moreover, the foliar litter C/N increased by 7.2% after elevated CO2 treatment for 3 years, which was lower than that after treatment for 10 years (16.9%) (p < 0.05, Fig. 1b).
The biomass and C and N stocks (a) and foliar litter C/N (b) of Quercus mongolica after treatment for 3 years (A3 and E3 for ambient and elevated CO2 respectively) and ten years (A10 and E10 for ambient and elevated CO2 respectively). Different letters indicate statistical significance at p < 0.05 among the four values according to one way ANOVA (Tukey’s HSD test). Annual mean increases in biomass and C and N stocks were calculated in (a) and the absolute increase in litter C/N was presented in (b). Data of foliar litter C/N and diameter at breast height of Quercus mongolica (for biomass calculation) after 3 years of CO2 treatment were from Zheng et al. (2010) and Zhou et al. (2010) respectively
Plant photosynthetic rates, leaf C and N concentrations and isotopic compositions
Plant photosynthetic rates generally decreased with time during the growing season under both treatments (Fig. 2). Elevated CO2 (7.2 ± 1.1 μmol CO2 m−2 s−1) increased mean plant photosynthetic rates by 34.9% compared with ambient CO2 (5.3 ± 0.9 μmol CO2 m−2 s−1) over the growing season (p = 0.05).
Plant photosynthetic rates of Quercus mongolica under ambient and elevated CO2 during the growing season of 2014. Values are mean ± standard error (n = 3). “Treat” represents ambient and elevated CO2 treatments, “Time” represents sampling time in repeated measures ANOVA (RM-ANOVA), F represents F ratios and df represents degree of freedom (the same below)
Elevated CO2 did not significantly change leaf C concentration (Fig. 3a). Leaf δ13C was significantly lower under elevated CO2 (p = 0.01), and decreased over the growing season from May to October under both treatments (Fig. 3c). Leaf N concentration gradually decreased from May to October under both treatments, and the mean leaf N concentration tended to decrease under elevated CO2 throughout the growing season (p = 0.06, Fig. 3b). Especially from May to August, a significant decrease in leaf N concentration was found under elevated CO2 (p < 0.05). Leaf δ15N gradually increased from May to July and declined thereafter under both treatments, and elevated CO2 significantly increased leaf δ15N during the growing season (p = 0.02, Fig. 3d).
Soil NH4 +, NO3 −, DON and DOC concentrations and isotopic compositions
Mean soil NH4+-N concentration (5.6 ± 0.2 mg N kg−1 dry soil) was 4.7 times higher than mean soil NO3−-N concentration (1.2 ± 0.1 mg N kg−1 dry soil) under both treatments throughout the growing season (Fig. 4a, c). Elevated CO2 did not change mean soil NO3− or NH4+ concentration throughout the growing season. However, elevated CO2 increased soil NO3− on June 26 and October 5, 10 and 15 (p < 0.05), and increased soil NH4+ in the early and late growing season (p < 0.05). Soil NH4+ concentration dropped from early to mid growing season, and remained constant thereafter. Neither δ15N of soil NO3− nor δ15N of NH4+ was different between treatments, and δ15N of soil NO3− was significantly lower than that of NH4+ (p < 0.05, Fig. S2). The δ15N of NH4+ was positively correlated with leaf δ15N (p = 0.04), but no relationship was found between δ15N of soil NO3− and leaf δ15N (Fig. S3a and c).
On average, elevated CO2 significantly increased soil DON (19.9 ± 0.7 mg N kg−1 dry soil) by 19.8% compared with ambient CO2 (16.6 ± 0.5 mg N kg−1 dry soil) during the growing season (p = 0.02, Fig. 4b). Mean increase of soil DOC under elevated CO2 (64.4 ± 2.0 mg C kg−1 dry soil) was 17.1% compared with that under ambient CO2 (55.0 ± 1.4 mg C kg−1 dry soil) (p = 0.01, Fig. 4d), and soil DOC generally decreased with time during the growing season under both treatments. When data under both treatments were combined, a significant positive correlation between photosynthetic rate and soil DOC was found (p < 0.01, Fig. S4).
Soil microbial biomass C and N and isotopic compositions
During the growing season, soil microbial N initially decreased and then increased from August 28 to October 15 under both treatments (Fig. 5a). Elevated CO2 did not change mean microbial N throughout the growing season. However, a significant increase was found on August 20 and September 5, 15 and 25 under elevated CO2 (p < 0.05). On average, elevated CO2 (210 ± 8 mg C kg−1 dry soil) significantly increased microbial C by 24.2% compared with ambient CO2 (169 ± 8 mg C kg−1 dry soil) over the growing season (p < 0.01, Fig. 5b). When data under both treatments were combined, microbial C was positively correlated with soil NH4+, NO3−, DOC and DON, and microbial N was positively correlated with soil NH4+ and DOC (Table S1).
Mean microbial δ15N was significantly higher under elevated CO2 (8.6 ± 0.3‰) than under ambient CO2 (7.0 ± 0.4‰) during the growing season (p = 0.02, Fig. 5c). Microbial δ15N was significantly higher than δ15N of soil NH4+ and NO3− (p < 0.05, Fig. S2), and was negatively correlated with δ15N of soil NH4+ (p = 0.03) (Fig. S3b). But no relationship between microbial δ15N and δ15N of soil NO3− was found (Fig. S3d). Microbial δ13C was not significantly changed under elevated CO2 throughout the growing season (Fig. 5d).
N transformation rates
Elevated CO2 did not affect mean net N transformation rates throughout the growing season, while significant interaction effect between treatment and time was found (Fig. 6). Net N mineralization and ammonification rates significantly increased in April and May, and significantly decreased in June, July and August under elevated CO2 (p < 0.05, Fig. 6a, b). Elevated CO2 significantly decreased net nitrification in April and May, but had no effect from June to September (Fig. 6c).
The lab assay results showed that potential gross N mineralization and nitrification increased by 41.4 and 237.5% respectively under elevated CO2 (Table 4). Elevated CO2 significantly increased NH4+ and NO3− immobilization by 82.6 and 68.8% respectively (p < 0.05). NH4+ immobilization was much higher than NO3− immobilization under both treatments. The \({\text{I}}_{{{\text{NH}}_{ 4}^{ + } }} /{\text{I}}_{{{\text{NO}}_{ 3}^{ - } }}\) (the ratio of gross NH4+ immobilization to gross NO3− immobilization) was higher under elevated CO2 (35.1) compared with that under ambient CO2 (32.4) (p < 0.05). The re-mineralization rate was 149% higher under elevated CO2 compared with that under ambient CO2 (p = 0.03).
Discussion
Effects of elevated CO2 on soil N cycling
Consistent with our hypothesis that elevated CO2 would accelerate soil N cycling, and we found elevated CO2 increased gross N mineralization by 41.1% and nitrification by 237.5%. We believe the increased gross N mineralization was mainly attributable to increased microbial biomass C under elevated CO2 (Fig. 5b) because mineralization process is an aggregated process driven by most microbes (Schimel and Bennett 2004). The significant relationship between microbial C and products of N mineralization (DON and NH4+) (Table S1) also indicated that the increased microbial biomass stimulated N mineralization under elevated CO2. Although previous studies demonstrated that increased soil moisture enhanced soil N mineralization under elevated CO2 (Hungate et al. 1997a; Dijkstra et al. 2008b), this was not the case in our research because soil moisture was not different between the treatments (Fig. S1). Earlier studies in temperate forest ecosystems suggested that increased labile C inputs via rhizodeposition under elevated CO2 enhanced microbial biomass and activity, which may cause a priming effect on SOM decomposition and N mineralization (Drake et al. 2011; Phillips et al. 2011; Zak et al. 2011). In our study, the increased photosynthesis, root biomass and soil DOC, combined with the significantly positive correlation between photosynthetic rate and soil DOC implied potential higher labile C inputs into the soil under elevated CO2 compared with under ambient CO2. In addition, elevated CO2 was found to increase fine root decomposition due to increased soil microbial biomass and enzyme activities in our study site (Li et al. 2010). Therefore, there also might be a priming effect on SOM decomposition and N mineralization induced by increased soil labile C inputs in our study (Li et al. 2010; Drake et al. 2011; Phillips et al. 2011). Although our result was contrary to other previous studies (Berntson and Bazzaz 1997; Hungate et al. 1999; Richter et al. 2003), which found decreased gross N mineralization under elevated CO2, the underlying mechanism was consistent between our results and those studies. They found elevated CO2 increased C partitioning to N2-fixing microbes and therefore decreased C partitioning to other soil microbes, resulting in unchanged microbial biomass (Hungate et al. 1999; Richter et al. 2003). Elevated CO2 decreased gross N mineralization in their studies (Berntson and Bazzaz 1997; Hungate et al. 1999; Richter et al. 2003), because of decreased litter quality (higher plant C/N) and the unchanged microbial activities. Therefore, the responses of belowground C inputs, microbial biomass and activity are central to the response of gross N mineralization under elevated CO2.
N mineralization can induce 15N enrichment in microbes and 15N depletion in mineralization products (Collins et al. 2008), and microbial 15N abundance is generally higher when N mineralization is higher (Dijkstra et al. 2006, 2008a). In the present study, higher microbial δ15N compared with δ15N of mineralization products (e.g. NH4+ and NO3−) indicated that N mineralization induced 15N enrichment in microbes. Thus, the higher microbial δ15N under elevated CO2 was consistent with the higher gross N mineralization under elevated CO2 compared with under ambient CO2. An indicator for the occurrence of progressive N limitation is the decrease in gross N mineralization (Luo et al. 2004). Our finding of increased potential soil gross N mineralization suggested that to date progressive N limitation has not occurred in our studied system.
We found elevated CO2 increased gross nitrification, which was likely due to increased gross N mineralization and the higher NH4+ availability (Fig. 4c), because the change in nitrification is strongly dependent on N mineralization (Booth et al. 2005). Nitrification is a critical process for long-term ecosystem N availability (Schimel and Bennett 2004) and its increase is beneficial to those plant species which preferentially use NO3− as their N source. Our previous study found oak in the study area prefers NO3− to NH4+ (Liu et al. 2017). Therefore, the increased nitrification under elevated CO2 could benefit its growth. Our result is consistent with Hungate et al. (1997a) which also indicated increased C supply and N mineralization under elevated CO2 were beneficial to nitrifiers and nitrification process in two different annual grassland ecosystems. However, some studies found decreased gross nitrification under elevated CO2 (Müller et al. 2009; Rütting et al. 2010), because of enhanced NH4+ immobilization and decreased NH4+ availability for nitrification (Rütting et al. 2010; Björsne et al. 2014). In our study, although NH4+ immobilization increased under elevated CO2, NH4+ availability did not decrease, even increased sometimes during the growing season due to the enhanced gross N mineralization.
The observed increase in the gross NH4+ and NO3− immobilization was consistent with increased microbial biomass and gross N mineralization under elevated CO2, which illustrated the higher microbial N demand under elevated CO2. The higher microbial biomass C and N corresponded to the higher soil respiration under elevated CO2 as reported in our previous study (Fig. 7, Sun et al. 2017). Many previous studies found higher gross N immobilization under elevated CO2 due to increased microbial biomass (Hungate et al. 1997b, 1999; de Graaff et al. 2006; van Groenigen et al. 2006; Müller et al. 2009; Rütting et al. 2010; Björsne et al. 2014). However, the enhanced competition between plants and microbes for available N may induce unchanged, or even decreased microbial N immobilization under elevated CO2 (Hungate et al. 1997a; Hu et al. 2006) because plants are found to out-compete microbes for the available N (Schimel and Bennett 2004; Hu et al. 2006). We found microbial N did not change during the early growing season (from June to mid August) under elevated CO2 when plants grew fast, while it increased during the late growing season, when plants N demand decreased. Therefore, the impact of elevated CO2 on microbial immobilization is not constant across different systems, or even is not constant in the same system at different times, and we should carefully consider the specific circumstances, especially the response of microbial N under elevated CO2.
Summary scheme showing the responses of ecosystem C and N pools and processes to consecutive 10 years of elevated CO2 in our studied oak dominated system. Dashed line represents C or N cycling processes. min mineralization, nit nitrification, immo immobilization. ↑, ↓ and ↔ refer to increases, decreases and no change under elevated CO2 respectively. Red color indicates significant difference between treatments, and black color indicates no significantly statistical discrepancy between treatments. “?” was marked because the processes were not evaluated. Data of soil respiration and N2O flux were from Sun et al. (2017). (Color figure online)
We found elevated CO2 increased re-mineralization of microbial N by 149%, which even exceeded the rate of N supply derived from gross N mineralization. It is not the size of the readily available N pool but rather the speed of replenishment of the N pool, which determines the availability of N for plants and microbes in N limited ecosystems (Thornley and Cannell 2000). Although the higher N immobilization occurred under elevated CO2, the faster microbial N turnover decreased the N limitation for plants and microbes. Microbial N re-mineralization was found to be higher when environmental conditions promote high microbial growth rates (e.g. increased higher quality C availability) (Bengtson and Bengtsson 2005). We believe the higher soil DOC under elevated CO2 suggested higher quality of soil C for microbes, which mainly contributed to the increased microbial N re-mineralization.
NH4+ was the dominating inorganic N form at our research site, which was 4.8 times higher than NO3− concentration on average under both treatments. The significant relationship between δ15N of soil NH4+ and plant/microbial δ15N, and no relationship between δ15N of soil NO3− and plant/microbial δ15N, further indicated the importance of NH4+ for ecosystem N demands. The increased plant N stock and \({\text{I}}_{{{\text{NH}}_{ 4}^{ + } }} /{\text{I}}_{{{\text{NO}}_{ 3}^{ - } }}\) under elevated CO2 implied increased plant and microbial NH4+ demand. Thus, although elevated CO2 increased gross N mineralization, the seasonal mean soil NH4+ did not change. The concentration of soil NH4+ was mainly controlled by the NH4+ production and consumption, and these processes are complex and changeable, which induced various responses of soil NH4+ to elevated CO2 in previous studies, including no change (Arnone and Bohlen 1998; Niklaus et al. 1998; Johnson et al. 2001; Niklaus et al. 2001; Carrillo et al. 2012; Schleppi et al. 2012; Liang et al. 2016), decrease (Berntson and Bazzaz 1997; Barnard et al. 2004) or increase (Barnard et al. 2004; Carrillo et al. 2012; Liang et al. 2016). In our study, because both the production (gross N mineralization and re-mineralization) and the consumption (microbial immobilization and plant uptake) of soil NH4+ increased, the unchanged soil NH4+ concentration was reasonable. Similarly, the interaction of different NO3− production and consumption processes may cause the unchanged seasonal mean soil NO3− in our study. The seasonal variations of the effects of elevated CO2 on the concentrations of soil NH4+ and NO3− also suggested that the effects were not constant and were determined by various N cycling processes.
Implications for our understanding of progressive N limitation
After 10 years of elevated CO2 treatment, the biomass and C and N stocks of Q. mongolica were significantly higher than those under control treatment, which indicated that elevated CO2 stimulated oak growth and enhanced plant C and N sequestration. It is hypothesized that after the initial increase of plant N sequestration, the stimulation of biomass would decline or disappear within a few years in strongly N limited systems due to decreased soil available N supply (Luo et al. 2004). However, we found soil inorganic N generally did not change, or even increased sometimes under elevated CO2. Combined with increased plant photosynthesis, these results suggested that to date progressive N limitation has not happened in our studied system. This was because increased gross N mineralization and hastened microbial N turnover under elevated CO2, providing additional available N for plants growth. However, this still did not prevent the increase in plant C/N under elevated CO2, hinting the potential limitation of N for plant growth. The increase in gross N mineralization does not provide new N inputs into the system and would cause the decrease in organic N. Although the pool size of organic N is large, the mechanism of supplying the plant N demand still may not be sustainable over the long term.
Indeed, our results showed that the mean annual increase rate of oak biomass, C and N stocks was lower after 10 years of treatment compared with that after 3 years of treatment (p < 0.05). Moreover, the enhancement of litter C/N under elevated CO2 increased after treatment for 10 years compared with that after treatment for 3 years. Collectively, these results indicated that soil N have constrained CO2 fertilization effect with increasing duration of treatment. The lower leaf N concentration under elevated CO2 compared with under ambient CO2 also implied the tendency of N deficiency under elevated CO2. Although some studies suggested that increased plant N use efficiency can decrease leaf N demand under elevated CO2, leading to lower leaf N concentration (Long et al. 2004; Ainsworth and Long 2005). This may not be the case for our study because our study area is typically N limited, which induced a gradual decrease in leaf N concentration from May to August, suggesting insufficient soil N supply. In N limited ecosystems, the higher temperate forest productivity under elevated CO2 was supported by increased plant N uptake rather than N use efficiency (Finzi et al. 2007). Indeed, elevated CO2 increased soil NH4+ and NO3− during the early and late growing seasons, but had no effect during the mid growing season when plant N demand was highest, which suggested increased plant N uptake under elevated CO2. Additionally, the lower net N mineralization during the mid growing season indicated higher microbial N demand under elevated CO2. Thus, the intensified competition between plants and microbes for available N under elevated CO2 decreased plant N acquisition. This was in line with Feng et al. (2015) who found that lower plant N concentration under elevated CO2 was due to deceased N acquisition, rather than decreased N demand. Together, these results suggested that although gross N mineralization and re-mineralization were accelerated under elevated CO2, both increase in plant and microbial N demand during the period of fast plant growth caused insufficient soil N supply, which retarded the pace of enhancement of plant productivity.
Conclusions
In the Quercus mongolica dominated system, elevated CO2 increased plant biomass, C and N stocks and C/N by 26.4, 26.2, 16.5 and 8.6% respectively, indicating increased ecosystem C and N sequestration, and plant N demand. Consistent with our hypothesis, elevated CO2 increased photosynthesis and microbial biomass, which accelerated soil N cycling and supplied additional N for plant growth. Thus, progressive N limitation for plant growth has not happened in this oak dominated system after 10 years of elevated CO2 treatment, but the annual and seasonal dynamics of ecosystem N status indicated that gradual N deficiency may be occurring.
References
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165(2):351–372
Arnone JA, Bohlen PJ (1998) Stimulated N2O flux from intact grassland monoliths after two growing seasons under elevated atmospheric CO2. Oecologia 116(3):331–335
Barnard R, Barthes L, Le Rous X, Leadley PW (2004) Dynamics of nitrifying activities, denitrifying activities and nitrogen in grassland mesocosms as altered by elevated CO2. New Phytol 162(2):365–376
Barnard R, Barthes L, Leadley PW (2006) Short-term uptake of 15N by a grass and soil micro-organisms after long-term exposure to elevated CO2. Plant Soil 280(1–2):91–99
Bengtson P, Bengtsson G (2005) Bacterial immobilization and remineralization of N at different growth rates and N concentrations. FEMS Microbiol Ecol 54(1):13–19
Berntson GM, Bazzaz FA (1997) Regenerating temperate forest mesocosms in elevated CO2: belowground growth and nitrogen cycling. Oecologia 113(1):115–125
Björsne AK, Rütting T, Ambus P (2014) Combined climate factors alleviate changes in gross soil nitrogen dynamics in heathlands. Biogeochemistry 120(1–3):191–201
Booth MS, Stark JM, Rastetter E (2005) Controls on nitrogen cycling in terrestrial ecosystems: a synthetic analysis of literature data. Ecol Monogr 75(2):139–157
Brookes PC, Kragt JF, Powlson DS, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: the effects of fumigation time and temperature. Soil Biol Biochem 17(6):831–835
Carrillo Y, Dijkstra FA, Pendall E, Morgan JA, Blumenthal DM (2012) Controls over soil nitrogen pools in a semiarid grassland under elevated CO2 and warming. Ecosystems 15(5):761–774
Chapin FS III, Matson PA, Mooney HA (2002) Principles of terrestrial ecosystem ecology. Springer, New York
Collins JG, Dijkstra P, Hart SC, Hungate BA, Flood NM, Schwartz E (2008) Nitrogen source influences natural abundance 15N of Escherichia coli. FEMS Microbiol Lett 282(2):246–250
De Graaff MA, van Groenigen KJ, Six J, Hungate BA, van Kessel C (2006) Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Global Change Biol 12(11):2077–2091
Dijkstra P, Menyailo OV, Doucett RR, Hart SC, Schwartz E, Hungate BA (2006) C and N availability affects the 15N natural abundance of the soil microbial biomass across a cattle manure gradient. Eur J Soil Sci 57(4):468–475
Dijkstra P, LaViolette CM, Coyle JS, Doucett RR, Schwartz E, Hart SC, Hungate BA (2008a) 15N enrichment as an integrator of the effects of C and N on microbial metabolism and ecosystem function. Ecol Lett 11(4):389–397
Dijkstra FA, Pendall E, Mosier AR, King JY, Milchunas DG, Morgan JA (2008b) Long-term enhancement of N availability and plant growth under elevated CO2 in a semi-arid grassland. Funct Ecol 22(6):975–982
Drake JE, Gallet-Budynek A, Hofmockel KS, Bernhardt ES, Billings SA, Jackson RB, Johnsen KS, Lichter J, McCarthy HR, McCormack ML, Moore DJP, Oren R, Palmroth S, Phillips RP, Pippen JS, Pritchard SG, Treseder KK, Schlesinger WH, DeLucia EH, Finzi AC (2011) Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecol Lett 14(4):349–357
Feng ZZ, Rütting T, Pleijel H, Wallin G, Reich PB, Kammann CI, Newton PCD, Kobayashi K, Luo YJ, Uddling J (2015) Constraints to nitrogen acquisition of terrestrial plants under elevated CO2. Glob Change Biol 21(8):3152–3168
Finzi AC, Norby RJ, Calfapietra C, Gallet-Budynek A, Gielen B, Holmes WE, Hoosbeek MR, Iversen CM, Jackson RB, Kubiske ME, Ledford J, Liberloo M, Oren R, Polle A, Pritchard S, Zak DR, Schlesinger WH, Ceulemans R (2007) Increases in nitrogen uptake rather than nitrogen-use efficiency support higher rates of temperate forest productivity under elevated CO2. Proc Natl Acad Sci USA 104(35):14014–14019
Gee GM, Bauder JW (1986) Physical and mineralogical methods. Methods of soil analysis. American Society of Agronomy, Madison, pp 383–411
Hikosaka K, Terashima I, Katoh S (1994) Effects of leaf age, nitrogen nutrition and photon flux density on the distribution of nitrogen among leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading of leaves. Oecologia 97(4):451–457
Hu S, Tu C, Chen X, Gruver JB (2006) Progressive N limitation of plant response to elevated CO2: a microbiological perspective. Plant Soil 289(1–2):47–58
Hungate BA, Chapin FS III, Zhong H, Holland EA, Field CB (1997a) Stimulation of grassland nitrogen cycling under carbon dioxide enrichment. Oecologia 109(1):149–153
Hungate BA, Lund CP, Pearson HL, Chapin FS III (1997b) Elevated CO2 and nutrient addition alter soil N cycling and N trace gas fluxes with early season wet-up in a California annual grassland. Biogeochemistry 37(2):89–109
Hungate BA, Dijkstra P, Johnson DW, Hinkle CR, Drake BG (1999) Elevated CO2 increases nitrogen fixation and decreases soil nitrogen mineralization in Florida scrub oak. Glob Change Biol 5(7):781–789
Huo CF, You WZ, Zhang HD, Yan TW, Wei WJ, Zhao G, Guo JS, Xing ZK (2011) Biomass and net primary productivity of Quecus mongolica plantation in Binglashan Mountains in Liaoning Province. J Liaoning For Sci Technol 4:4–11
IPCC (2013) Climate Change 2013: The physical science basis: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 465–470
Iversen CM, Hooker TD, Classen AT, Norby RJ (2011) Net mineralization of N at deeper soil depths as a potential mechanism for sustained forest production under elevated CO2. Glob Change Biol 17(2):1130–1139
Jaeger CH III, Monson RK, Fisk MC, Schmidt SK (1999) Seasonal partitioning of nitrogen and soil microorganisms in an alpine ecosystem. Ecology 80(6):1883–1891
Johnson DW, Hungate BA, Dijkstra P, Hymus G, Drake B (2001) Effects of elevated carbon dioxide on soils in a Florida scrub oak ecosystem. J Environ Qual 30(2):501–507
Leuzinger S, Luo YQ, Beier C, Dieleman W, Vicca S, Körner C (2011) Do global change experiments overestimate impacts on terrestrial ecosystems? Trends Ecol Evol 26(5):236–241
Li XF, Han SJ, Guo ZL, Shao DK, Xin LH (2010) Changes in soil microbial biomass carbon and enzyme activities under elevated CO2 affect fine root decomposition processes in a Mongolian oak ecosystem. Soil Biol Biochem 42(7):1101–1107
Liang JY, Qi X, Souza L, Luo YQ (2016) Processes regulating progressive nitrogen limitation under elevated carbon dioxide: a meta-analysis. Biogeosciences 13(9):2689–2699
Liu J, Peng B, Xia ZW, Sun JF, Gao DC, Dai WW, Jiang P, Bai E (2017) Different fates of deposited NH4 + and NO3 − in a temperate forest in northeast China: a 15N tracer study. Glob Change Biol 23(6):2441–2449
Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide: plants face the future. Annu Rev Plant Biol 55:591–628
Lu Y, Chen YM, Cao Y, Song C (2015) C, N and P stoichiometric characteristics of plants and soil in Quercus liaotungensis forest on Ziwuling mountain of Loess Plateau. J Soil Water Conserv 29(3):196–201
Luo YQ, Su B, Currie WS, Dukes JS, Finzi A, Hartwig U, Hungate BA, McMurtrie RE, Oren R, Parton WJ, Pataki DE, Rebecca Shaw M, Zak DR, Field CB (2004) Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 54(8):731–739
Luo YQ, Hui DF, Zhang DQ (2006) Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: a meta-analysis. Ecology 87(1):53–63
Mary B, Recous S, Robin D (1998) A model for calculating nitrogen fluxes in soil using 15N tracing. Soil Biol Biochem 30(14):1963–1979
McKinley DC, Romero JC, Hungate BA, Drake BG, Megonigal JP (2009) Does deep soil N availability sustain long-term ecosystem responses to elevated CO2? Glob Change Biol 15(8):2035–2048
Müller C, Rütting T, Abbasi MK, Laughlin RJ, Kammann C, Clough TJ, Sherlock RR, Kattge J, Jäger HJ, Watson CJ, Stevens RJ (2009) Effect of elevated CO2 on soil N dynamics in a temperate grassland soil. Soil Biol Biochem 41(9):1996–2001
Niklaus PA, Leadley PW, Stöcklin J, Körner C (1998) Nutrient relations in calcareous grassland under elevated CO2. Oecologia 116(1):67–75
Niklaus PA, Kandeler E, Leadley PW, Schmid B, Tscherko D, Körner C (2001) A link between plant diversity, elevated CO2 and soil nitrate. Oecologia 127(4):540–548
Phillips RP, Finzi AC, Bernhardt ES (2011) Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol Lett 14(2):187–194
Raison RJ, Connell MJ, Khanna PK (1987) Methodology for studying fluxes of soil mineral N in situ. Soil Biol Biochem 19(5):521–530
Reich PB, Hobbie SE, Lee T, Ellsworth DS, West JB, Tilman D, Knops JMH, Naeem S, Trost J (2006) Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440(7086):922–925
Richter M, Hartwig UA, Frossard E, Nösberger J, Cadisch G (2003) Gross fluxes of nitrogen in grassland soil exposed to elevated atmospheric CO2 for seven years. Soil Biol Biochem 35(10):1325–1335
Rütting T, Andresen LC (2015) Nitrogen cycle responses to elevated CO2 depend on ecosystem nutrient status. Nutr Cycl Agroecosyst 101(3):285–294
Rütting T, Clough TJ, Müller C, Lieffering M, Newton PCD (2010) Ten years of elevated atmospheric carbon dioxide alters soil nitrogen transformations in a sheep-grazed pasture. Glob Change Biol 16(9):2530–2542
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85(3):591–602
Schleppi P, Bucher-Wallin I, Hagedorn F, Körner C (2012) Increased nitrate availability in the soil of a mixed mature temperate forest subjected to elevated CO2 concentration (canopy FACE). Glob Change Biol 18(2):757–768
Schneider MK, Lüscher A, Richter M, Aeschlimann U, Hartwig U, Blum H, Frossard E, Nösberger J (2004) Ten years of free-air CO2 enrichment altered the mobilization of N from soil. Glob Change Biol 10(8):1377–1388
Sebilo M, Mayer B, Grably M, Billiou D, Mariotti A (2004) The use of the ‘ammonium diffusion’ method for δ15N-NH4 + and δ15N-NO3 − measurements: comparison with other techniques. Environ Chem 1(2):99–103
Sun JF, Peng B, Li W, Qu GF, Dai WW, Dai GH, Jiang P, Han SJ, Bai E (2016) Effects of nitrogen addition on potential soil nitrogen cycling processes in a temperate forest ecosystem. Soil Sci 181(1):29–38
Sun JF, Xia ZW, He TX, Dai WW, Peng B, Liu J, Gao DC, Jiang P, Han SJ, Bai E (2017) Ten years of elevated CO2 affects soil greenhouse gas fluxes in an open top chamber experiment. Plant Soil 420(1–2):435–450
Thornley JHM, Cannell MGR (2000) Dynamics of mineral N availability in grassland ecosystems under increased CO2: hypotheses evaluated using the Hurley Pasture Model. Plant Soil 224(1):153–170
Van Groenigen KJ, de Graaff MA, Six J, Harris D, Kuikman P, van Kessel C (2006) The impact of elevated atmospheric CO2 on soil C and N dynamics: a meta-analysis. Managed ecosystems and CO2 case studies, processes and perspectives. Springer, Berlin, pp 373–392
Vance ED, Brookes PC, Jenkinson DS (1987) Microbial biomass measurements in forest soils: determination of kC values and tests of hypotheses to explain the failure of the chloroform fumigation-incubation method in acid soils. Soil Biol Biochem 19(6):689–696
Williams MA, Rice CW, Owensby CE (2000) Carbon dynamics and microbial activity in tallgrass prairie exposed to elevated CO2 for 8 years. Plant Soil 227(1–2):127–137
Xia JY, Wan SQ (2008) Global response patterns of terrestrial plant species to nitrogen addition. New Phytol 179(2):428–439
Yoccoz NG (1991) Use, overuse, and misuse of significance tests in evolutionary biology and ecology. Bull Ecol Soc Am 72(2):106–111
Zak DR, Pregitzer KS, Curtis PS, Teeri JA, Fogel R, Randlett DL (1993) Elevated atmospheric CO2 and feedback between carbon and nitrogen cycles. Plant Soil 151(1):105–117
Zak DR, Pregitzer KS, Kubiske ME, Burton AJ (2011) Forest productivity under elevated CO2 and O3: positive feedbacks to soil N cycling sustain decade-long net primary productivity enhancement by CO2. Ecol Lett 14(12):1220–1226
Zheng JQ, Han SJ, Wang Y, Zhang CG, Li MH (2010) Composition and function of microbial communities during the early decomposition stages of foliar litter exposed to elevated CO2 concentrations. Eur J Soil Sci 61(6):914–925
Zhou YM, Li MH, Cheng XB, Wang CG, Fan AN, Shi LX, Wang XX, Han SJ (2010) Soil respiration in relation to photosynthesis of Quercus mongolica trees at elevated CO2. PLoS ONE 5(12):e15134
Acknowledgements
This study was supported by National Key R&D Program of China (2016YFA0600804), the National Natural Science Foundation of China (31522010), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB15010301), and the Key Research Program of Frontier Sciences, CAS (QYZDB-SSWDQC006). We are very grateful to Dr. B. Mary for kindly providing the FLUAZ model, and Lufu Zhao for maintaining the OTC facility.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Stephen Porder.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, J., Dai, W., Peng, B. et al. Does the accelerated soil N cycling sustain N demand of Quercus mongolica after decade-long elevated CO2 treatment?. Biogeochemistry 139, 197–213 (2018). https://doi.org/10.1007/s10533-018-0463-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-018-0463-9