Abstract
Background
The production and consumption of greenhouse gases (GHGs) in soils are largely regulated by biological processes. Increasing atmospheric CO2 may alter these processes, thereby affecting GHG emissions and their feedbacks to climate.
Methods and aims
Here, we used an open top chamber (OTC) experiment to examine the effects of elevated CO2 for ten years on soil GHG fluxes in a Quercus mongolica dominated system in northeastern China.
Results
Our results showed that elevated CO2 increased soil CO2 emissions, consistent with increased microbial biomass and the abundance of arbuscular mycorrhizal fungi and actinomycetes. Additionally, elevated CO2 increased CH4 uptake due to stimulated growth of methanotrophs. The seasonal mean soil N2O flux was not changed by elevated CO2, consistent with unchanged ammonia oxidizing bacteria, archaea and denitrifiers, which was probably due to large variations between the individual OTCs and with time. However, seasonal cumulative soil N2O emissions increased by 64.7% under elevated CO2. Our results also hinted that nitrification by ammonia oxidizing archaea was the major process of soil N2O emissions.
Conclusions
In our study elevated CO2 increased soil GHG emissions and the cumulative global warming potential by 27.8%, causing an important positive feedback to climate change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atmospheric concentrations of the greenhouse gases (GHGs) carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O) have reached 391 ppm, 1803 ppb, and 324 ppb in 2011, which exceeded the pre-industrial levels by about 40%, 150%, and 20%, respectively (IPCC 2013). CO2 is by far the most abundant GHG in the atmosphere, while CH4 and N2O are powerful GHGs which are 25 and 298 times more potent than CO2 over a 100 year lifespan (Forster et al. 2007).
Terrestrial ecosystems are large sources and sinks of GHGs. Increasing atmospheric CO2 may alter the direction and strength of GHG fluxes in terrestrial ecosystems, which in turn could feedback to our climate. Elevated atmospheric CO2 concentrations could increase plant photosynthesis (Ainsworth and Long 2005) and water use efficiency (Jackson et al. 1994), and indirectly alter soil properties (e.g. soil carbon (C) and nitrogen (N) availability, soil moisture and oxygen content), all of which may affect soil GHG fluxes. Most previous studies found that elevated CO2 generally stimulated soil CO2 emissions in different ecosystems, including grasslands (Luo et al. 1996; Zak et al. 2000; Reich et al. 2001), forests (Zak et al. 2000; Deng et al. 2010) and croplands (Smith et al. 2010). However, the effects of elevated CO2 on soil-atmosphere exchange of CH4 and N2O varied widely among different ecosystems (Phillips et al. 2001; Dijkstra et al. 2010; van Groenigen et al. 2011; Dijkstra et al. 2013) and have not reached a consensus.
The source of soil CO2 emissions is mainly from soil respiration, which includes autotrophic respiration (mainly respiration of plant roots) and heterotrophic microbial respiration. Three potential reasons of increased soil CO2 emissions under elevated CO2 have recently been explored. First, increased belowground C allocation under elevated CO2 could stimulate plant root growth and increase autotrophic respiration (Zak et al. 2000; Deng et al. 2010; Zhou et al. 2010). Second, elevated CO2 could increase root exudation and cause more labile C inputs into the rhizosphere (Zak et al. 1993), resulting in a priming effect on soil organic matter (SOM) decomposition and thereby an increase of heterotrophic respiration (Zak et al. 1993; Cheng 1999). Third, increased plant residues under elevated CO2 could also stimulate heterotrophic respiration (Zak et al. 1996; Deng et al. 2010). Additionally, elevated CO2 may increase plant water use efficiency (Jackson et al. 1994), which could induce higher soil moisture and benefit SOM decomposition (Hungate et al. 1997a; Dijkstra et al. 2008).
Soil net CH4 flux is the balance between CH4 production and uptake by soil microbes, and both processes are regulated by climatic and soil conditions. Elevated CO2 may influence soil CH4 flux by altering soil C, N availability and soil moisture (Dijkstra et al. 2010; Dijkstra et al. 2013). For soil CH4 uptake which occurs in aerobic soils via oxidation of CH4 by methanotrophs, previous studies found it could be stimulated by elevated CO2 due to increased labile C (Dijkstra et al. 2010). However, this stimulation may be counterbalanced by increased soil moisture under elevated CO2 which may limit CH4 and/or O2 diffusion (Ambus and Robertson 1999; Phillips et al. 2001). On the other hand, CH4 can be produced in anaerobic soils by methanogens during SOM decomposition. Increased soil moisture and labile C under elevated CO2 were beneficial to CH4 production (Dijkstra et al. 2012). Therefore, the net soil CH4 flux is the complex result of CH4 uptake and production and its responses to elevated CO2 still needs to be further explored.
Soil N2O is mainly produced by microbial processes such as nitrification, denitrification, and nitrifier denitrification (Zhu et al. 2013). Altered soil C, N availability and soil moisture under elevated CO2 could influence microbial activities and therefore soil N2O emissions. Increased labile C inputs under elevated CO2 were linked to increased denitrification rates, thereby increasing soil N2O emissions, in particular when soil N availability was high (Ineson et al. 1998; Kammann et al. 2008; Dijkstra et al. 2012). Increased soil moisture or soil respiration under elevated CO2 was also found to stimulate soil N2O emissions due to their beneficial effects on denitrification (Hungate et al. 1997a; Robinson and Conroy 1998; Kammann et al. 2008). However, increased N limitation under elevated CO2 could often offset those stimulation effects, resulting in unchanged N2O fluxes (Hungate et al. 1997b; Mosier et al. 2002; Dijkstra et al. 2013). Additionally, elevated CO2 may decrease nitrification due to increased NH4 + limitation for autotrophic nitrifiers growth, reducing nitrification-derived N2O flux (Müller et al. 2009; Rütting et al. 2010). Therefore, the effects of elevated CO2 on soil N2O emissions are regulated by many processes and factors, and are most likely system-specific.
The above introduction shows that microorganisms are key drivers of soil C and N cycling and play an important role in regulating terrestrial ecosystem GHG emissions (Singh et al. 2010). Thus, a robust prediction of future climate change requires mechanistic understanding of how elevated CO2 affects soil microbial activities and community composition (Carney et al. 2007; He et al. 2010). Generally, elevated CO2 increases soil C inputs and alters soil physico-chemical properties, which influences soil microbes and thereby the corresponding microbially regulated GHG processes. However, depending on the direction and magnitude of changes in soil C, N availability, soil moisture and oxygen content, and other soil properties under elevated CO2 (He et al. 2010; Garcia-Palacios et al. 2015), different responses of soil microbes to elevated CO2 were observed. For instance, elevated CO2 was found to stimulate the growth of fungi (Carney et al. 2007; Cheng et al. 2012; Lipson et al. 2014) and actinomycetes (Billings and Ziegler 2005; Drigo et al. 2010) and alter the microbial community composition (de Graaff et al. 2006; He et al. 2010). Contrary, in a Populus grandidentata dominated ecosystem, Zak et al. (1996) found unchanged microbial community composition under elevated CO2. In a temperate forest ecosystem, elevated CO2 stimulated the growth of ammonium oxidizing archaea (AOA), but had no effect on ammonium oxidizing bacteria (AOB), probably due to N limitations (Long et al. 2012). However, He et al. (2010) found elevated CO2 increased AOB, but decreased AOA in a grassland ecosystem. Recently, Bodelier and Steenbergh (2014) found that elevated CO2 enhanced competition between methanotrophs and nitrifiers for NH4 + which altered soil methane uptake and N cycling. These potential changes of soil microbes under elevated CO2 are important to understand the mechanisms underlying the responses of GHGs to the rising atmospheric CO2.
The aims of this study was to investigate the effect of ten consecutive years of elevated atmospheric CO2 concentrations on soil GHG fluxes from a Quercus mongolica (oak) dominated system and to explore factors affecting changes of soil GHG fluxes under elevated CO2. We hypothesized that (1) elevated CO2 increases soil CO2 emissions due to increased belowground C inputs, which stimulates microbial activity; (2) Elevated CO2 increases soil CH4 uptake due to increased labile C inputs and well-aerated soil conditions, which are beneficial to methanotrophs; (3) Elevated CO2 would not change soil N2O fluxes, because our research site is typically N limited.
Materials and methods
Site description
The open top chamber (OTC) experiment was established in Changbai Forest Ecosystem Research Station in Jilin province, Northeastern China (42°24′N, 128°06′E). The study area is characterized by a typically temperate climate, with a mean annual temperature of 3.6 °C and mean annual precipitation of 745 mm (Wang et al. 2009; Hu et al. 2010). Six hexagonal OTC experimental plots (4.2 m in diameter and 4 m in height originally, and due to the growth of oak, the height was increased to 6 m in 2010) were utilized for CO2 fumigation. The plots were arranged in a completely randomized design to lessen the effects of topographic variation and potential soil properties heterogeneity. Three chambers were dispensed with pure CO2 to elevate the CO2 concentration by 180 umol mol−1 above ambient, while the other three chambers were maintained at ambient CO2 concentration. Fans equipped in OTCs were used to increase air circulation. Infrared gas analyzers (A-SENSE-D, SenseAir, Delsbo, Sweden) placed in OTCs were used to monitor the CO2 concentration. A computerized control system recorded 10-s averages of CO2 concentration every 3 min, and periodically adjusted the flow of pure CO2 into the OTCs to maintain the elevated CO2 concentration. More details about the OTC can be found in Li et al. (2010).
Two year old oak seedlings were transplanted into these OTCs in the autumn of 2004. Twenty-two oak seedlings were planted in each OTC, and the seedlings came from the same nursery and had the same genotype (Li et al. 2010). The CO2 fumigation experiment started in April 2005, and was carried out for ten years until October 2014. Oaks were exposed to elevated CO2 during daytime in the growing season, from May to October every year.
The soil at our experimental site is a dark brown soil developed from volcanic ash (Albic Luvisol), approximately neutral (pH = 6.7), with a well-drained loamy sand texture (clay, silt and sand content were about 10.4%, 13.6% and 76.0%, respectively in control plots). The above soil properties were unchanged under elevated CO2 (Table S1).
Measurement of greenhouse gas fluxes
Soil CO2, CH4 and N2O fluxes were measured using the static chamber method (Dijkstra et al. 2013). One squared stainless steel base (0.3 m × 0.3 m × 0.05 m) was placed in the center of each OTC in order to reduce any edge effects of the CO2 fumigation system and inserted into soil 3 cm depth. The bases were installed into the soil approximately two months before the initial gas sampling to avoid disturbance on soil. A stainless steel chamber (0.3 m × 0.3 m × 0.3 m) was placed on the base every time gases were sampled, and the connection between the chamber and the base was sealed by water to prevent gas leakage. Gas samples (100 ml) were collected from each chamber every 20 min over a 1 h period, and stored in aluminum foil air bags (150 ml) for GHG measurements within 12 h using a gas chromatograph (HP 5890-II, Agilent, USA) equipped with FID and ECD detectors combined with Nickel converter. The carrier gas was high pure N2, and the standard gas concentrations were 0.362, 2.03 and 455 ppm for N2O, CH4 and CO2 respectively. The minimum detectable fluxes of CO2, CH4 and N2O were 10, 0.4 and 0.1 ppm respectively.
Gas samples were taken six times (5th, 10th, 15th, 20th, 25th, 30th) each month from June to October, in 2014. Samples were taken between 9:00 and 10:00 as this time period was shown to represent daily average GHGs fluxes best (Xu and Qi 2001; Alves et al. 2012; Dijkstra et al. 2013). Furthermore this time slot was verified on two measurement occasions of the diurnal dynamics of GHG fluxes at our research site (Table S2).
Air and soil temperatures at 10 cm depth were recorded in situ at each sampling event using a digital thermometer. CO2, CH4 and N2O fluxes were calculated as:
where F is the CO2, CH4 flux (mg or μg C m−2 h−1) or N2O flux (μg N m−2 h−1); ρ is the density of C in CO2 and CH4 (0.54 mg C cm−3) or the density of N in N2O (1.25 mg N cm−3) in the standard atmosphere environment; V is the volume of the static chamber (cm3); A is the base area of the static chamber (cm2); ∆c/∆t is the slope of the linear correlation between CO2 (ppm min−1), CH4 (ppb min−1) or N2O (ppb min−1) concentration and time; T is air temperature (°C); 0.6 is conversion coefficient due to the different unit between ∆c/∆t of CO2, CH4 or N2O and F.
CO2, CH4 and N2O fluxes measured on each sampling date were extrapolated to diurnal fluxes according to Eq. (2):
where Fdiurnal is the diurnal GHG flux; F9 is the GHG fluxes measured from 9:00 to 10:00 at each sampling time. The above calculated diurnal GHG fluxes were integrated into the growing season cumulative GHG emissions using Origin 8.5 software (Origin, OriginLab, USA). In details, using the time interval as X axis, and the diurnal flux as Y axis, we calculated the integral area as the cumulative GHG emissions during the time interval. The cumulative global warming potential (GWP) was calculated by summing the GWP of CO2, CH4 and N2O (in g CO2 eq. per m2). The GWP of CH4 was calculated by cumulative emissions of CH4 multiplied by 25 and the GWP of N2O was calculated by cumulative emissions of N2O multiplied by 298 (Dijkstra et al. 2013).
Measurement of soil NH4 + and NO3 − concentrations
Two soil subsamples (0–10 cm) were randomly taken close to the center of each OTC every time fluxes were measured. The two soil subsamples were fully mixed and sieved (< 2 mm) for analysis of soil NH4 +, NO3 −, dissolved organic C (DOC), dissolved organic N (DON), microbial C, N and community composition and the abundance of five specific functional bacteria genes (ammonia oxidizing bacteria-AOB amoA, ammonia oxidizing archaea-AOA amoA, methanotroph-pmoA, N2 fixing bacteria-nifH, denitrifer-nirS). NH4 + and NO3 − were extracted from 20 g soil with 100 ml of 2 M KCl, and filtered with ash-less filter papers (Qualitative Filter Paper, BH92410262) and measured with a Continuous Flow Analyzer (Bran-Luebbe Inc., Germany).
Measurement of soil microbial C, N and community composition
The chloroform fumigation method was used for microbial C and N analysis (Brookes et al. 1985). Un-fumigated and fumigated soils (20 g) were extracted with 100 ml of 0.05 M K2SO4, and the extract solutions were dried in a ventilated oven at 60 °C, and ground to fine powder and analyzed for C and N concentrations using an Elementar Vario EL Cube (Elementar Analysis system GmbH, Hanau, Germany) instrument interfaced to a PDZ Europa 20–20 isotope ratio mass spectrometer (Sercon Ltd., Cheshire, UK) at the Stable Isotope Faculty of University of California, Davis. Microbial C and N were calculated as:
where M(C) and M(N) are microbial C and N respectively; e and f represent before and after chloroform fumigation respectively. DOC and total dissolved N concentrations were obtained from the C and N concentrations in the K2SO4 extract before chloroform fumigation. DON was calculated by subtracting dissolved inorganic nitrogen from the total dissolved N. The extraction efficiency factors were 0.45 for microbial C (Vance et al. 1987) and 0.54 for microbial N (Brookes et al. 1985).
PLFA analysis
Phospholipids were extracted and fractionated from lyophilized soil (4 g) according to Wang et al. (2013), and identified by gas chromatography (Agilent 7890, Agilent technologies, USA) equipped with FID detector and the soil microbial identification system (MIDI Inc., Newark, DE). Methyl nonadecanoate (19:0, Sigma-Aldrich, St. Louis, MO, USA) was used as the internal standard for quantifying the PLFAs. The abundance of specific PLFAs was calculated according to Eq. (4). Terminally branched saturated PLFAs (i14:0, i15:0, a15:0, i16:0, i17:0 and a17:0), monounsaturated PLFAs (16:1 w7c, 16:1 w9c, 18:1 w5c, 18:1 w7c, cy 17:0 and cy 19:0), and the 14:0, 15:0, 16:0, 17:0, 18:0 were used as bacterial indicators (Zak et al. 1996; Moore-Kucera and Dick 2008; Wang et al. 2013); 16:1 w5c was used to represent arbuscular mycorrhizal fungi (AM fungi) (Olsson et al. 1995); 18:1 w9c and 18:2 w6c represent fungi (Zak et al. 1996). This interpretation requires some caution because 18:1 w9c can also exist in some bacteria (Frostegård et al. 2011). Nevertheless, 18:1 w9c is a relatively good indicator of fungi in forest soil (Frostegård et al. 2011); 10Me 16:0, 10Me 17:0 and 10Me 18:0 represent actinomycetes (Moore-Kucera and Dick 2008).
where CPLFAs (nmol g−1 dry soil) is specific microbial biomass; B is the characteristic peak area of specific PLFA; A is the characteristic peak area of 19:0; 40 ng ul−1 is the concentration of 19:0; 400 ul is the volume of the sample; MPLFAs (g mol−1) is PLFA molecular weight.
Soil DNA extraction
Soil DNA was extracted from 0.25 g lyophilized soil sample with MOBIO PowerSoil® DNA Isolation Kit (Carlsbad, USA) according to the manufacturer’s instructions. The extracted DNA which had high quality with the ratio of A260/A280 in the range of 1.78 to 1.85 was stored at −20 °C for quantitative real time PCR analysis.
Quantitative real time PCR
Quantitative PCR was conducted on a LightCycler 1.0 (Roche, Switzerland) and each measurement was performed in triplicates. Data analysis was carried out using the LightCycler software (version 5.23). The plasmids containing each target gene were extracted using Takara MiniBEST plasmid purification Kit (Takara), and quantified with a NANODROP 2000 UV-Vis Spectrophotometer (Thermo scientific, USA). Real time PCR was performed in 20 ul volumes which contained 10 ul SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) (Takara), 0.8 ul forward and reverse primers respectively (0.4 uM), 2 ul extracted DNA solution, and sterile water to fill up the volume to 20 μl. The target genes which were related to the processes producing or consuming soil N2O and CH4, including ammonia oxidizing bacteria (AOB amoA), ammonia oxidizing archaea (AOA aomA), denitrifier (nirS), N2 fixing bacteria (nifH) and methanotroph (pmoA), and the lengths of target genes, primer sets, nucleotide sequences of primers, annealing temperature and elongation time are described in Table 1. The thermal cycling conditions for the assays were: denaturation of DNA at 95 °C for 30 s, followed by 45 cycles of denaturation at 95 °C for 5 s, annealing at the specific temperature (see Table 1) for 30 s and elongation at 72 °C for 30 s or 60 s (Table 1). The fluorescence signals were measured once per cycle at the end of the elongation step. In order to verify no nonspecific and primer dimer amplification, a melting curve was obtained by gradually increasing the temperature from 65 °C to 95 °C. A sample was only considered positive if it exhibited a log-linear amplification in the fluorescence curve (r2 = 0.999) with an amplification efficiency at about 0.95 and a specific peak in the melting curve (between 85 and 92 °C).
Measurement of soil C and N concentrations, moisture, texture and pH
Soil organic C (SOC) and total N (TN) were measured using an Elementar Vario EL Cube (Elementar Analysis system GmbH, Hanau, Germany). Standard oven-drying method was carried out to measure soil gravimetric water content. Soil texture was determined by the pipette sedimentation method (Gee and Bauder 1986). Bulk density (BD) was determined by the core method (Burke et al. 1986). Soil pH was measured in a 1:5 (w/v) soil to water (CO2-free) ratio using a pH detector (E-201-C, Leici, China). Water filled pore space (WFPS) was calculated based on Eq. (5):
where PS is soil pore space and was calculated from Eq. (6) according to Parton et al. (2001).
Q10 was calculated using Eq. (8) according to Epron et al. (2001).
where R represents soil respiration; T is soil temperature; A is the basic soil respiration rate at soil temperature 0 °C; B represents the temperature sensitivity of soil respiration.
Statistical analyses
Repeated measures ANOVA was performed to examine the effects of elevated CO2 on soil properties, PLFAs, microbial C, N, functional bacteria genes and GHG fluxes during the growing season in 2014 using CO2 fumigation treatments as the between subject factor and sampling time as the within subject factor. One way ANOVA was used to identify elevated CO2 effects on cumulative GHG emissions and GWP. Principal components analysis (PCA) was used to analyze the effect of elevated CO2 on microbial community composition. Data were transformed to normalized variance across treatments before analysis when necessary. Repeated measures ANOVA was carried out with R 3.2.2 software, and other analyses were carried out using SPSS 17.0 software (SPSS, Chicago, IL, USA).
Results
Effects of elevated CO2 on greenhouse gas fluxes
Elevated CO2 significantly increased mean oak height and diameter at breast height (4.5 ± 0.1 m and 2.5 ± 0.1 cm, respectively) compared to that under ambient CO2 (4.2 ± 0.1 m and 2.2 ± 0.1 cm, respectively), and increased soil C/N, DON and DOC (p < 0.05), but had no effect on SOC, TN, NH4 +, NO3 −, soil temperature (10 cm in depth), soil moisture and WFPS (Table 2).
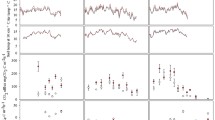
The seasonal mean soil CO2 flux under elevated CO2 was 78.7 ± 6.0 mg C m−2 h−1, and 61.7 ± 4.9 mg C m−2 h−1 under ambient CO2, meaning a significant increase rate of 27.5% by elevated CO2 (p = 0.04, Fig. 1a). During the growing season, seasonally cumulative CO2 emissions increased by 27.7% under elevated CO2 (250.4 ± 19.2 g C m−2) compared to that under ambient CO2 (196.0 ± 14.8 g C m−2) (p < 0.05, Fig. 2c). When data for both treatments were combined, the soil CO2 flux had significant positive correlations with soil temperature, WFPS, DOC, the photosynthetic rate (p < 0.01, Table S3, Fig. S3), and with the microbial C and the biomass of AM fungi and actinomycetes (p < 0.05, Fig. 3a. b and c). Q10 was higher under elevated CO2 (2.83) than under ambient CO2 (2.69) (Fig. S3a). Elevated CO2 significantly increased soil CO2 fluxes throughout the sampling days (p < 0.01, Fig. S2a, d) and diurnal cumulative soil CO2 emissions (p < 0.05, Fig. S2 g) during both diurnal measurements.
Soil CO2 (a), N2O (b) and CH4 (c) fluxes under ambient and elevated CO2 during the growing season in 2014. Negative values indicate soil uptake and positive values indicate soil emission. Values are mean ± standard error (n = 3). “CO2” represents ambient and elevated CO2 treatments, “Time” represents sampling time in repeated measures ANOVA (the same below)
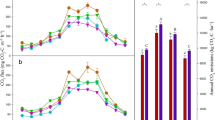
Cumulative CH4 (a), N2O (b) [in mg C or N m−2 (left y-axis) and in g CO2 eq. m−2 (right y-axis)], CO2 emissions (c) and their global warming potential (GWP) (d) under ambient and elevated CO2 during the growing season in 2014. Values are mean ± standard error (n = 3). Different letters above bars indicate significant differences between ambient and elevated CO2 treatments at p < 0.05 based on one way ANOVA
Relationships between soil CO2 flux and microbial biomass C (a) and the biomass of arbuscular mycorrhizal fungi (b) and actinomycetes (c), and relationship between soil CH4 flux and the abundance of methanotrophs pmoA (d), and relationships between soil N2O flux and the abundance of AOA amoA (e) and nirS (f). Each data point in the figures is the mean of the three replicates at each sampling time in Jun. and Sep. for microbial biomass C and PLFAs, and in Jun., Sep. and Oct. for functional genes under each treatment. Regression lines are only shown when significant (p ≤ 0.05)
CH4 fluxes were always negative under both treatments throughout the growing season in 2014, and varied between −45.1 ± 8.3 and −7.4 ± 3.1 μg C m−2 h−1 under ambient CO2 and between −77.3 ± 9.8 and −17.7 ± 2.6 μg C m−2 h−1 under elevated CO2 (Fig. 1c), suggesting net soil uptake of CH4. Elevated CO2 significantly increased mean CH4 uptake (−29.3 ± 2.2 μg C m−2 h−1) by 32.4% compared to ambient CO2 (−22.1 ± 1.4 μg C m−2 h−1) (p = 0.02), and significantly increased seasonally cumulative CH4 uptake by 34.7% (p < 0.05, Fig. 2a). When data under both treatments were combined, soil CH4 flux was significantly positively correlated with WFPS (p = 0.01), and negatively correlated with NH4 + (p = 0.01), DOC (p = 0.03) (Fig. S4) and the abundance of methanotroph pmoA (p = 0.01, Fig. 3d). Two diurnal changes also showed that elevated CO2 significantly increased both daily mean CH4 uptake (p < 0.01, Fig. S2b, e) and cumulative CH4 uptake (p < 0.05, Fig. S2 h).
Elevated CO2 had no effect on mean soil N2O flux across the growing season (Fig. 1b). However, the seasonally cumulative N2O was 64.7% higher under elevated CO2 compared to that under ambient CO2 (p < 0.05, Fig. 2b). When data were aggregated across both treatments, the soil N2O flux was significantly negatively correlated with WFPS (p = 0.05) and microbial N (p = 0.04) (Fig. S5a, d), and positively correlated with soil NH4 + concentration (p = 0.06, Fig. S5c). The soil N2O flux did not correlate with soil NO3 − (Fig. S5b). Our results also showed that the soil N2O flux was significantly positively correlated with the abundance of AOA amoA (p = 0.05), but not with the abundance of nirS (Fig. 3e, f). The two diurnal changes showed that elevated CO2 significantly increased both daily mean N2O fluxes (p < 0.01, Fig. S2c, f) and cumulative N2O emissions (p < 0.05, Fig. S2i).
Elevated CO2 significantly increased cumulative GWP by 27.8% (p < 0.05), which was mainly due to increased soil CO2 emissions (Fig. 2d). The GWP caused by increased N2O emissions was mostly offset by increased CH4 uptake.
Effects of elevated CO2 on soil microbial C, N and community composition
During the growing season microbial C and N concentrations were on average 16.7% and 26.3% higher under elevated CO2 than that under ambient CO2, respectively (p < 0.05, Fig. 4). Elevated CO2 significantly increased the mean abundance of AM fungi, actinomycetes and bacteria by 11.5%, 16.7% and 11.0% respectively (p < 0.05), and decreased the ratio of bacteria to actinomycetes by 4.8% compared to ambient CO2 throughout the growing season (p < 0.01, Table 3). The microbial community composition was not changed by elevated CO2 in Apr. and Jun., whereas in Sep., elevated CO2 significantly changed the microbial community composition on both PC1 and PC2 which accounted for 67% and 21% of the total variation respectively in the PCA analysis (Fig. S6).
The seasonal mean abundance of the methanotroph pmoA gene was 4.4 ± 0.4 × 107 copies g−1 dry soil under elevated CO2, which was significantly higher than under ambient CO2 (2.8 ± 0.2 × 107 copies g−1 dry soil) (p < 0.01, Fig. 5a). The abundance of nifH was 8.5 ± 1.6 × 107 copies g−1 dry soil under elevated CO2, which was not significantly different from that under ambient CO2 (5.7 ± 1.0 × 107 copies g−1 dry soil) throughout the growing season. However, one way ANOVA showed elevated CO2 significantly increased the abundance of the N2 fixing nifH gene in Sep. (p = 0.02, Fig. 5b). The mean abundance of the denitrifier nirS gene was 1.9 ± 0.3 × 106 copies g−1 dry soil under ambient CO2, and 1.6 ± 0.2 × 106 copies g−1 dry soil under elevated CO2 during the growing season, which was not affected by elevated CO2 (Fig. 5c). Elevated CO2 did not significantly change the abundance of AOB amoA (2.6 ± 0.4 × 106 copies g−1 dry soil) compared to ambient CO2 (2.7 ± 0.5 × 106 copies g−1 dry soil) (Fig. 5d). The abundance of AOA amoA under elevated CO2 (3.5 ± 0.7 × 107 copies g−1 dry soil) was not significantly different compared to that under ambient CO2 (2.6 ± 0.4 × 107 copies g−1 dry soil) based on repeated measures ANOVA, while in Sep. a significant increase in the abundance of AOA amoA was found under elevated CO2 based on one way ANOVA (p = 0.03, Fig. 5e). The abundance of AOA amoA was one order magnitude higher than that of AOB amoA with the mean AOA/AOB amoA ratios at 10.2 and 15.9 under ambient and elevated CO2 respectively (Fig. 5f). Results of the one way ANOVA showed that the ratio of AOA/AOB tended to increase under elevated CO2 (p = 0.06, Fig. 5f).
Soil methanotroph gene (pmoA) (a), N2 fixing bacteria gene (nifH) (b), denitrifier gene (nirS) (c), ammonia oxidizing bacteria gene (AOB amoA) (d), ammonia oxidizing archaea gene (AOA aomA) (e) and AOA/AOB (f) under ambient and elevated CO2 during the growing season in 2014. Values are mean ± standard error (n = 3). *: significant difference at p < 0.05 based on one way ANOVA for each sampling time separately
Discussion
Effect of elevated CO2 on soil CO2 emissions
Elevated CO2 significantly increased the mean soil CO2 flux and its cumulative emissions during the growing season in 2014 (p < 0.05), which was consistent with our first hypothesis. Some previous studies found that higher soil moisture under elevated CO2 stimulated SOM decomposition (Hungate et al. 1997a; Dijkstra et al. 2008), resulting in increased CO2 fluxes. Our result also suggested a positive correlation between soil CO2 flux and WFPS, indicating important effects of soil moisture on soil respiration. However, we did not find significant differences of soil moisture or WFPS between the two treatments, indicating that the increased soil respiration under elevated CO2 was not caused by soil moisture. Elevated CO2 significantly increased plant photosynthesis (Zhou et al. 2010), soil DOC and microbial biomass, and these factors were significantly positively correlated with soil CO2 flux, which hinted that enhanced C inputs was the dominant reason for increased soil respiration under elevated CO2. A previous study in the same site as our study found elevated CO2 stimulated root growth and increased autotrophic respiration (Zhou et al. 2010), which further supported this conclusion. Microbes benefit from increased C inputs, especially AM fungi and actinomycetes (Treseder 2004; Billings and Ziegler 2005; Cheng et al. 2012). Indeed, our results showed that elevated CO2 increased the biomass of AM fungi and actinomycetes, and a significant positive correlation was found between soil CO2 flux and the abundance of AM fungi and actinomycetes (p < 0.05). Because AM fungi and actinomycetes were found to decompose more recalcitrant SOM (Killham 1994; Cheng et al. 2012), their increases partly contributed to the higher soil CO2 emissions under elevated CO2.
Elevated CO2 did not affect soil temperature, but did increase Q10, which indicated that soil respiration got more sensitive to the change of soil temperature under elevated CO2. This was possibly because more recalcitrant C was decomposed due to increased AM fungi and actinomycetes under elevated CO2, and recalcitrant C generally has higher Q10 than labile C (Xu et al. 2014; Leitner et al. 2016). Therefore, our results suggest the interaction of soil warming and elevated CO2 would accelerate the decomposition of SOM more than a single factor would.
Effect of elevated CO2 on soil CH4 uptake
WFPS was the dominating factor regulating the net CH4 flux in our study (Table S3). Previous studies showed that higher WFPS may constrain and slow down the diffusion of CH4 and O2 from the atmosphere to the water-film covered microbes and suppress CH4 uptake (Phillips et al. 2001; McLain et al. 2002). Moreover, the anaerobic conditions induced by higher soil moisture may stimulate methanogens activity and increase CH4 production (McLain and Ahmann 2008), counterbalancing the amount of CH4 uptake. The relatively dry soil conditions at our site were beneficial for aerobic methanotrophs, but not for anaerobic methanogens, resulting in net CH4 uptake.
Higher N availability was previously reported to alleviate N limitation of methanotrophs and stimulate CH4 uptake (Bodelier et al. 2000; Dijkstra et al. 2010; Bodelier and Steenbergh 2014). However, when NH4 + concentrations are too high, it may inhibit methanotrophic activity due to competitive inhibition of the key enzyme (methane monooxygenase) (Bédard and Knowles 1989; Bodelier and Steenbergh 2014). Our study area is typically N limited, and thus, higher NH4 + concentration was found to stimulate CH4 uptake (Fig. S4b).
The increased CH4 uptake under elevated CO2 was associated with the increase of the methanotroph pmoA gene, which was mainly due to increased soil DOC under elevated CO2. Benstead et al. (1998) and Goldman et al. (1995) also found increased labile C substrates stimulated soil CH4 uptake. In a semiarid grassland ecosystem, Dijkstra et al. (2010) speculated that increased labile C inputs stimulated methanotrophs to consume CH4 under elevated CO2. Our results provided direct evidence of the increase of both methanotrophs and DOC, and therefore supported their viewpoints.
Effect of elevated CO2 on soil N2O emissions
The N2O flux was low in our study due to N limitations. The elevated CO2 therefore had no effect on the seasonal mean N2O flux during the growing season in 2014. Previous studies in N limited ecosystems also found low N2O fluxes and non-significant effects of elevated CO2 (Ambus and Robertson 1999; Billings et al. 2002; Mosier et al. 2002). Some studies found that the greater plant and microbial N utilization under elevated CO2 decreased N availability, resulting in unchanged soil N2O fluxes (Hungate et al. 1997b; Mosier et al. 2002), or even reduced N2O fluxes (Pleijel et al. 1998; Kettunen et al. 2007). The significant negative correlation between microbial N and N2O flux in our research site suggested that the higher microbial N immobilization under elevated CO2 may have reduced N2O emissions, counterbalancing the potential stimulation effect of increased C inputs on N2O emissions. However, we found seasonally cumulative N2O emissions were higher under elevated CO2 than that under ambient CO2. We speculated that the large variations among individual OTC partly contributed to the lack of significant effects of elevated CO2 on the seasonal mean N2O flux. Moreover, the interaction effects between “time” and “elevated CO2” based on repeated measures ANOVA were significant (Fig. 1b), which means the effects of elevated CO2 were influenced by time. Thus, the significant difference in seasonal cumulative fluxes was probably due to elevated CO2 effects on a few days, when soil temperature and/or soil NH4 + concentrations were larger under elevated CO2 compared to ambient CO2 (Fig. S7). These conditions may have benefited the nitrifying population, and thereby significantly increased soil N2O fluxes. Therefore, the stimulation effect of elevated CO2 on cumulative N2O emissions should not be ignored.
Soil N2O production is mainly controlled by nitrifiers and denitrifiers which regulate nitrification and denitrification processes respectively. The non-significant effect of elevated CO2 on seasonal mean N2O flux was associated with the unchanged AOB amoA, AOA amoA and nirS. In a temperate forest ecosystem, Long et al. (2012) found elevated CO2 did not affect AOB due to increased N limitation, but stimulated AOA growth, indicating that elevated CO2 and N limitation benefited AOA growth. At our site, the abundance of AOA amoA was one order of magnitude higher than that of AOB amoA and denitrifier nirS, and the AOA/AOB ratio tended to increase under elevated CO2. Especially, in Sep., elevated CO2 significantly stimulated AOA growth. Therefore in this study the increased seasonal cumulative N2O emissions under elevated CO2 was most likely caused by the AOA community and increased nitrification rates. The significantly positive correlation between soil N2O flux and AOA amoA abundance further supported this conclusion. Furthermore, we found a positive correlation between NH4 + and N2O flux, but found no relationship between NO3 − and N2O flux, which also indicated that most N2O production at our site was derived from nitrification. The soil at our study site was of well-aerated loamy sand texture, which is considered unfavourable for denitrifiers. Soil WFPS ranged from 16.1% to 50.9%, and N2O is thought to originate mostly from nitrification when WFPS is below 60% (Linn and Doran 1984). The significant negative correlation between WFPS and N2O flux also provided evidence of nitrification dominating N2O production in our study site (Fig. S5a).
Although on average the N2 fixing bacteria gene nifH was not affected by elevated CO2 during the study period, in Sep. a significant increase in N2 fixing bacteria occurred under elevated CO2. This was probably because N limitation was most severe in Sep. under elevated CO2, inducing N2 fixation. The stimulation of the growth of N2 fixing bacteria under elevated CO2 has been reported previously, which could relieve N limitations (Berthrong et al. 2014; Liang et al. 2016).
Conclusions
Elevated CO2 increased soil CO2 emissions and CH4 uptake throughout the growing season in the Quercus mongolica dominated OTC system. Although the effect of elevated CO2 on seasonal mean soil N2O flux was not significant during the growing season, on some individual measurement dates N2O emissions were increased by elevated CO2. Changes in GHG fluxes were closely related to changes in soil microbial biomass and community composition, which drive the GHG production and consumption processes. The increased microbial biomass and the abundance of arbuscular mycorrhizal fungi and actinomycetes partly contributed to the increased soil CO2 emissions under elevated CO2. Elevated CO2 increased CH4 uptake rates due to stimulated growth of methanotrophs. Our results also hinted that nitrification by AOA was the major process of soil N2O emissions in our studied system. We found elevated CO2 increased cumulative GWP from CH4, N2O and CO2, pointing to positive feedbacks to global warming.
Abbreviations
- N:
-
Nitrogen
- C:
-
Carbon
- GHG:
-
Greenhouse gas
- CO2 :
-
Carbon dioxide
- N2O:
-
Nitrous oxide
- CH4 :
-
Methane
- GWP:
-
Global warming potential
- SOM:
-
Soil organic matter
- SOC:
-
Soil organic carbon
- TN:
-
Total nitrogen
- DOC:
-
Dissolved organic carbon
- DON:
-
Dissolved organic nitrogen
- NH4 + :
-
Ammonium
- NO3 − :
-
Nitrate
- WFPS:
-
Water filled pore space
- AOA:
-
Ammonium oxidizing archaea
- AOB:
-
Ammonium oxidizing bacteria
- OTC:
-
Open top chamber
References
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–372
Alves BJR, Smith KA, Flores RA et al (2012) Selection of the most suitable sampling time for static chambers for the estimation of daily mean N2O flux from soils. Soil Biol Biochem 46:129–135
Ambus P, Robertson GP (1999) Fluxes of CH4 and N2O in aspen stands grown under ambient and twice-ambient CO2. Plant Soil 209:1–8
Bédard C, Knowles R (1989) Physiology, biochemistry, and specific inhibitors of CH4, NH4 +, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev 53:68–84
Benstead J, King GM, Williams HG (1998) Methanol promotes atmospheric methane oxidation by methanotrophic cultures and soils. Appl Environ Microbiol 64:1091–1098
Berthrong ST, Yeager CM, Gallegos-Graves L, Steven B, Eichorst SA, Jackson RB, Kuske CR (2014) Nitrogen fertilization has a stronger effect on soil nitrogen-fixing bacterial communities than elevated atmospheric CO2. Appl Environ Microbiol 80:3103–3112
Billings SA, Ziegler SE (2005) Linking microbial activity and soil organic matter transformations in forest soils under elevated CO2. Glob Chang Biol 11:203–212
Billings SA, Schaeffer SM, Evans RD (2002) Trace N gas losses and N mineralization in Mojave desert soils exposed to elevated CO2. Soil Biol Biochem 34:1777–1784
Bodelier PLE, Steenbergh AK (2014) Interactions between methane and the nitrogen cycle in light of climate change. Curr Opin Environ Sustain 9-10:26–36
Bodelier PLE, Hahn AP, Arth IR, Frenzel P (2000) Effects of ammonium-based fertilisation on microbial processes involved in methane emission from soils planted with rice. Biogeochemistry 51:225–257
Brookes PC, Kragt JF, Powlson DS, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: the effects of fumigation time and temperature. Soil Biol Biochem 17:831–835
Burke W, Gabriels D, Bouma J (1986) Soil structure assessment. A. A. Balkema Publishers, Rotterdam, p 92
Carney KM, Hungate BA, Drake BG, Megonigal JP (2007) Altered soil microbial community at elevated CO2 leads to loss of soil carbon. Proc Natl Acad Sci U S A 104:4990–4995
Cheng WX (1999) Rhizosphere feedbacks in elevated CO2. Tree Physiol 19:313–320
Cheng L, Booker FL, Tu C et al (2012) Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 337:1084–1087
De Graaff MA, Van Groenigen KJ, Six J, Hungate B, Van Kessel C (2006) Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Glob Chang Biol 12:2077–2091
Deng Q, Zhou G, Liu J, Liu S, Duan H, Zhang D (2010) Responses of soil respiration to elevated carbon dioxide and nitrogen addition in young subtropical forest ecosystems in China. Biogeosciences 7:315–328
Dijkstra FA, Pendall E, Mosier AR, King JY, Milchunas DG, Morgan JA (2008) Long-term enhancement of N availability and plant growth under elevated CO2 in a semi-arid grassland. Funct Ecol 22:975–982
Dijkstra FA, Morgan JA, LeCain DR, Follett RF (2010) Microbially mediated CH4 consumption and N2O emission is affected by elevated CO2, soil water content, and composition of semi-arid grassland species. Plant Soil 329:269–281
Dijkstra FA, Prior SA, Runion GB, Torbert HA, Tian H, Lu C, Venterea RT (2012) Effects of elevated carbon dioxide and increased temperature on methane and nitrous oxide fluxes: evidence from field experiments. Front Ecol Environ 10:520–527
Dijkstra FA, Morgan JA, Follett RF, LeCain DR (2013) Climate change reduces the net sink of CH4 and N2O in a semiarid grassland. Glob Chang Biol 19:1816–1826
Drigo B, Pijl AS, Duyts H et al (2010) Shifting carbon flow from roots into associated microbial communities in response to elevated atmospheric CO2. Proc Natl Acad Sci U S A 107:10938–10942
Epron D, Le Dantec V, Dufrene E, Granier A (2001) Seasonal dynamics of soil carbon dioxide efflux and simulated rhizosphere respiration in a beech forest. Tree Physiol 21:145–152
Forster P, Ramaswamy V, Artaxo P et al (2007) Changes in atmospheric constituents and in radiative forcing. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 130–234
Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB (2005) Ubiquity and diversity of ammonia oxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci U S A 102:14683–14688
Frostegård Å, Tunlid A, Bååth E (2011) Use and misuse of PLFA measurements in soils. Soil Biol Biochem 43:1621–1625
Garcia-Palacios P, Vandegehuchte ML, Shaw EA et al (2015) Are there links between responses of soil microbes and ecosystem functioning to elevated CO2, N deposition and warming? A global perspective. Glob Chang Biol 21:1590–1600
Gee GM, Bauder JW (1986) Part I: physical and mineralogical methods. In: Klute A (ed) Methods of soil analysis. American Society of Agronomy, Inc., Madison, pp 383–411
Goldman MB, Groffman PM, Pouyat RV, Mcdonnell MJ, Pickett STA (1995) CH4 uptake and availability in forest soils along an urban to rural gradient. Soil Biol Biochem 27:281–286
He Z, Xu M, Deng Y et al (2010) Metagenomic analysis reveals a marked divergence in the structure of belowground microbial communities at elevated CO2. Ecol Lett 13:564–575
Hu NF, Wang AZ, Guan DX et al (2010) Multiple time scale analysis of precipitation series in Changbai Mountain region in 1959-2006. Chin J Appl Ecol 21:549–556
Hungate BA, Chapin FS III, Zhong H, Holland EA, Field CB (1997a) Stimulation of grassland nitrogen cycling under carbon dioxide enrichment. Oecologia 109:149–153
Hungate BA, Lund CP, Pearson HL, Chapin FS III (1997b) Elevated CO2 and nutrient addition alter soil N cycling and N trace gas fluxes with early season wet-up in a California annual grassland. Biogeochemistry 37:89–109
Ineson P, Coward PA, Hartwig UA (1998) Soil gas fluxes of N2O, CH4 and CO2 beneath Lolium perenne under elevated CO2: the Swiss free air carbon dioxide enrichment experiment. Plant Soil 198:89–95
IPCC (2013) In: Stocker TF, Qin D, Plattner GK et al (eds) Climate change 2013: the physical science basis: contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, pp 465–470
Jackson RB, Sala OE, Field CB, Mooney HA (1994) CO2 alters water use, carbon gain, and yield for the dominant species in a natural grassland. Oecologia 98:257–262
Kammann C, Müller C, Grünhage L, Jäger HJ (2008) Elevated CO2 stimulates N2O emissions in permanent grassland. Soil Biol Biochem 40:2194–2205
Kettunen R, Saarnio S, Martikainen PJ, Silvola J (2007) Can a mixed stand of N2 fixing and non-fixing plants restrict N2O emissions with increasing CO2 concentration? Soil Biol Biochem 39:2538–2546
Killham K (1994) Soil Ecology. Cambridge University Press, Cambridge
Kolb S, Knief C, Stubner S, Conrad R (2003) Quantitative detection of methanotrophs in soil by novel pmoA-targeted real-time PCR assays. Appl Environ Microbiol 69:2423–2429
Leitner S, Sae-Tun O, Kranzinger L, Zechmeister-Boltenstern S, Zimmermann M (2016) Contribution of litter layer to soil greenhouse gas emissionsin a temperate beech forest. Plant Soil 403:455–469
Li XF, Han SJ, Guo ZL, Shao DK, Xin LH (2010) Changes in soil microbial biomass carbon and enzyme activities under elevated CO2 affect fine root decomposition processes in a Mongolian oak ecosystem. Soil Biol Biochem 42:1101–1107
Liang J, Qi X, Souza L, Luo Y (2016) Processes regulating progressive nitrogen limitation under elevated carbon dioxide: a meta-analysis. Biogeosciences 13:2689–2699
Linn DM, Doran JW (1984) Effect of water filled pore space on carbon dioxide and nitrous oxide production in tilled and non-tilled soils. Soil Sci Soc Am J 48:1267–1272
Lipson DA, Kuske CR, Gallegos-Graves LV, Oechel WC (2014) Elevated atmospheric CO2 stimulates soil fungal diversity through increased fine root production in a semiarid shrubland ecosystem. Glob Chang Biol 20:2555–2565
Long X, Chen CR, Xu ZH, Oren R, He JZ (2012) Abundance and community structure of ammonia-oxidizing bacteria and archaea in a temperate forest ecosystem under ten-years elevated CO2. Soil Biol Biochem 46:163–171
Luo YQ, Jackson RB, Field CB, Mooney HA (1996) Elevated CO2 increases belowground respiration in California grasslands. Oecologia 108:130–137
McLain JET, Ahmann DM (2008) Increased moisture and methanogenesis contribute to reduced methane oxidation in elevated CO2 soils. Biol Fertil Soils 44:623–631
McLain JET, Kepler TB, Ahmann DM (2002) Belowground factors mediating changes in methane consumption in a forest soil under elevated CO2. Glob Biogeochem Cycles 16:23–37
Moore-Kucera J, Dick RP (2008) PLFA profiling of microbial community structure and seasonal shifts in soils of a Douglas-fir chronosequence. Microb Ecol 55:500–511
Mosier AR, Morgan JA, King JY, Lecain D, Milchunas DG (2002) Soil-atmosphere exchange of CH4, CO2, NOx, and N2O in the Colorado shortgrass steppe under elevated CO2. Plant Soil 240:201–211
Müller C, Rütting T, Abbasi MK et al (2009) Effect of elevated CO2 on soil N dynamics in a temperate grassland soil. Soil Biol Biochem 41:1996–2001
Olsson PA, Bääth E, Jakobsen I, Söderström B (1995) The use of phospholipid and neutral lipid fatty-acids to estimate biomass of arbuscular mycorrhizal fungi in soil. Mycol Res 99:623–629
Parton WJ, Holland EA, Del Grosso SJ et al (2001) Generalized model for NOx and N2O emissions from soils. J Geophys Res 106:17403–17419
Phillips RL, Whalen SC, Schlesinger WH (2001) Influence of atmospheric CO2 enrichment on methane consumption in a temperate forest soil. Glob Chang Biol 7:557–563
Pleijel H, Sild J, Danielsson H, Klemedtsson L (1998) Nitrous oxide emissions from a wheat field in response to elevated carbon dioxide concentration and open top chamber enclosure. Environ Pollut 102:167–171
Poly F, Monrozier LJ, Bally R (2001) Improvement in the RFLP procedure for studying the diversity of nifH genes in communities of nitrogen fixers in soil. Res Microbiol 152:95–103
Reich PB, Knops J, Tilman D et al (2001) Plant diversity enhances ecosystem responses to elevated CO2 and nitrogen deposition. Nature 410:809–812
Robinson D, Conroy JP (1998) A possible plant-mediated feedback between elevated CO2, denitrification and the enhanced greenhouse effect. Soil Biol Biochem 31:43–53
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine scale analysis of natural ammonia oxidizing populations. Appl Environ Microbiol 63:4704–4712
Rütting T, Clough TJ, Müller C, Lieffering M, Newton PCD (2010) Ten years of elevated atmospheric carbon dioxide alters soil nitrogen transformations in a sheep-grazed pasture. Glob Chang Biol 16:2530–2542
Singh BK, Bardgett RD, Smith P, Reay DS (2010) Microorganisms and climate change: terrestrial feedbacks and mitigation options. Nat Rev Microbiol 8:779–790
Smith KE, Runion GB, Prior SA, Rogers HH, Torbert HA (2010) Effects of elevated CO2 and agricultural management on flux of greenhouse gases from soil. Soil Sci 175:349–356
Throbäck IN, Enwall K, Jarvis A, Hallin S (2004) Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol Ecol 49:401–417
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355
Van Groenigen KJ, Osenberg CW, Hungate BA (2011) Increased soil emissions of potent greenhouse gases under increased atmospheric CO2. Nature 475:214–218
Vance ED, Brookes PC, Jenkinson DS (1987) Microbial biomass measurements in forest soils: determination of kC values and tests of hypotheses to explain the failure of the chloroform fumigation-incubation method in acid soils. Soil Biol Biochem 19:689–696
Wang JJ, Pei TP, Wang AZ, Guan DX, Jin CJ (2009) Changes in the mean maximum and minimum temperatures in Changbai Mountain, northeastern China in the past 50 years. Journal of Beijing Forestry University 31:50–57
Wang QK, He TX, Wang SL, Liu L (2013) Carbon input manipulation affects soil respiration and microbial community composition in a subtropical coniferous forest. Agric For Meteorol 178:152–160
Xu M, Qi Y (2001) Soil-surface CO2 efflux and its spatial and temporal variations in a young ponderosa pine plantation in northern California. Glob Chang Biol 7:667–677
Xu WH, Li W, Jiang P, Wang H, Bai E (2014) Distinct temperature sensitivity of soil carbon decompostion in forest organic layer and mineral soil. Sci Rep 4:6512–6518
Zak DR, Pregitzer KS, Curtis PS, Teeri JA, Fogel R, Randlett DL (1993) Elevated atmospheric CO2 and feedback between carbon and nitrogen cycles. Plant Soil 151:105–117
Zak DR, Ringelberg DB, Pregitzer KS, Randlett DL, White DC, Curtis PS (1996) Soil microbial communities beneath Populus grandidentata crown under elevated atmospheric CO2. Ecol Appl 6:257–262
Zak DR, Pregitzer KS, King JS, Holmes WE (2000) Elevated atmospheric CO2, fine roots and the response of soil microorganisms: a review and hypothesis. New Phytol 147:201–222
Zhou Y, Li MH, Cheng XB et al (2010) Soil respiration in relation to photosynthesis of Quercus mongolica trees at elevated CO2. PLoS One 5:e15134
Zhu X, Burger M, Doane TA, Horwath WR (2013) Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc Natl Acad Sci U S A 110:6328–6333
Acknowledgements
This study was supported by the Major State Basic Research Development Program of China (973 Program) (2014CB954400), National Natural Science Foundation of China (31522010) and Key Research Program of Frontier Sciences, CAS (QYZDB-SSW-DQC006) and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB15010301). We are very grateful to Jidong Zhou for the quantitative real time PCR assay, and Lufu Zhao for maintaining the OTC facility.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Per Ambus
Electronic supplementary material
ESM 1
(DOCX 1.38 mb)
Rights and permissions
About this article
Cite this article
Sun, J., Xia, Z., He, T. et al. Ten years of elevated CO2 affects soil greenhouse gas fluxes in an open top chamber experiment. Plant Soil 420, 435–450 (2017). https://doi.org/10.1007/s11104-017-3414-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3414-7