Abstract
A novel degradation pathway of chloroaniline in Diaphorobacter sp. PCA039 entails initial hydroxylation. A novel gene cluster (pca), involved in the degradation of p-chloroaniline, was identified from Diaphorobacter sp. PCA039 capable of utilizing p-chloroaniline as sole carbon, nitrogen and energy source for growth. There were 29 ORFs in the pca cluster, in the order of pcaTRKLMNOPR 1 D 1 C I U 1 R 2 D 2 C 2 U 2 EFGXJHYWZI 1 I 2 QS. Based on sequence analysis, they were putatively identified as encoding a multicomponent phenol-hydroxylating oxygenase (pcaKLMNOP), two extradiol ring-cleavage dioxygenases, transcriptional regulatory proteins, enzymes mediating chlorocatechol degradation, and transportation functions. The genes pcaKLMNOP exhibited significant sequence identity (94%) to those of phenol hydroxylases (PH) from other bacteria, inferring that they might encode a multicomponent PH. This PH activity was also functionally characterized with the recombinant strain E. coli TOP10-S201 showing only PH activity, indicating that the degradation of p-chloroaniline by strain PCA039 was initiated by hydroxylation instead of normal dioxygenation. The other nineteen genes, pcaR 1 D 1 C I U 1 R 2 D 2 C 2 U 2 EFGXJHYWZI 1 I 2 , encode for further degradation of p-chloroaniline to intermediates of the TCA-cycle and transposases. RT-PCR revealed that the hydrolytic (pcaF) and dehydrogenetic pathways (pcaE, pcaH), the two degrading branches, are both necessary for complete degradation of aniline, p-chloroaniline, phenol and also 4-aminophenol; and of the two C23O sets, PcaR1D1C1U1 and PcaR2D2C2U2, PcaC1 is produced in the degradation of above four substrates, while PcaC2 is only expressed in p-chloroaniline degradation, suggesting that both C23O sets are needed for complete degradation of p-chloroaniline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chloroanilines constitute a group of xenobiotics that have been in industrial use for a long time in the production of paints, pesticides, plastics, and pharmaceuticals (Meyer 1981). This group of compounds is considered as important environmental pollutants and accumulation in the long run may be detrimental to human health as a result of their persistence, toxicity, and transformation into hazardous intermediates (Pieper and Reineke 2004; Pizon et al. 2009). A number of bacterial species, capable of utilizing chloroanilines as sole source of nutrients, have been isolated from various environments such as a bioreactor (Kaminski et al. 1983), activated sludge (Boon et al. 2001; Ren et al. 2005) and soil (Vangnai and Petchkroh 2007). In bacteria, aerobic catabolic pathways for aromatic hydrocarbon degradation can schematically be divided into two major biochemical steps. First, early reactions, the so-called upper pathways or peripheral routes, channel the hydrocarbons towards the formation of partially oxidized aromatic intermediates. Then, dihydroxylated aromatic molecules that can undergo the cleavage of the ring are produced and further processed to give compounds that can enter the tricarboxylic acid (TCA) cycle. Although a wide variety of very different peripheral routes for the oxidation of many different aromatic hydrocarbons exist, only a limited number of dihydroxylated compounds that can be cleaved and productively processed to enter the TCA cycle are known. However, until now, the peripheral routes for the bacterial aerobic degradation of aniline and substituted anilines have been found only to be initiated by the reaction catalyzed by aniline dioxygenase among those bacteria, such as Frateuria species ANA-18 (Murakami et al. 2003), Delftia acidovorans strain 7N (Urata et al. 2004), D. tsuruhatensis AD9 (Liang et al. 2005) and Delftia sp. AN3 (Zhang et al. 2008); and never by any other peripheral routes such as hydroxylation, which has normally been found in the degradation of phenol and toluene (Divari et al. 2003; Griva et al. 2003). The reactions for oxygenative ring fission of catechol and the subsequent conversion to TCA intermediates are limited to one of two metabolic alternatives: i.e., (1) intradiol (ortho and modified ortho)-cleavage, and (2) extradiol (meta)-cleavage. The use of these two cleavage routes is dependent upon the microbial species and/or the nature of the growth substrate. Ortho-cleavage is the dominant cleavage mechanism in the degradation of chlorinated compounds, as extradiol cleavage of halocatechols may produce dead-end or suicide metabolites (Klecka and Gibson 1981; Surovtseva et al. 1980).
Here we report gene cloning, DNA sequencing, and partial functional analysis of the complete chloroaniline-degrading cluster (pca), from Diaphorobacter sp. PCA039; and homology analysis and enzyme assays indicate that the degradation of p-chloroaniline by strain PCA039 is initiated by hydroxylation instead of dioxygenation.
Materials and methods
Chemicals
Aniline, p-aminophenol, phenol, and p-chloroaniline were from Sinopharm Chemical Reagent Beijing Co., Ltd. Catechol and 4-chlorocatechol were from Sigma–Aldrich® Co.
Bacterial strains, plasmids, and culture conditions
The bacterial strains and plasmids used in this study are listed in Table 1. All Escherichia coli strains were grown in Luria–Bertani (LB) medium (Sambrook and Russell 2001). Diaphorobacter sp. PCA039 was able to utilize p-chloroaniline as sole carbon, nitrogen and energy source for growth and was grown at 30°C in mineral salt medium (Ren et al. 2005).
DNA preparation and manipulation
Escherichia coli was grown aerobically in a Luria–Bertani medium, and chemically competent cells were transformed by heat shock. Extraction of genomic DNA was carried out following the procedures of Sambrook and Russell (2001). Recombinant plasmid DNA was isolated with a Tian-prep Mini kit (TianGen Biotech CO., Ltd). Restriction enzymes, T4 DNA ligase and calf intestinal alkaline phosphatases were from New England Biolabs (USA) or Takara (Tokyo, Japan).
Construction of genomic DNA library of strain PCA039
Genomic DNA of Diahorobacter sp. PCA039 was partially sheared with a Hamilton syringe to obtain approximately 40 kb-size fragments. The partially treated DNA fragments were end-repaired by End-repair enzyme mix of Copycontrol™ Fosmid Library Production Kit (Cat. No. CCFOS059) and then ligated to the cloning-ready Copycontrol pCC2FOS vector, and the recombinant molecules were packaged into λ phage followed by phage transfection to E. coli EPI300 strain by using protocols described in the MaxPlax™ Lambda packaging kit (Epicentre Biotechnologies, Madison, Wisconsin, USA).
Cloning of the catechol 2, 3-dioxygenase gene from strain PCA039 and library screening
DNA sequences for catechol 2, 3-dioxygenase (C23O) gene from Acidovorax sp. JS42 (accession number YP_984548) and other bacteria were used to design a pair of degenerate primers, CDf65: 5′-AACCAYGTDGCWTACAAGGT-3′ and CDr235: 5′- GHCTTGAGBACRTCRTGCCA-3′ (S=C, G; Y=C, T; B=C, G, T; H=A, C, T; R=A, G; W=A, T; D=A, G, T) for PCR amplification of a part (510 bp) of C230 gene from genomic DNA of Diahorobacter sp. PCA039, and it was confirmed by sequencing and BLAST search. To screen the positive clones, colony PCR was performed using this pair of primers. Recombinant E. coli strains with PCR-positive were further confirmed by spraying catechol solution (10 mM) onto the colonies for detection of C23O activity.
DNA sequencing and sequence analysis
DNA sequencing of the recombinant Fosmid was performed by the shotgun method by SinoGenoMax Co., Ltd (Chinese National Human Genome Center, Beijing). The ORFs were analysed using DNAstar (Lynnon Biosoft) and Vector NTI 10.0 software (Invitrogen, USA); homology search for protein sequences was carried out using the BLAST and FASTA programs (Altschul et al. 1990; Pearson 1990). The phylogenetic tree were generated using the neighbor joining method of Saitou and Nei (1987) with MEGA 4.0 software (Tamura et al. 2007), and multiple sequence alignment was done using Clustal_X (Thompson et al. 1997).
Preparation of intact cell suspension, crude enzymes and assays of enzyme activity
Recombinant strains (E. coli TOP10-S201, E. coli TOP10-S202 and E. coli TOP10-S203, Table 1) were grown to a density of A 600 about 1.0–1.5, and then the cells were harvested by centrifugation at 10,000×g for 10 min. The cell pellets were washed twice in 50 mM sodium phosphate buffer (pH 7.6) containing 1 mM 2-mercaptoethanol, and resuspended in the same buffer. Cells were disrupted sonically by twenty 4-s 200-W bursts on ice with a Braun-Sonic 1510 apparatus. Cellular debris was removed by centrifugation at 120,000×g at 4 °C for 15 min, and the supernatant was used immediately for enzyme assays.
Phenol hydroxylase (EC 1.14.13.7) (PH) activity was assayed by measuring the decrease in A 340, using NADPH as the co-substrate (Cafaro et al. 2004). One unit of enzyme activity is defined as the amount of enzyme caused the oxidation of 1 μmol of NADPH per min.
Dioxygenase activity was determined with a Clark oxygen electrode (YSI, Ohio, USA), according to the method of Fukumori and Saint (1997). To estimate the endogenous respiratory rate, 0.3 ml of sterile distilled water instead of substrate solution was used in a parallel experiment.
Catechol 2,3-dioxygenase (EC 1.13.11.2) (C23O) activity was measured according to Nakazawa and Yokota (1973) using a DU-7 spectrophotometer (Beckman, USA). The absorption coefficient of 2-hydroxymuconic semialdehyde was 12,000 l mol−1 cm−1. Enzyme specific activities are reported as μmol of 2-hydroxymuconic semialdehyde produced per minute per mg of protein.
Protein was determined according to the Bradford method with bovine serum albumin as the standard.
Transcription detection of genes in degradation of four substrates by RT-PCR
Diaphorobacter sp. PCA039 was grown on chloroaniline, aniline, phenol and 4-aminophenol for 72 h, respectively. Then the cells were harvested by centrifugation and frozen by immersion in liquid N2, respectively. Total RNA was prepared by using TRIZOL kit (Invitrogen) according to the manufacturer’s instructions, further purified by Axyprep™ Multisource total RNA miniprep kit (Axygen) according to the manufacturer’s instructions, and used as template for gene expression analysis by reverse transcriptase-PCR (RT-PCR) according to the methods of Sambrook and Russell (2001) and the primers as listed in Table 2. The RT-PCR products were then authenticated by sequencing.
Nucleotide sequence accession number
The nucleotide sequence of the p-chloroaniline degradation gene cluster (pca) of Diaphorobacter sp. PCA039 has been deposited in the DDBJ/EMBL/GenBank under the accession number of FJ601374.
Results
Screening for C230 gene from genomic library of strain PCA039
A genomic library of strain PCA039 was constructed with more than 13, 000 recombinant strains of E. coli EPI300 harboring about 40 kb-size genomic DNA from Diaphorobacter sp. PCA039. From three of them, the specific fragment (510 bp) could be amplified, showing that they might carry the C23O gene from strain PCA039. A recombinant strain, E. coli EPI300-PCA1, contained a recombinant Fosmid, named FosB12, which carried a 28 kb DNA fragment from strain PCA039, was obtained (Table 1).
The pca cluster and the genes involved in p-chloroaniline degradation
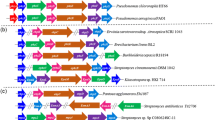
We obtained the DNA sequence of the ecombinant Fosmid, FosB12, identified 29 ORFs in a cluster spanning ~28 kb (Fig. 1) and named it as the pca cluster. Sequence and ORF analyses of this total 28 kb fragment indicated that there were 29 intact ORFs (Fig. 1a; Table 3), and the deduced products showed significant identity (73–100%, Table 3) to functionally identified proteins from other bacteria, especially to those from Acidovorax sp. JS42 (Copeland et al. 2006), a phenol-degrader. The high identity might explain the functions of these orfs. Among them, at least 20 (pcaRKLMNOPR 1 D 1 C I U 1 R 2 D 2 C 2 U 2 EFGJI 1 I 2 ) were expected to contribute towards complete metabolism of p-chloroaniline to TCA-cycle intermediates via the meta-cleavage pathway as shown in Fig. 1b and summarized in Table 3. Homology analysis of the pca cluster revealed that it consists at least three parts as follows:
Genetic organization of pca cluster (a) and putative chloroaniline degradation pathway (c) of Diaphorobacter sp. PCA039. The sequenced region of 28.4 kb is shown. Black arrows indicate PH genes, open and grey arrows indicate meta-cleavage pathway genes. b Details of plasmid construction are provided in Table 1. The function of other genes was explained in Table 3. Abbreviations: PH Phenol hydroxylase, AD aniline dioxygenase, C23O catechol 2, 3-dioxygenase, HMSD 2-hydroxymuconic semialdehyde dehydrogenase (EC), HMSH 2-hydroxymuconic semialdehyde hydrolase (EC 3.7.1.9), OCD 4-oxalocrotonate decarboxylase (EC 4.1.1.77), OEH 2-oxopent-4-dienoate hydratase, HOA 4-hydroxy-2-oxovalerate aldolase (EC 4.1.3.39), ADA acetaldehyde dehydrogenase. + Positive, − negative, S Sau 3AI site
Degradative genes of the pca cluster
The products of pcaKLMNOP, PcaKLMNOP, showed significant sequence homology (73–94%) with a multicomponent phenol hydroxylase (PH), present in phenol-degrading strains such as Alcaligenes faecalis IS-46 (Zhu et al. 2008) and Acidovorax sp. JS42 (Copeland et al. 2006), especially with those from Acidovorax sp. JS42. The remaining 14 gene products (PcaR1D1CIU1R2D2C2U2EFGJI1I2) exhibited considerable amino acid identity (73–100%) to enzymes of central catechol metabolism via the meta-cleavage pathway found in aniline-degrading and other aromatic-degrading bacteria. Interestingly, in the pca cluster, there were two C23O sets, PcaR1D1CIU1 and PcaR2D2C2U2. Phylogenetic analysis (data not shown) indicated that PcaC1 and PcaC2 belonged to two different branches in the phylogenetic tree, and shared only 41.9% identity and 57.5% similarity between themselves.
Regulatory genes
There are at least three regulatory genes (pcaR, pcaR 1 and pcaR 2 ) and one potential candidate orfZ in the pca cluster. The deduced product (PcaR) of pcaR is a large protein with 596 amino acid residues. PcaR shows as high as 94% sequence identity (Table 3) to a Sigma 54 specific transcriptional regulator from Acidovorax sp. JS42 (Copeland et al. 2006). A conserved domain (CD) search reveals that a region of 180 residues at the C terminus homologous to the DNA-binding domains of various regulators (Calogero et al. 1994; Canellakis et al. 1993), in addition to a region corresponding to a proteobacterial transcriptional activator domain (PAD) at the N terminus and the V4R (vinyl 4 reductase) domain predicted to bind small molecules such as hydrocarbons. Sigma 54 specific regulator is a transcriptional activator found in the aromatic-compound-degrading cluster from bacteria such as Acidovorax sp. JS42. The predicted products of pcaR 1 and pcaR 2 show 98 and 93% sequence identity, respectively, to a LysR family transcriptional regulator found in aromatic compound degradation from Comamonas sp. JS765 and other homologous activators found in a variety of Proteobacteria. It is likely that PcaR1 and PcaR2 function by regulating two C23O systems. OrfZ shows high identity (55–100%) to the GntR family transcriptional regulator found in proteobacterial strains such as Acidovorax sp. JS42 (Copeland et al. 2006) and R. eutropha JMP134 (Kim et al. 1996).
Transposase genes and genes with unknown function
Besides the degradative genes and regulatory genes, in the pca cluster, orfU1 and orfU2 are located in the two C23O systems, which is a common phenomenon in the meta-cleavage pathway, though their exact function is unknown. Another gene, orfY, encodes a putative membrane transport protein similar to aromatic aminotransferase and transmembrane transport proteins found in other bacteria such as Delftia sp. AN3 (Zhang et al. 2008). Moreover, on the up- and down-stream of the pca cluster were located the transposases and hypothesized exodeoxyribonuclease III Xth, which were encoded by pcaT and pcaQ, respectively. These gene products are believed to function in horizontal gene transfer and protection among the strains. In addition, two orfs (orfX, pcaQ) also found with high similarity to those existed in the meta degradation pathway of an aniline degrader, D. tsuruhatensis AD9 (Liang et al. 2005) and a phenol degrader, Acidovorax sp. JS42 (Copeland et al. 2006), in addition to P. putida UCC22 (Fukumori and Saint 1997). However, the functions of their products have still not been identified.
Function of the genes pcaKLMNOP
As revealed in the homologous analysis, the products of genes pcaKLMNOP exhibited significant homology (Table 3) to those of multicomponent PH found in phenol degraders such as Alcaligenes faecalis IS-46 (Zhu et al., the afp products). However, they were not homologous to those of multicomponent aniline dioxygenase (AD) commonly found in aniline and substituted aniline degraders such as D. tsuruhatensis AD9 (Liang et al. 2005), Delftia sp. AN3 (Zhang et al. 2008) and D. acidovorans strain 7N(Urata et al. 2004). This might infer that the degradation of p-chloroaniline by strain PCA039 is unusually initiated by hydroxylation, as the degradation of phenol, not initiated by dioxygenation as the degradation of aniline and substituted anilines. To date, the degradation of aniline and substituted anilines was found only to be started with the peripheral reaction catalyzed by AD (Fujii et al. 1997; Liang et al. 2005; Murakami et al. 2003; Urata et al. 2004; Zhang et al. 2008); never started with any other peripheral steps. In order to confirm the function of the genes pcaKLMNOP, they, as a whole, were subcloned into plasmid pUC118 (Table 1) and transformed into E. coli TOP10. A recombinant strain, E. coli TOP10-S201, was obtained. Then recombinant strain E. coli TOP10-S201 was used for PH and AD assays.
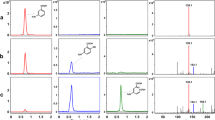
As shown in Figs. 1 and 2, recombinant strain E. coli TOP10-S201 did only exhibit PH activity on substrates such as aniline, phenol, 4-aminophenol and chloroaniline, especially on aniline. However, it never exhibited AD activity on any substrates (Fig. 1). This result suggested that the degradation of p-chloroaniline by Diaphorobacter sp. PCA039 was initiated by hydroxylation instead of normal dioxygenation. After the regulatory gene pcaR knock-out, the mutant strain indeed lost the ability of degrading chloroaniline (data not shown), also suggesting the products of genes pcaKLMNOP are necessary for chloroaniline degradation. This is a novel peripheral route for the degradation of chloroanilines that has never been reported before.
Phenol hydroxylase assay of recombinant strain E. coli TOP10-S201 on various substrates, as expressed by absorption at 340 nm (A
340) due to the consumption of NADPH. A
340 was recorded every 30 s and NADPH was added to all samples.  control l, E. coli TOP10/PH only;
control l, E. coli TOP10/PH only;  control 2, E coli TOP10 + aniline;
control 2, E coli TOP10 + aniline;  E. coli TOP10/PH + 4-aminophenol;
E. coli TOP10/PH + 4-aminophenol;  E. coli TOP10/PH + chloroaniline;
E. coli TOP10/PH + chloroaniline;  E. coli TOP10/PH + phenol;
E. coli TOP10/PH + phenol;  E. coli TOP10/PH + aniline
E. coli TOP10/PH + aniline
Prelude to gene expression and transcripts analysis of pca cluster when strain PCA039 grew on different substrates.
Transcripts of several genes of the pca cluster when strain PCA039 was grown on different substrates are represented in Fig. 3. This illustrates that all of the detected genes except pcaC2 were expressed when strain PCA039 grew on aniline, phenol, 4-aminophenol and chloroaniline; however, the gene pcaC 2 was only expressed when strain PCA039 grew on chloroaniline. These results confirmed the degradation of all above four substrates to be initiated with the “hydroxylation” peripheral route instead of the “dioxygenation” peripheral route, then followed the meta-cleavage pathway in strain PCA039. Both the expression of the genes pcaF, and pcaE and pcaH means that the two degrading branches (hydrolysis and dehydrogenation) are both necessary for complete degradation of these substrates. Furthermore, the two C23O sets (pcaC 1 and pcaC 2 ) in pca cluster, pcaC 1 were expressed in the degradation of all above four substrates, while pcaC2 was only expressed in the degradation of chloroaniline. It was strongly suggested that pcaC 2 might necessarily function in chlorocatechol cleavage, in turn PcaC1 was enough for catechol metabolism. In addition, PcaC1 and PcaC2 have distinct substrate specificity, PcaC2 showed relatively high activity on chlorocatechol (Catechol, 100%; chlorocatechol, 140%), while TdnC1 showed less activity on chlorocatechol (Catechol, 100%; chlorocatechol, 7.5%). According to these results, a hypothetic pathway for the degradation of chloroaniline by strain PCA039 was proposed (Fig. 1c).
RT-PCR detection of gene expression when strain PCA039 grew on different substrates. a RNA preparations after purification (RL10000, RNA markers, Takara) from cells grown on: 1 chloroaniline, 2 aniline, 3 phenol, 4 4-aminophenol. b RT-PCR products on 1.5% agarose gel. DL2000, DNA marker (Takara). Four substrates used for growing strain PCA039 were indicated. pcaL, phenol hydroxylase subunit L; pcaC1, catechol 2, 3-dioxygenase (C1); pcaC2, catechol 2,3-dioxygense (C2); pcaE, HMSD; pcaF, HMSH; pcaH, 4-oxalocrotonate decarboxylase; and pcaJ, 4-hydroxy-2-oxovalerate aldolase gene
Discussion
Previous studies had pointed out that the first step in the degradation of aniline and substituted anilines was always dioxygenation, never degradation by any other peripheral routes (Fukumori and Saint 1997; Liang et al. 2005; Murakami et al. 2003; Urata et al. 2004; Zeyer et al. 1985; Zhang et al. 2008). Here, it can be concluded that the degradation of p-chloroaniline by Diaphorobacter sp. PCA039 is a novel peripheral route for the metabolism of anilines. Zeyer et al. deemed that the “normal” ortho ring fission pathway widely existed in the microbial degradation of aromatic compounds, chloroanilines, benzoate, phenol (Zeyer et al. 1985). And also, this led Janke et al. (1988, 1989) to propose that complete degradation of chloroaromatics should satisfy the following qualifications, i.e., (1) it should be a low specificity oxygenase system; (2) be short of, or with a blocked meta-pathway; and (3) have a modified ortho-cleavage pathway. However, productive meta-cleavage pathways in several strains have recently been shown to exist, capable of degrading chloroaromatic compounds via meta-cleavage systems (Surovtseva et al. 1980; Arensdorf and Focht 1995; Ren et al. 2005). The different phylogenetic position and low identity of PcaC1 and PcaC2 inferred that they might have evolved from different ancestors. In addition, it was reported that TdnC and TadC1 had relatively high activity on substituted catechols (methylcatechols), while TdnC2 and TadC2 have showed less activity on these substituted catechols (Fukumori and Saint 1997; Liang et al. 2005), and the authors assumed that it might be necessary for cells to acquire another C23O for these methylcatechols to expand the assimilation range for toluidines. In contrast, in strain PCA039, PcaC1 was produced on all four substrates, while PcaC2 was only produced on chloroaniline, as revealed by RT-PCR (Fig. 3). This might be due to only chloroaniline being able to induce the expression of pcaC 2 . Furthermore, PcaC2 showed very high activity on chlorocatechol, while PcaC1 had very low activity on chlorocatechol, suggesting that both PcaC1 and PcaC2 are necessary for the vigorous degradation of chloroaniline by strain PCA039.
References
Altschul SF, Gish W, Miller W et al (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Arensdorf JJ, Focht DD (1995) A meta cleavage pathway for 4-chlorobenzoate, an intermediate in the metabolism of 4-chlorobiphenyl by Pseudomonas cepacia P166. Appl Environ Microbiol 61:443–447
Boon N, Goris J, De Vos P et al (2001) Genetic diversity among 3-chloroaniline- and aniline-degrading strains of the Comamonadaceae. Appl Environ Microbiol 67:1107–1115
Cafaro V, Izzo V, Scognamiglio R et al (2004) Phenol hydroxylase and toluene/o-xylene monooxygenase from Pseudomonas stutzeri OX1: interplay between two enzymes. Appl Environ Microbiol 70:2211–2219
Calogero S, Gardan R, Glaser P et al (1994) RocR, a novel regulatory protein controlling arginine utilization in Bacillus subtilis, belongs to the NtrC/NifA family of transcriptional activators. J Bacteriol 176:1234–1241
Canellakis ES, Paterakis AA, Huang SC et al (1993) Identification, cloning, and nucleotide sequencing of the ornithine decarboxylase antizyme gene of Escherichia coli. Proc Natl Acad Sci USA 90:7129–7133
Copeland A, Lucas S, and Lapidus A (2006) Complete sequence of chromosome 1 of Acidovorax sp. Js42. Submitted to the embl/genbank/ddbj databases
Divari S, Valetti F, Caposio P et al (2003) The oxygenase component of phenol hydroxylase from Acinetobacter radioresistens S13. Eur J Biochem 270:2244–2253
Fujii T, Takeo M, Maeda Y (1997) Plasmid-encoded genes specifying aniline oxidation from Acinetobacter sp. strain YAA. Microbiology 143:93–97
Fukumori F, Saint CP (1997) Nucleotide sequences and regulational analysis of genes involved in conversion of aniline to catechol in Pseudomonas putida UCC22(pTDN1). J Bacteriol 179:399–408
Griva E, Pessione E, Divari S et al (2003) Phenol hydroxylase from Acinetobacter radioresistens S13. Isolation and characterization of the regulatory component. Eur J Biochem 270:1434–1440
Janke D, Almofarji T, Straube G et al (1988) Critical steps in degradation of chloroaromatics by Rhodococci. 1. Initial enzyme-reactions involved in catabolism of aniline, phenol and benzoate by Rhodococcus sp. AN-117 and AN-213. J Basic Microbiol 28:509–518
Janke D, Ihn W, Tresselt D (1989) Critical steps in degradation of chloroaromatics by Rhodococci. 4. Detailed kinetics of substrate removal and product formation by resting pre-adapted cells. J Basic Microbiol 29:305–314
Kaminski U, Janke D, Prauser H et al (1983) Degradation of aniline and monochloroanilines by Rhodococcus sp. An 117 and a pseudomonad: a comparative study. Z Allg Mikrobiol 23:235–246
Kim Y, Ayoubi P, Harker AR (1996) Constitutive expression of the cloned phenol hydroxylase gene(s) from Alcaligenes eutrophus JMP134 and concomitant trichloroethylene oxidation. Appl Environ Microbiol 62:3227–3233
Klecka GM, Gibson DT (1981) Inhibition of catechol 2, 3-dioxygenase from Pseudomonas putida by 3-chlorocatechol. Appl Environ Microbiol 41:1159–1165
Liang O, Takeo M, Chen M et al (2005) Chromosome-encoded gene cluster for the metabolic pathway that converts aniline to TCA-cycle intermediates in Delftia tsuruhatensis AD9. Microbiology 151:3435–3446
Meyer U (1981) Biodegradation of synthetic organic colorants. In: Leisinger T, Cook AM, Hutter R, Nuesch J (eds) Microbial degradation of xenobiotic and recalcitrant compounds, FEMS Symposium 12. Academic Press, London, pp 371–385
Murakami S, Hayashi T, Maeda T et al (2003) Cloning and functional analysis of aniline dioxygenase gene cluster, from Frateuria species ANA-18, that metabolizes aniline via an ortho-cleavage pathway of catechol. Biosci Biotechnol Biochem 67:2351–2358
Nakazawa T, Yokota T (1973) Benzoate metabolism in Pseudomonas-putida (arvilla) mt-2—demonstration of 2 benzoate pathways. J Bacteriol 115:262–267
Pearson WR (1990) Rapid and sensitive sequence comparison with Fastp and Fasta. Methods Enzymol 183:63–98
Pieper DH, Reineke W (2004) Degradation of chloroaromatics by Pseudomona(d)s. In: Ramos JL (ed) The pseudomonads. Kluwer Academic/Plenum, New York, pp 509–574
Pizon AF, Schwartz AR, Shum LM et al (2009) Toxicology laboratory analysis and human exposure to p-chloroaniline. Clin Toxicol 47:132–136
Ren HF, Li SQ, Liu SJ et al (2005) Isolation and characterization of a p-chloroaniline-degrading bacterial strain. Huan jing ke xue 26:154–158
Saitou N, Nei M (1987) The neighbor-joining method—a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 8.46–8.53
Surovtseva EG, Vasilieva GK, Volnova AI et al (1980) Destruction of monochloroanilines in the meta-cleavage of Alcaligenes faecalis. Doklady Akademii Nauk Sssr 254:226–230
Tamura K, Dudley J, Nei M et al (2007) Mega4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thompson JD, Gibson TJ, Plewniak F et al (1997) The Clustal_x windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Urata M, Uchida E, Nojiri H et al (2004) Genes involved in aniline degradation by Delftia acidovorans strain 7 N and its distribution in the natural environment. Biosci Biotechnol Biochem 68:2457–2465
Vangnai AS, Petchkroh W (2007) Biodegradation of 4-chloroaniline by bacteria enriched from soil. FEMS Microbiol Lett 268:209–216
Zeyer J, Wasserfallen A, Timmis KN (1985) Microbial mineralization of ring-substituted anilines through an ortho-cleavage pathway. Appl Environ Microbiol 50:447–453
Zhang T, Zhang J, Liu S et al (2008) A novel and complete gene cluster involved in the degradation of aniline by Delftia sp. An3. J Environ Sci (China) 20:717–724
Zhu CG, Zhang LY, Zhao LP (2008) Molecular cloning, genetic organization of gene cluster encoding phenol hydroxylase and catechol 2, 3-dioxygenase in Alcaligenes faecalis IS-46. World J Microbiol Biotechnol 24:1687–1695
Acknowledgments
This work was supported by grants of Hi-Tech Research and Development Program of China (“863” program, No. 2006AA06Z316) and the Knowledge Innovation Program of the Chinese Academy of Sciences, No. KSCS2-YW-G-055-01.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, T., Ren, HF., Liu, Y. et al. A novel degradation pathway of chloroaniline in Diaphorobacter sp. PCA039 entails initial hydroxylation. World J Microbiol Biotechnol 26, 665–673 (2010). https://doi.org/10.1007/s11274-009-0221-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-009-0221-1