Abstract
In this study, a distinct inoculum was investigated as an isolated variable within sequencing batch reactors via a comparison of the 4-fluoroaniline (4-FA) or 2,4-difluoroaniline (2,4-DFA) removal amounts. The inocula were derived from a treatment plant for treating pharmaceutical wastewater plus a small amount of municipal sewage (PMS), a treatment plant for treating fluoridated hydrocarbon wastewater (FHS), and a treatment plant for treating the comprehensive wastewater in an industrial park (CIS). There were slight differences among the degradation patterns of the 4-FA for the three inocula, whether during the enrichment period or the high concentration shock period. In contrast, it was observed that the degradation efficiency of 2,4-DFA initially varied with the inocula. The FHS-derived inoculum was determined to be optimal, exhibiting the earliest degradation reaction only after an acclimation of 7 days had the highest degradation rate constant of 0.519 h−1, and had the fastest recovery time of three weeks after high concentration shock. Additionally, compared with the PMS-derived inoculum, the CIS-derived inoculum exhibited an earlier degradation reaction within three weeks, and a higher microbial diversity, but a lower shock resistance and degradation rate constant of 0.257 h−1. High-throughput sequencing demonstrated that each final consortium was different in composition, and the microbial consortia developed well on the inoculum and substrate. In comparison of the similarity among the three 2,4-DFA enrichment cultures, the higher similarity (63.9–70.0%) among three final consortia enriching with 4-FA was observed. The results indicated that the inoculum played an important role in the degradation of FAs and the microbial bacterial communities of final consortia, and the effect extent might well depend on the fluorinated level of FAs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluoroaromatics have increasingly contributed to environmental pollution in recent decades due to the wide use of fluorinated pharmaceuticals and agrochemicals (Kiel and Engesser 2015). The carbon–fluorine bond is one of the strongest bonds in nature, which makes the fluoroaromatics recalcitrant to chemical and biological degradation, and easily accumulating in water and soil (Adams and Halden 2010). Considering the toxicity of fluoroanilines (FAs) to the environment (Eadsforth et al. 1984; Mosneang et al. 2014; Smulek et al. 2017), these substances have been listed as a priority to control organic pollutants in the United States and China (Cui et al. 2017). Obviously, it is an urgent and fascinating challenge for researchers to eliminate or reduce FAs contamination.
Compared with other treatment methods, biological treatment methods provide a safer, environmentally friendly, and cheaper option (Kiel and Engesser 2015). Despite the fluoroaromatics have low natural biodegradability owing to the stability of their molecular structures, there have been some efforts towards their biological treatment. To date, several reports in the literature have confirmed that some strains can degrade FAs (Amorim et al. 2013; Song et al. 2014; Zhao et al. 2019b). Using microbial consortia instead of single strains for the biodegradation of FAs allows one to take advantage of the microbial interactions, by making optimal use of their intricate regulatory systems, which may bypass problems of feedback regulation and metabolite repression that are often posed by single strain (Carvalho et al. 2010; Cortes-Tolalpa et al. 2016; Cui et al. 2017; Khehra et al. 2005). As for new or unusual chemicals, the microorganisms typically can acquire new metabolic pathways by pre-adaptation, which is referred to as acclimation (Movahedyan et al. 2008; Orozco et al. 2013; Sahinkaya and Dilek 2005; Wosman et al. 2016). Among the literature regarding fluoroaromatic degradation, the acclimation duration ranges from several weeks to months even years (Duque et al. 2011; Zhao et al. 2015). The initial seeding source is a critical factor for determining the dominant community that is responsible for the key function during acclimation, which would influence the acclimation duration and degradation efficiency (Alexandrino et al. 2018; Cortes-Tolalpa et al. 2016; Ding et al. 2018; Kim et al. 2017). Thus, the seeding sources are likely a critical variable that controls the success of fluoroaromatics degradation. However, little is known about effects of seeding sources on FAs degradation and microbial communities of the final consortia.
In recent years, negligible effects of seeding sources on pollutants removal and the microbial communities of the final consortia has been reported, such as anaerobic ammonia oxidation(Cho et al. 2018) and 5-fluorouracil degradation(Kim et al. 2017). Also, several literatures show that effects of the seeding sources on pollutant removal depend on substance type (Alexandrino et al. 2018; Kim et al. 2017). To our knowledge, it remains unclear whether effect extent of the seeding sources on FAs degradation depends on fluorinated level.
Besides, the xenobiotic concentrations easily fluctuate in actual industrial wastewater (Osuna et al. 2008). As one of the important fluctuant factors, the pollutant concentration can not only affect the characteristics of microorganisms, but also have an impact on metabolisms of microorganisms for utilizing substrates in wastewater, which results in variation in the pollutant removal performance (Jiang et al. 2019a). In particular, when the concentrations of FAs increase, the insufficient degradation capability is also prevalent due to their toxicity, even if the microorganisms are successfully acclimated and achieve a steady state at a certain concentration (Alves et al. 2017; Movahedyan et al. 2008). Thus, the capacity to respond to high concentration FAs shock should be considered for the final consortia obtained from the distinct inocula.
In this study, two different fluorine substitution FAs including 4-fluoroaniline (4-FA) and 2,4-difluoroaniline (2,4-DFA) are focused on. This study is conducted in six sequencing batch reactors (SBRs) inoculated three typical activated sludge, (i) to evaluate influence of the seeding inocula on the degradation efficiencies of 4-FA or 2,4-DFA during acclimation and high concentration shock period, and its respond to fluorinated level of FAs; (ii) to assess whether the final consortia enriched from the distinct inocula has similar degradation capacity to high concentration 4-FA or 2,4-DFA and their homologues;(iii) to determine the relevance of the microbial inoculum as the driver of the final enrichment FAs-degradative consortia. This research provides insights into the role of the inoculum source during degradation of FAs, and its response to fluorinated level of FAs. Additionally, it provides important information on the choice of the seeding sources for actual wastewater treatment system stability and efficient operations.
Materials and methods
Inoculum sources and mineral salt medium
In general, the inoculation from industrial wastewater treatment station has more advantages than that from domestic municipal wastewater treatment plant. Thus, the inoculum sources were taken from (1) the activated sludge return line from the secondary clarifier of a mixed pharmaceutical-municipal wastewater treatment plant (PMS, R1); (2) the aerobic pool of a fluorinated hydrocarbon wastewater treatment system (FHS, R2); and (3) the oxidation ditch of a comprehensive wastewater treatment plant in an industrial park (CIS, R3). All three factories were located in Quzhou, Zhejiang Province, China, with no FAs exposure. The sludge volume indexes (SVI) of the PMS-derived, FHS-derived, and CIS-derived inocula were 120 mL/g, 59 mL/g, and 81 mL/g, respectively. The components of the medium were as follows (in mg/L): sucrose 0–500, sodium acetate 0–850, 4-FA or 2,4-DFA 25–500, NH4Cl 237, KH2PO4 27.69, K2HPO4·3H2O 46.15, FeSO4·7H2O 6.25, MgCl2·7H2O 5.1, CaCl2 6, and 1 mL/L of micronutrient solutions which was described in our previous report(Zhao et al. 2015). The initial pH was adjusted to 7.2 ± 0.2 with 1 M HCl or NaOH.
Enrichment of microbial consortia degrading FAs from three inoculum sources
Six reactors with a working volume of 2.0 L were set up. They were cylindrical and made of polymethyl methacrylate. A total of 2.0 L liquid, including the medium and the mixed liquor volatile suspended solids (MLVSS), was added to each reactor to support microbial growth. The reactors containing the same inoculum source were supplied with 4-FA or 2,4-DFA (reagent grade; Shanghai Sheng Chemical Technology Co., Ltd.). The trace mineral solution was prepared according to the method of Zhao Zhiqing et al. (2015). Each reactor was seeded with approximately 1500 mg/L of MLVSS. A stable reactor temperature was maintained at 28 ± 2 °C using a thermostat-controlled electric heating rod. The operating cycle of every 24 h included: 5 min of feeding, 23 h of aerobic reaction with an O2 concentration of 3 mg/L (maintained by mechanical aeration), 45 min of sedimentation, and 10 min of effluent withdrawal, during which 70% of the liquid volume was replaced with fresh medium. According to the model compound (4-FA, and 2,4-DFA) and inocula (PMS, FHS, and CIS), the reactors were named FR1, FR2, FR3, DFR1, DFR2, and DFR3.
Stepwise acclimation was applied during the operation. Whole acclimation included two stages. In phase I, the FAs concentration at the beginning of the acclimation was 25 mg/L, then gradually increased to 50 mg/L, 100 mg/L, 150 mg/L, and finally 200 mg/L, when the accompanying supplementary carbon resources gradually decreased from 1350 mg/L (Wosman et al. 2016). In phase II, the concentration was further increased to 300 mg/L without supplementary carbon resources. Every time the removal efficiency of the FAs reaches ≥ 85%, the concentration of FAs will further increase. During the entire acclimation, samples were periodically taken out to determine the residual 4-FA and 2,4-DFA concentration, as well as the released fluoride ion (F−) concentration. At the end of acclimation, nine samples were collected, including seed inocula (R1seed, R2seed, R3seed), 4-FA enrichment culture (FR1, FR2 and FR3), and 2,4-DFA enrichment culture (DFR1, DFR2 and DFR3), to analyze the microbial community structure at the end of the experiment.
FAs are toxic to many kinds of microorganisms, resulting in inhibition to their growth (Vazquez and Rial 2014). To date, how the final enriched consortia obtained from the distinct inocula show responses to high concentration shock becomes unknown. Thus, when the SBRs successfully achieved a steady state at 300 mg/L, the initial concentration was further increased to 400 mg/L and 500 mg/L that were above the median lethal concentration (EC50) value (Zhao et al. 2019a), namely phase III.
Batch degradation experiments
FAs degradation kinetics
After successful acclimation, the enriched culture was washed three times with 0.01 M sodium phosphate buffer (pH 7.2) and then placed in a new 2.0 L bioreactor. The initial MLVSS and FAs (4-FA or 2,4-DFA) concentrations in the reactor were 1000 mg/L and 500 mg/L, respectively. The operating temperature was maintained at 28 ± 2 °C, and the O2 concentration was controlled at 3 mg/L. To monitor the variation in concentration over time, after 0, 2, 4, 6, 8, 10, 12, and 24 h of incubation, 25 mL of the supernatant was sampled from each reactor to perform the FAs and F− concentrations analyses. The residual amount of 4-FA or 2,4-DFA obtained at the different time intervals was fitted to the first-order kinetics to determine its degradation efficiency (Chettri and Singh 2019), as shown below.
where Ct is the concentration of 4-FA or 2,4-DFA at time t; t is time; k is the first-order rate constants; and C0 is a constant of the biodegradation by three enriched mixed cultures.
Degradation potential of the enriched microbial consortia for high concentration FAs
Typically, the concentrations of FAs in wastewater range from tens to hundreds, but the concentrations in actual wastewater can also reach high as the thousands (Wang and Huang 2005). Thus, to further investigate the degradation potential of the final enriched microbial consortia for high concentration FAs, they were inoculated in 100 mL medium with FAs concentrations varying from 300 to 1500 mg/L for three days at pH 7.0, 28 ± 2 °C and 120 rpm, respectively. The equivalent biomass (approximately 1000 mg/L MLVSS) of the enriched microbial consortia was added to each flask. Prior to inoculation, the enriched microbial consortia was washed three times with a 0.01 M sodium phosphate buffer (pH 7.2).
Degradation potential of the enriched microbial consortia for homologues
To test the degradation potential of the enriched microbial consortia for five homologues including 2-fluoroaniline (2-FA), 3-fluoroaniline (3-FA), 4-FA, 2,4-DFA, and 2,3,4-trifluoroaniline (2,3,4-TFA), a batch incubation experiment was established. Triplicate batch reactors were set up in 250 mL flasks containing 100 mL medium with a concentration of 100 mg/L FAs for three days at pH 7.0, 28 ± 2 °C, and 120 rpm. The equivalent biomass (approximately 1000 mg/L MLVSS) of the enriched microbial consortia was provided in each flask. Also, controlled experiments were conducted along with the primary experiments without biomass or using the autoclaved biomass.
The degradation efficiency and defluorination rate were calculated according to the following equations:
where η1 is the degradation efficiency; and Ct (mg/L) and C0 (mg/L) represent the residual concentrations of 4-FA or 2,4-DFA at time t (h) and initially, respectively (Cortes-Tolalpa et al. 2016).
where η2 is the defluorination rate; mt (mg/L) and m0 (mg/L) represent the concentrations of fluoride ions (F−) at time t (h) and initially, respectively; and RF (%) represents the theoretical mass percent of F− in 4-FA or 2,4-DFA (Li et al. 2020a).
Microbial characterization of the final microbial consortia enriched with 4-FA or 2,4-DFA
Genomic DNA was extracted by using a Fast DNeasy® PowerSoil® Kit (Carlsbad, CA, USA) according to the manufacturer's instructions. Primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) with different barcodes were used to amplify the hypervariable regions V3-V4 of bacterial 16S rRNA gene in an Applied Biosystems 9700 PCR System in triplicate. The PCR productions were sequenced on an Illumina Miseq PE300 platform (Illumina,USA) in Majorbio Co., Ltd (Shanghai, China). Raw sequencing data in this study have been deposited in Sequence Read Archive (SRA) database with accession number PRJNA531176.
Sequences were analyzed using the Quantitative Insights into Microbial Ecology (QIIME) software (Fan et al. 2018). The operational taxonomic units (OTUs) were defined using the Usearch algorithm (version 7.0 http://drive5.com/uparse/) on the basis of 97% sequence identity (Edgar 2010). OTUs containing less than two sequences (singletons) were removed from the analysis. Based on these clusters, Alpha diversity indices, including Shannon index, and Chao 1, were calculated. Reads were classified using the Classifier tool from the Ribosomal Database Project (version 2.2 http://sourceforge.net/projects/rdp-classifier/).
Analytical methods
According to the procedure described by Zhao et al. (2019a), FAs were analyzed by Waters high performance liquid chromatography (HPLC) coupled with UV absorbance detector at a wavelength of 230 nm, with C18 reversed-phase column.The F− was analyzed by using the ion selective electrode method (composed of PF-1-01 fluorine ion selective electrode and 232-01 calomel electrode). The determination procedures of MLVSS was detailed in the Standard Methods (APHA 2005).
Results and discussion
Removal of 4-FA or 2,4-DFA during the enrichment and the high concentration shock periods
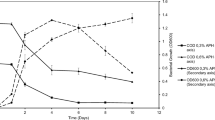
Obviously, all six reactors had been successfully initiated and operated stably (Figs. 1, 2). For 4-FA, the degradation efficiency (FADE) and defluorination rate (DEFR) on day 7 reached nearly 100%, and a high FADE and DEFR were even maintained while increasing the initial concentration without supplementary carbon resources in Phase II, regardless of the inoculum sources (Fig. 1). Also, in Phase III, when the concentrations were further increased to 400 mg/L and 500 mg/L, the removal performance of 4-FA was nearly stable until day 121. On day 121, the temperature in FR2 decreased from 28 °C to room temperature (about 23 °C) due to damage of the heat rod. To maintain the operation condition consistent for the three 4-FA enriched microbial consortia, the temperature in the FR1 and FR3 was also controlled at room temperature until day 135. During this period, the DEFR slightly decreased for FR2 and FR3, but obviously decreased for FR1.
Evolution of 4-FA removal performances for a FR1, b FR2, and c FR3 during enrichment and high concentrations FAs shock periods. Sucrose and sodium acetate were used as supplementary substrates in Phase I, no supplementary substrates were employed in Phase II, whereas high concentrations FAs were applied in Phase III after the enrichment
Evolution of 2,4-DFA removal performances for a DFR1, b DFR2, and c DFR3 during enrichment and high concentrations FAs shock periods. Sucrose and sodium acetate were used as supplementary substrates in Phase I, no supplementary substrates were employed in Phase II, whereas high concentrations FAs were applied in Phase III after the enrichment
In contrast, degradation of 2,4-DFA was strongly dependent on the inoculum sources. It was observed that the degradation efficiency of 2,4-DFA initially varied with the inoculum (Fig. 2). The degradation of 2,4-DFA in DFR2 began without a lag period (Fig. 2b), but the initial FADE and DEFR in DFR1 and DFR3 were low (30–60%) (Fig. 2a, c) until 6 weeks and 3 weeks later, respectively. Moreover, nearly every increase in the 2,4-DFA concentration significantly inhibited the DEFR in DFR1. It is important to note that when the 2,4-DFA concentration increased to 300 mg/L without supplementary carbon resources, the FADE and DEFR regardless of the inocula decreased by greater than 10% and 30%, respectively (Phase II in Fig. 2). Moreover, DFR3 required nearly five weeks for recovery, and only one week was required for DFR1 and DFR2.This could be attributed to the high toxicity of 2,4-DFA, and the disappearance of the biogenic substrates, as well as the distinct seeding sources. Generally speaking, with the concurrent presence of xenobiotic and biogenic substrates, provisions for growth and energy can be obtained from easily-utilized substrates, providing better resistance against toxicity, and also can enhance the ability of certain heterogeneous microbial communities to degrade xenobiotic (Chong and Chen 2007). Additionally, the role of biogenic substrates and the resistance capacity to toxicity depends on the microbial community structure (Kim et al. 2017; Li et al. 2020a; Xu et al. 2018a).Furthermore, the concentrations of 2,4-DFA increased to 400 mg/L and 500 mg/L, and the reactors were successively administered with each concentration shock for fifteen days and above, considering 7–9 days of mean cell residence time (Chong and Chen 2007).The mixed cultures kept stable degradation efficiency for 2,4-DFA at 400 mg/L and 500 mg/L ( Phase III in Fig. 2).
Batch degradation tests
First-order reaction kinetics of 4-FA and 2,4-DFA degradation
According to the literature on the conversion of fluoroanilines (Song et al. 2014) and chloroanilines (Li et al. 2020a), typically, the halogenated anilines biodegradation is initially catalyzed by hydroxylation, resulting in the formation of halocatechols. Then the halocatechols are transformed into halomuconates through a catechol 1,2-dioxygenase or 2,3-dioxygenase, and subsequently the intermediates are recycled through several reaction types. The elimination of fluorine as F− is a critical step during fluoroaniline degradation. Therefore, the samples were collected from the six reactors at different time to analyze the 4-FA, 2,4-DFA, and F− concentration (Figs. 3a–c, 4a–c). The complete degradation of 4-FA in FR1, FR2, and FR3 required 12 h, 8 h, and 24 h, respectively, while the complete degradation of 2,4-DFA required 10–12 h. The degradation of 4-FA and 2,4-DFA was very consistent with the first-order kinetic model (R2 = 0.903–0.947, Figs. 3d–f, 4d–f). The degradation rate constants of 4-FA and 2,4-DFA were both the highest in FR2 and DFR2 (0.725 h−1and 0.519 h−1, respectively), followed by FR1 and DFR2 (0.299 h−1 and 0.349 h−1, respectively), and the lowest in FR3 and DFR3 (0.148 h−1 and 0.257 h−1, respectively). During the 4-FA degradation, the F− release also occurred quickly, and also the similar results have been reported (Song et al. 2014). When the amount of 4-FA decreased from nearly 4.50 mM to 0 mM, the amount of release F− increased from 0 mM to approximately 4.50 mM. Conversely, when the amount of 2,4-DFA decreased from nearly 3.87 mM to 0 mM at 12 h, the release of F− increased constantly until 24 h, and the amounts of F− were 4.61–5.88 mM. Obviously, the amount of 4-FA degradation should be approximately equivalent to the amount of F− release. However, it was also noticed that the F− detection amount only reached about 59.56–75.97% of the theoretical release amount(7.74 mM) in 2,4-DFA, which could be caused by the low defluorination rate.
Obviously, during the degradation of 4-FA and 2,4-DFA, the enriched microbial consortium from the FHS-derived inoculum had the maximum rate constant, and the lowest rate constants for the enriched microbial consortium from CIS-derived inoculum. It can be concluded that the degradation rate constant depended on the inoculated sources, and this result was consistent with previous studies (Alexandrino et al. 2018; Kim et al. 2017; Paule et al. 2015). In general, the inoculum source primarily determines the structure of the final effective consortia, each final consortium has a unique combination of specific enzymatic activities (Cortes-Tolalpa et al. 2016), leading to the differences of the degradation efficiencies.
Degradation potential of the enriched microbial consortia for high concentration FAs
The degradation potential of the enriched microbial consortia to high concentration of FAs was further studied, and the results are shown in Figure.S1 and Figure.S2. When the initial concentrations of 4-FA increased from 300 to 1500 mg/L, the degradation efficiencies remained nearly 100% for all the treatments except the final consortium in FR3 at 1500 mg/L (84.05%). However, with increasing FAs concentration, the DEFR decreased significantly, and only reached 54.77%, 50.18%, and 48.23% for the final consortia in FR1, FR2, and FR3 at 1500 mg/L, respectively. For 2,4-DFA, when the concentrations increased from 300 to 1500 mg/L, the degradation efficiencies decreased from 97.36%, 93.21% and 97.62% to 83.10%, 81.34% and 90.90% for the final consortia in DFR1, DFR2 and DFR3, respectively. Moreover, the defluorination rates in DFR1, DFR2 and DFR3 decreased to 8.80%, 16.05% and 12.05%, respectively. According to the literature, 1250 mg/L is the highest tolerance concentration for the FAs degradation strains (Amorim et al. 2013; Song et al. 2014; Zhao et al. 2019b). These results showed that the six enriched microbial consortia regardless of inoculum sources had good degradation potential for high concentration FAs, though the high concentration had a negative impact on DEFR.
When the exposure concentrations of pollutants increase, microorganisms require a longer adaptation period to produce enough enzymes to metabolize target compounds (Wang et al. 2007), or higher initial inoculum concentrations are required (Guo et al. 2020).In addition, the high concentration of a pollutant might increase toxicity to microorganisms, and decrease metabolic activity and degradation rates, as C.V. Eadsforth reported (Eadsforth et al. 1984). Additionally, regarding their degradability, each microbial consortium enriched with 4-FA or 2,4-DFA showed a similar overall degradation pattern, but only small differences were found between them. The degradation pattern in each consortium may be related to the specific microbial composition, because each organism may be related to different enzymes that attack the substrate. Also, some microorganisms identified as the same or closely related were found in the distinct enriched microbial consortia, which was the reason for the similar removal efficiencies. This will be discussed later.
Degradation potential of the enriched microbial consortia for homologues
Table 1 compares the removal efficiencies of the five homologues using the six enriched microbial consortia. For the three 4-FA enriched microbial consortia, they completely degraded 3-FA and 4-FA within 72 h, and both the degradation efficiency and defluorination rate reached greater than 99.0%. The final microbial consortia in FR1 and FR3 performed well in the degradation of 2-FA, 4-FA and 2,4-DFA (FADE = 98.2–100%, DEFR = 88.7–97.8%), while the final microbial consortium in FR2 had a lower degradation capacity to 2-FA (FADE = 85.5%, DEFR = 57.9%) and 2,4-DFA (FADE = 79.2%, DEFR = 43.8%). Additionally, the three enriched microbial consortia partially degraded 2,3,4-TFA, with 38.6%–68.5% of FADE and 17.4–36.1% of DEFR. However, the final microbial consortia enriched with 2,4-DFA showed a high degradation capacity to the five homologues tested, including 2,3,4-DFA, with greater than a 95.0% degradation efficiency and greater than a 83.0% defluorination rate.
Generally, the resistance of microbial biodegradation depends on the number and position of the halogen atom on the aromatic ring as well as the type of microorganisms involved (Murphy 2016). Compared with the other four homologues, 2,3,4-DFA has the worst biodegradability, because the stoichiometric release of F− provides conclusive evidence of nearly complete degradation in the medium (Kiel and Engesser 2015). However, three final microbial consortia enriched with 4-FA had poorer capacity to 2,3,4-TFA than those enriched with 2,4-DFA, which might attribute to a high similarity between 2,4-DFA enriched culture and 2,3,4-TFA enriched culture (Zhao et al. 2015). Additionally, the degradation capacity of the final microbial consortium in FR2 was poorer than those in FR1 and FR3, but only small differences were found among three final microbial consortia from DFR1, DFR2, and DFR3.
Overall, after acclimation, the final microbial consortia enriched with 4-FA or 2,4-DFA showed significant differences among the degradation rate constants of 4-FA or 2,4-DFA, and similar degradation potential to the high concentration of FAs and the homologs, which mint have been due to the selection of unique and similar communities through acclimation (Cortes-Tolalpa et al. 2016), which will be discussed in the following section.
Composition of the final enriched microbial consortia from different inoculum sources
Alpha diversity
The estimated rarefaction curves of all the samples reached a plateau (Fig.S3), which indicated that the microbial diversity of each sample had been appropriately measured. The indices of Shannon and Chao1 indices were analyzed to evaluate the community richness and diversity respectively. The results are shown in Table 2. The good coverage of all the samples always exceeds 0.97, indicating that each sample has a sufficient sequencing depth. When three inocula were enriched with 500 mg/L 4-FA or 2,4-DFA, the Shannon diversity index decreased from 5.25, 3.35 and 4.46 in the R1seed, R2seed and R3seed, respectively, to 2.98, 2.56, 2.82, 1.95, 2.70, and 2.72 in FR1, FR2, FR3, DFR1, DFR2, and DFR3, respectively, Similarly, all the Chao1 richness index values had the same variation. FAs-tolerant microbes were likely pre-selected during acclimation, and acclimation resulted in decreased diversity. Interestingly, significant differences were observed for the microbial diversity in the six final enrichment mixtures. In detail, the final consortium in FR1 showed the highest microbial diversity, followed by FR3 and FR2, which was consistent with the three inoculation sources of the microorganisms. In contrast, the final microbial consortium in DFR1 showed the lowest microbial diversity, while no difference was observed between the final microbial consortia in DFR2 and DFR3.
OTU is defined as a 16S RNA sequence with a similarity of over 97% (Zhou et al. 2020). After rarefied each sample to 30,979, 1351 OTUs were grouped together from 836,433 sequences, and the similarity was 97%. The initial inoculum of the R1seed (884) had the highest OTUs, which was 1.5 times and 2.5 times that of the R3seed (602) and the R2seed (352) (p < 0.05, Fig. S4). Enrichment with 4-FA or 2,4-DFA will severely deplete the OTUs content in all inoculums (p < 0.05), and at the end of the experiment, it was found that the differences among the final enriched microbial consortia from the different inocula were small (Fig. S4a). This result was also observed for the Shannon index (Fig. S4b). The results showed that regardless of the form of inoculation sources and the type of fluoroaniline, enrichment led to depletion and similar bacterial diversity. Principal coordinates analysis (PCoA) based on the Bray–Curtis distance showed that the bacterial communities in these systems were strongly influenced by the enrichment substrates (p < 0.05), while the influence of the inoculum sources was significant only when the substrate was the same (p < 0.05) (Fig. 5a). Similar results are also shown in the cluster heatmap (Fig. 5b).
The three inocula shared 17.3% (214 out of 1234) of the total OTUs, which was 0.3 and 0.4 times of that enriched with 4-FA (51.2%, 177 out of 346) and 2,4-DFA (41.8%, 142 out of 340), respectively (Fig. 6a–c). The unique OTU in the inocula decreased significantly with the degree of enrichment. For example, 267 OTUs were unique in the R1seed, with only 28 OTUs and 19 OTUs in FR1 and DFR1 (Fig. 6a), respectively. These results indicated that the bacterial community structures in the three seeding sources were significantly different, and the bacterial diversity decreased after acclimation due to the non-degraders endogenous decay. Also, it was found that the bacterial community structures in three final microbial consortia enriched with 4-FA or 2,4-DFA had a higher degree of similarity than that of the three seeding sources, which might be due to the same operation conditions during the long-time enrichment (Cho et al. 2018).
The Venn plots showing the shared and unique OTU numbers among R1, R2 and R3 of seed (a), 4-FA enriched consortia (b) and 2,4-DFA (c) enriched consortia, and among seed, 4-FA enriched consortia and 2,4-DFA enriched consortia of R1 (d), R2 (e) and R3 (f), as well as the relative abundance of the top 10 phyla (g) and genera (h) of bacterial community in all treatments
The 15.0% (150 out of 1006), 25.77% (109 out of 423), 17.77% (139out of 782), 11.1%(112 out of 1006), 26.27% (113 out of 423), and 15.87% (124 out of 782)of the total OTUs in the three inocula were supplied for the corresponding enrichment consortia, namely FR1, FR2, FR3, DFR1, DFR2, and DFR3, respectively (Fig. 6d–f).After enrichment with 4-FA or 2,4-DFA, the OTUs shared with the corresponding inoculum accounted for 59.4% (151 out of 254), 43.1% (109 out of 253), 50.2%(139 out of 277), 56.8%(112 out of 197), 42.2%(113 out of 268), and 50.82%(124 out of 244) of the total OUTs in FR1, FR2, FR3, DFR1, DFR2 and DFR3, respectively (Fig. 6b–c). Additionally, 177 OTUs were shared among the OTUs of three enrichment cultures enriching with 4-FA, these shared OTUs accounted for 69.7% (177 out of 254), 70.0% (177 out of 253), and 63.9% (177 out of 277) of that in FR1, FR2, and FR3, respectively (Fig. 6b). A total of 142 OTUs were shared among the OTUs of the three final microbial consortia enriching with 2,4-DFA, and these shared OTUs accounted for 72.1% (142 out of 197), 53.0% (142 out of 268), and 58.2% (142 out of 244)of that in DFR1, DFR2, and DFR3, respectively (Fig. 6c).These results suggested 85.0–88.9% of OTUs in R1seed, 73.7–74.2% of OTUs in R2seed, and 82.2–84.13% of OTUs in the R3seed disappeared after acclimation with 4-FA or 2,4-DFA, explaining why the acclimation time required for three seeding sources is in the order of PMS > CIS > FHS. It was also found that some unique OTUs formed after acclimation with 4-FA or 2,4-DFA, and the differences among the OTUs of 2,4-DFA enriched consortia were more significant than that among the OTUs of the 4-FA enriched consortia. This indicated that the bacterial communities depended on the seeding sources (Cortes-Tolalpa et al. 2016; Song et al. 2015) and substrates (Chen et al. 2019).
Obviously, similar to the α-diversity, acclimation led to a more homogeneous microbial community among the three seeds, with decreasing unique OTUs and an increasing ratio of shared OTUs. Approximately half of the OTUs in the 4-FA-accilimated mixtures were not detected in the inocula, indicating an enrichment of rare OTUs from the inocula. Notably, the summary relative abundance of these OTUs was as high as 97.9% widely distributed in 17 phyla and 24 classes (Table 3). The OTUs affiliated to Chlorobi were most generally shared in 4-FA-enriched mixtures, accounting for 94% of the total reads from this phylum. Actinobacteria (86.0%), Proteobacteria (81.7%), and Gemmatimonadetes (70.4%) were also highly shared after enriched by 4-FA. However, less than 20% of reads from Firmicutes, Planctomycetes and Verrucomicrobia were shared, indicating a low defluorination capacity to these phyla. Compared with β- (80.8%), γ- (93.1%) and δ-proteobacteria (82.5%), α-proteobacteria (54.3%) was much less shared in the enrichments. Similar results were found in the 2,4-DFA-enriched mixtures. Moreover, nearly all the reads shared among the final consortia enriched with 4-FA were also shared among that of the three 2,4-DFA enriched consortia. This is reasonable because the release of a fluoride ion into the medium is the most critical step for the decomposition of all organic fluorides (Kiel and Engesser 2015).
Microbial community composition
Shifts in the relative abundance of the top ten phyla (Fig. 6g) and genera (Fig. 6h) as a result of the FAs acclimation were also analyzed. Chloroflexi, Acidobacteria, Planctomycetes, Deinococcus-Thermus and SBR1093 nearly all decreased in each final enrichment consortium, indicating these phyla played a very small role during the degradation of 4-FA and 2,4-DFA. Conversely, Proteobactoria, Gemmatimonadetes, and Bacteroidetes nearly all increased in each final enrichment consortium (Table S1). Proteobacteria and Bacteroidetes have been previously found to be responsible for the degradation of aniline (Jiang et al. 2019b; Li et al. 2020b). Therefore, they might be important during the degradation of 4-FA and 2,4-DFA. Additionally, Chlorobi was increased in the three 4-FA enrichment microbial consortia and the final consortium in DFR2, and Actinobacteria only increased in the three 2,4-DFA enrichment microbial consortia (p < 0.05, Table S1). Actinobacteria is thought to have a potential capacity to fluorotelomer alcohols (Yu et al. 2018) and aniline (Zhao et al. 2016) degradation. These results provide further evidence for this speculation.
After enrichment, six genera including Thermomonas, Dokdonella, Gemmatimonas, Terrimonas and Tahibacter increased in each final consortium. Moreover, Gemmatimonas and Tahibacter were not found in any seeding source. In addition, the shifts in some other relatively highly abundant unique genera were observed for the final consorti. Six genera including Variovorax, Diaphorobacteria, Ferruginibacteria, Pseudoxanthomonas, Woodsholea and unclassified_f__Chitinophagaceae significantly increased only in the three 4-FA enrichment consortia, while these genera including Microbacterium, Flavihumibacter, Bosea, Niabella, Filimonas, Taibaiella and unclassified_f__Comamonadaceae only increased in the 2,4-DFA enrichment consortia (p < 0.05, Table S1 and Table 4). This indicated that these genera were unique for the 4-FA enrichment cultures and 2,4-FA enrichment cultures. The relatively higher abundance of Variovorax in the three 4-FA enrichment consortia was 1.19%, 40.27% and 35.20%, that of Dokdonella was 18.05%, 8.72% and 6.45%, that of Diaphorobacter was 22.20%, 8.22% and 4.71%, and that of Ferruginibacter was 6.11%, 16.49% and 8.50%, respectively. The relatively higher abundance of Thermomonas in the three 2,4-DFA enrichment consortia was 55.26%, 23.51% and 33.08%, that of Gemmatimonas was 0.52%, 17.45% and 6.54%, that of Microbacterium was 11.93%, 13.34%and 3.95% and 4.71%, and that of Flavihumibacter was 2.77%, 2.09% and 19.44%, respectively. Obviously, there were many same genera were found among three final consortia enriched with 4-FA or 2,4-DFA, and the abundances were distinct. This result might explain the differences and the similarity among the biodegradation capacity of the final enrichment microbial consortia.
As previously reported, Thermomonas has been proven to be an important aniline-degrading microorganism (Hou et al. 2018); Variovorax and Diaphorobacter species are known to degrade chloroanilines (Breugelmans et al. 2010; Zhang et al. 2010); Pseudoxanthomonas is found to be responsible for reduction of aniline (Jiang et al. 2019b); Microbacterium specie is known to degrade chloroaniline (Dejonghe et al. 2002) and aniline (Kafilzadeh and Khezri 2015); Niabella can play a very important role in the biodegradation of polycyclic aromatic hydrocarbons (Wang et al. 2016) and Bisphenol S (Wang et al. 2019); Gemmatimonas has been reported in polycyclic aromatic hydrocarbon degradation system (Jiao et al. 2016) and azo dyes degradation system (Xu et al. 2018b). These results suggested that these genera, especially Gemmatimonas that was found only in the six final microbial consortia, might be significant for 4-FA or 2,4-DFA degradation, and the further study on these genera would be required in the future.
Effects of the inoculum source
Acclimation time, degradation rate constant, and degradation capacity to different initial concentrations and homologs can be used as indicators for choosing the optimal inoculum source. The inoculum source was found to have a major impact on the rates and efficiencies of 2,4-DFA biodegradation. For 2,4-DFA, the FHS-derived inoculum showed the shortest acclimation time and the strongest resistance to shock compared to the PMS-derived and CIS-derived inocula. In general, activated sludge from industrial wastewater treatment plants is affected by a variety of complex organics. With xenobiotic as C resources, sludge microorganisms must produce various exoenzymes to convert them into readily available forms. Therefore, the obtained results are not surprising given that the existing literature on xenobiotic biodegradation has often documented a requirement for specific microbial phylotypes to degrade specific contaminants (Moreno et al. 2005; Zhao et al. 2015). As described above, there were significant differences among the microbial communities of the three inocula, and the final enriched consortia shared the different OUTs percent with the corresponding inocula, which can explain, in part, the varied 2,4-DFA removals.
Conversely, the inocula had minor impacts on 4-FA biodegradation during the acclimation period, which might attribute to the smaller toxicity and the higher biodegradability (Zhao et al. 2015), as well as the shared OTUs among the three inocula. Similar results have been reported. For example, a similar fate of polycyclic aromatic hydrocarbons in anaerobic digesters inoculated with three microbial communities has also been observed (Braun et al. 2015). Cortes-Tolalpa et al. (2016) reported that different inocula produced distinctive microbial consortia with a similar lignocellulose degradation capacity. Combined with previous research about 2,3,4-TFA degradation using the distinct inocula (Zhao et al. 2019a), it could be inferred that the effect extents of the inocula on FAs degradation might depend on the fluorinated level of FAs. Hence, further studies are required in the future.
The bacterial communities from each seeding source were clearly distinct, which contributed to the differences in the overall community structures. Thermomonas was present in the PMS-derived inoculum, and a higher abundance appeared in the corresponding final cultures (2.25% in FR1, 55.26% in DFR1) in comparison with the other final consortis. Terrimonas was present in the PMS-derived and CIS-derived inocula, and a high abundance appeared in the corresponding final consortia even including the other final consortia over time. Taibaiella, and Dokdonella only appeared in DFR1 and DFR3 from FHS- derived and CIS-derived inocula.
In addition, the same inoculum source will establish distinct microbial communities after enrichment with 4-FA or 2,4-DFA. For example, Variovorax, Diaphorobacteria, Ferruginibacteria, Pseudoxanthomonas, Woodsholea, norank_f_P3OB-42 and unclassified_f__Chitinophagaceae only appeared in the 4-FA enrichment cultures, while Microbacterium, Flavihumibacter, Bosea, Niabella, Filimonas, Taibaiella, and unclassified_f__Comamonadaceae only appeared in the 2,4-DFA enrichment cultures. Whereas, Thermomonas, Microbacterium and Gemmatimonas increased in all 2,4-DFA reactors (p < 0.05, Table S1). Moreover, a more similar structure was found among three final consortia enriching with 4-FA in comparison to that among the three 2,4-DFA enrichment consortia These findings further confirmed the effect extent of the inocula might depend well on the fluorinated level of FAs.
Conclusions
In this study, information regarding the effects of three distinct seeding sources on the degradation of FAs, and the response to the fluorinated level of FAs was presented. The source of the microbial community was found to have a major impact on the rates and efficiencies of 2,4-DFA transformations, and this was found by comparing the performance of reactors inoculated with sludge with different compositions. Only a minor effect on the biodegradation of 4-FA was found during the acclimation and high concentration shock periods, while a major effect on the degradation rate constants of 4-FA using the three final enrichment consortia was observed. The 4-FA and 2,4-DFA removals were found to be substantially higher for the FHS-derived inoculum compared with the PMS- and CIS -derived inocula despite comparable biomasses. After acclimation, there was the lower biodiversity and similarity among the three 2,4-DFA enrichment consortia in comparison with that among the 4-FA enriched consortia. Thermomonas, Dokdonella, Gemmatimonas, Terrimonas, and Tahibacter could play critical roles in degrading 4-FA or 2,4-DFA.Variovorax, Diaphorobacteria, Ferruginibacteria, Pseudoxanthomonas, Woodsholea and unclassified_f__Chitinophagaceae could be linked with 4-FA degradation. Microbacterium, Flavihumibacter, Bosea, Niabella, Filimonas, Taibaiella and unclassified_f__Comamonadaceae could be responsible for 2,4-DFA degradation. The results showed that the inoculum source is an important factor that affects the treatment performance of FAs. In addition, the composition of the microbial community in the final consortia and the influence degree of the inoculum source depends on fluorinated level of the FAs.
References
Adams DEC, Halden RU (2010) Fluorinated chemicals and the impacts of anthropogenic use. ACS Symp Ser 1048:539–560
Alexandrino DAM, Ribeiro I, Pinto LM, Cambra R, Oliveira RS, Pereira F, Carvalho MF (2018) Biodegradation of mono-, di- and trifluoroacetate by microbial cultures with different origins. New Biotechnol 43:23–29. https://doi.org/10.1016/j.nbt.2017.08.005
Alves APA, Lima PS, Dezotti M, Bassin JP (2017) Impact of phenol shock loads on the performance of a combined activated sludge-moving bed biofilm reactor system. Int Biodeter Biodegr 123:146–155. https://doi.org/10.1016/j.ibiod.2017.06.015
Amorim CL, Carvalho MF, Afonso CMM, Castro PML (2013) Biodegradation of fluoroanilines by the wild strain Labrys portucalensis. Int Biodeter Biodegr 80:10–15. https://doi.org/10.1016/j.ibiod.2013.02.001
APHA, AWWA (2005) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, DC
Braun F, Hamelin J, Bonnafous A, Delgenes N, Steyer JP, Patureau D (2015) Similar PAH fate in anaerobic digesters inoculated with three microbial communities accumulating either volatile fatty acids or methane. PLoS ONE 10(4):1–20
Breugelmans P, Leroy B, Bers K, Dejonghe W, Wattiez R, De Mot R, Springael D (2010) Proteomic study of linuron and 3,4-dichloroaniline degradation by Variovorax sp WDL1: evidence for the involvement of an aniline dioxygenase-related multicomponent protein. Res Microbiol 161:208–218. https://doi.org/10.1016/j.resmic.2010.01.010
Carvalho G et al (2010) Biological treatment of propanil and 3,4-dichloroaniline: kinetic and microbiological characterisation. Water Res 44:4980–4991. https://doi.org/10.1016/j.watres.2010.08.06
Chen YW et al (2019) Microbial community assembly in detergent wastewater treatment bioreactors: influent rather than inoculum source plays a more important role. Bioresour Technol 287:121467. https://doi.org/10.1016/j.biortech.2019.121467
Chettri B, Singh AK (2019) Kinetics of hydrocarbon degradation by a newly isolated heavy metal tolerant bacterium Novosphingobium panipatense P5:ABC. Bioresour Technol 294:122190
Cho K, Choi M, Lee S, Bae H (2018) Negligible seeding source effect on the final ANAMMOX community under steady and high nitrogen loading rate after enrichment using poly(vinyl alcohol) gel carriers. Chemosphere 208:21–30. https://doi.org/10.1016/j.chemosphere.2018.05.155
Chong NM, Chen YS (2007) Activated sludge treatment of a xenobiotic with or without a biogenic substrate during start-up and shocks. Bioresour Technol 98:3611–3616. https://doi.org/10.1016/j.biortech.2006.11.031
Cortes-Tolalpa L, Jimenez DJ, Brossi MJD, Salles JF, van Elsas JD (2016) Different inocula produce distinctive microbial consortia with similar lignocellulose degradation capacity. Appl Microbiol Biotechnol 100:7713–7725. https://doi.org/10.1007/s00253-016-7516-6
Cui DZ, Shen D, Wu CR, Li C, Leng DJ, Zhao M (2017) Biodegradation of aniline by a novel bacterial mixed culture AC. Int Biodeter Biodegr 125:86–96. https://doi.org/10.1016/j.ibiod.2017.08.010
Dejonghe W, Goris J, Dierickx A, De Dobbeleer V, Crul K, De Vos P, Verstraete W, Top EM (2002) Diversity of 3-chloroaniline and 3,4-dichloroaniline degrading bacteria isolated from three different soils and involvement of their plasmids in chloroaniline degradation. Fems Microbiol Ecol 42:315–325. https://doi.org/10.1111/J.1574-6941.2002.Tb01021.X
Ding AQ, Zhao D, Ding F, Du SW, Lu HJ, Zhang M, Zheng P (2018) Effect of inocula on performance of bio-cathode denitrification and its microbial mechanism. Chem Eng J 343:399–407. https://doi.org/10.1016/j.cej.2018.02.119
Duque AF, Bessa VS, Carvalho MF, de Kreuk MK, van Loosdrecht MCM, Castro PML (2011) 2-Fluorophenol degradation by aerobic granular sludge in a sequencing batch reactor. Water Res 45:6745–6752. https://doi.org/10.1016/j.watres.2011.10.033
Eadsforth CV, Logan CJ, Morrison BJ, Warburton PA (1984) 2,4-difluoroaniline and 4-fluoroaniline exposure - monitoring by methemoglobin and urine analyses. Int Arch Occup Environ Health 54:223–232. https://doi.org/10.1007/Bf00379051
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. https://doi.org/10.1093/bioinformatics/btq461
Fan XY, Gao JF, Pan KL, Li DC, Zhang LF, Wang SJ (2018) Shifts in bacterial community composition and abundance of nitrifiers during aerobic granulation in two nitrifying sequencing batch reactors. Bioresour Technol 251:99–107. https://doi.org/10.1016/j.biortech.2017.12.038
Guo GY, Guan J, Sun SQ, Liu J, Zhao YJ (2020) Nutrient and heavy metal removal from piggery wastewater and CH4 enrichment in biogas based on microalgae cultivation technology under different initial inoculum concentration. Water Environ Res 92:922–933. https://doi.org/10.1002/wer.1287
Hou LF, Wu QP, Gu QH, Zhou Q, Zhang JM (2018) Community structure analysis and biodegradation potential of aniline-degrading bacteria in biofilters. Curr Microbiol 75:918–924. https://doi.org/10.1007/s00284-018-1466-4
Jiang Y, Wei L, Yang K, Wang HY (2019a) Investigation of rapid granulation in SBRs treating aniline-rich wastewater with different aniline loading rates. Sci Total Environ 646:841–849. https://doi.org/10.1016/j.scitotenv.2018.07.313
Jiang Y, Yang K, Shang Y, Zhang HN, Wei L, Wang HY (2019b) Response and recovery of aerobic granular sludge to pH shock for simultaneous removal of aniline and nitrogen. Chemosphere 221:366–374. https://doi.org/10.1016/j.chemosphere.2018.12.207
Jiao S, Chen WM, Wang ET, Wang JM, Liu ZS, Li YN, Wei GH (2016) Microbial succession in response to pollutants in batch-enrichment culture. Sci Rep 6:2179110
Kafilzadeh F, Khezri A (2015) Isolation and molecular characterization of aniline resistant bacteria from soil around shiraz refinery. Fresenius Environ Bull 24:2232–2237
Khehra MS, Saini HS, Sharma DK, Chadha BS, Chimni SS (2005) Decolorization of various azo dyes by bacterial consortium. Dyes Pigm 67:55–61
Kiel M, Engesser KH (2015) The biodegradation vs. biotransformation of fluorosubstituted aromatics. Appl Microbiol Biotechnol 99:7433–7464. https://doi.org/10.1007/s00253-015-6817-5
Kim S, Rossmassler K, Broeckling CD, Galloway S, Prenni J, De Long SK (2017) Impact of inoculum sources on biotransformation of pharmaceuticals and personal care products. Water Res 125:227–236. https://doi.org/10.1016/j.watres.2017.08.041
Li C, Zhang X, Lu Y, Fan Z, Wang TC, Zhang GL (2020a) Cometabolic degradation of p-chloroaniline by the genus Brevibacillus bacteria with extra carbon sources. J Hazard Mater 383:121198
Li Y, Zhang Q, Li M, Sang WJ, Wang Y, Wu LF, Yang YQ (2020b) Bioaugmentation of sequencing batch reactor for aniline treatment during start-up period: investigation of microbial community structure of activated sludge. Chemosphere 243:125426
Moreno B, Gomez MA, Ramos A, Gonzalez-Lopez J, Hontoria E (2005) Influence of inocula over start up of a denitrifying submerged filter applied to nitrate contaminated groundwater treatment. J Hazard Mater 127:180–186. https://doi.org/10.1016/j.jhazmat.2005.07.002
Mosneang CL, Grozea A, Oprescu I, Dumitrescu E, Muselin F, Gal D, Cristina RT (2014) Assessment of 2,4-difluoroaniline aquatic toxicity using A Zebrafish (Danio rerio) model. Thai J Vet Med 44:445–452
Movahedyan H, Assadi A, Amin MM (2008) Effects of 4-chlorophenol loadings on acclimation of biomass with optimized fixed time sequencing batch reactor. Iran J Environ Health 5:225–234
Murphy CD (2016) Microbial degradation of fluorinated drugs: biochemical pathways, impacts on the environment and potential applications. Appl Microbiol Biotechnol 100:2617–2627. https://doi.org/10.1007/s00253-016-7304-3
Orozco AMF, Lobo CC, Contreras EM, Zaritzky NE (2013) Biodegradation of bisphenol-A (BPA) in activated sludge batch reactors: analysis of the acclimation process. Int Biodeter Biodegr 85:392–399. https://doi.org/10.1016/j.ibiod.2013.09.005
Osuna MB, Sipma J, Emanuelsson MAE, Carvalho MF, Castro PML (2008) Biodegradation of 2-fluorobenzoate and dichloromethane under simultaneous and sequential alternating pollutant feeding. Water Res 42:3857–3869. https://doi.org/10.1016/j.watres.2008.05.011
Paule A, Biaz A, Leflaive J, Lawrence JR, Rols JL (2015) Fate of the herbicide alachlor exposed to different microbial consortia in aquatic systems. Water Air Soil Pollut 226:3
Sahinkaya E, Dilek FB (2005) Biodegradation of 4-chlorophenol by acclimated and unacclimated activated sludge - Evaluation of biokinetic coefficients. Environ Res 99:243–252. https://doi.org/10.1016/j.envres.2004.11.005
Smulek W, Zdarta A, Kwiczak J, Zgola-Grzeskowiak A, Cybulski Z, Kaczorek E (2017) Environmental biodegradation of halophenols by activated sludge from two different sewage treatment plants. J Environ Sci Health Part A 52:1240–1246. https://doi.org/10.1080/10934529.2017.1356197
Song EX, Wang MZ, Shen DS (2014) Isolation, identification and characterization of a novel Ralstonia sp FD-1, capable of degrading 4-fluoroaniline. Biodegradation 25:85–94. https://doi.org/10.1007/s10532-013-9642-5
Song ZW, Li T, Wang QX, Pan Y, Li LX (2015) Influence of microbial community structure of seed sludge on the properties of aerobic nitrifying granules. J Environ Sci 35:144–150. https://doi.org/10.1016/j.jes.2015.01.033
Vazquez JA, Rial D (2014) Inhibition of selected bacterial growth by three hydrocarbons: mathematical evaluation of toxicity using a toxicodynamic equation. Chemosphere 112:56–61. https://doi.org/10.1016/j.chemosphere.2014.03.008
Wang C, Lu GH, Zhou YJ (2007) Biodegradability of chlorinated anilines in waters. Biomed Environ Sci 20:141–145
Wang FK, Li C, Wang HJ, Chen WL, Huang QY (2016) Characterization of a phenanthrene-degrading microbial consortium enriched from petrochemical contaminated environment. Int Biodeter Biodegr 115:286–292. https://doi.org/10.1016/j.ibiod.2016.08.028
Wang XW, Chen JQ, Ji R, Liu YH, Su Y, Guo RX (2019) Degradation of bisphenol S by a bacterial consortium enriched from river sediments. Bull Environ Contamin Toxicol 103:630–635. https://doi.org/10.1007/s00128-019-02699-7
Wang ZL, Huang JSH (2005) The treatment of wastewater with high concentration of aniline. J Northeast For Univ 33:108–113 ((In chinese))
Wosman A et al (2016) Effect of operational strategies on activated sludge’s acclimation to phenol, subsequent aerobic granulation, and accumulation of polyhydoxyalkanoates. J Hazard Mater 317:221–228. https://doi.org/10.1016/j.jhazmat.2016.05.074
Xu H et al (2018a) Evaluation of microbial p-chloroaniline degradation in bioelectrochemical reactors in the presence of easily-biodegrading cosubstrates: degradation efficiency and bacterial community structure. Bioresour Technol 270:422–429. https://doi.org/10.1016/j.biortech.2018.09.064
Xu JL, Zheng C, Mou YY, Xu B, Zhu YL (2018b) Development and application of mixed cultures capable for decolorating and mineralizing azo dyes with an anaerobic-aerobic circle method. Desalin Water Treat 132:317–328. https://doi.org/10.5004/dwt.2018.23126
Yu XL, Nishimura F, Hidaka T (2018) Effects of microbial activity on perfluorinated carboxylic acids (PFCAs) generation during aerobic biotransformation of fluorotelomer alcohols in activated sludge. Sci Total Environ 610:776–785. https://doi.org/10.1016/j.scitotenv.2017.08.075
Zhang T, Ren HF, Liu Y, Zhu BL, Liu ZP (2010) A novel degradation pathway of chloroaniline in Diaphorobacter sp PCA039 entails initial hydroxylation. World J Microb Biot 26:665–673. https://doi.org/10.1007/s11274-009-0221-1
Zhao YS, Qu D, Zhou R, Ma YG, Wang H, Ren HJ (2016) Bioaugmentation with GFP-Tagged Pseudomonas migulae AN-1 in aniline-contaminated aquifer microcosms: cellular responses, survival and effect on indigenous bacterial community. J Microbiol Biotechnol 26:891–899. https://doi.org/10.4014/jmb.1511.11070
Zhao ZQ, Shen XL, Zheng TC, Abbas G, Fan R, Li YM (2019a) Evaluation of inoculum sources for aerobic treatment of 2,3,4-trifluoroaniline during start-up and shock. Water Air Soil Pollut. https://doi.org/10.1007/s11270-019-4346-z
Zhao ZQ, Tian BH, Zhang X, Ghulam A, Zheng TC, Shen DS (2015) Aerobic degradation study of three fluoroanilines and microbial community analysis: the effects of increased fluorine substitution. Biodegradation 26:1–14. https://doi.org/10.1007/s10532-014-9704-3
Zhao ZQ, Zheng TC, Zhang WJ, Shen XL, Lv L, Li YM (2019b) Degradation of 3-fluoroanilne by Rhizobium sp. JF-3. Biodegradation 30:433–445. https://doi.org/10.1007/s10532-019-09885-8
Zhou YK, Zou QP, Fan MJ, Xu Y, Chen YW (2020) Highly efficient anaerobic co-degradation of complex persistent polycyclic aromatic hydrocarbons by a bioelectrochemical system. J Hazard Mater 381:120945
Acknowledgements
This work was partially supported by the National Natural Science Foundation of China (No. 21607092); the Public Technology Research Program of Zhejiang Province (No. LZY21E080001; LGG19B020001). We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, ZQ., Wei, XM., Shen, XL. et al. Aerobic degradation of 4-fluoroaniline and 2,4-difluoroaniline: performance and microbial community in response to the inocula. Biodegradation 32, 53–71 (2021). https://doi.org/10.1007/s10532-021-09925-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-021-09925-2