Abstract
Urban environments generally have reduced biodiversity, nevertheless they may contribute to biodiversity conservation. The presence of epiphytes on urban trees indicates viability of urban areas as repositories of biodiversity; however the influence of biophysical and local habitat variables on the occurrence and diversity of epiphytes on urban trees has not been studied. Epiphytes were surveyed on 1170 roadside Albizia saman trees along 39 roads in the tropical city-state of Singapore. Eighty-seven percent of trees were host to at least one epiphyte species. A total of 51 epiphyte species were encountered, 33 of which were native and represented one-third of Singapore’s 113 extant native epiphyte species. Generalized linear mixed models revealed that at the tree level, DBH, fork structure (branch multiplicity), and distance of the host tree from nearest forest patch were associated with epiphyte richness, whereas at the road level, surrounding habitat type, fork structure, and average tree height were associated with epiphyte diversity (Simpson’s reciprocal index [1/λ]). Canonical correspondence analysis showed that the presence of a species was often influenced by a single factor rather than multiple factors, e.g. distance to forest, host tree trunk diameter or height, or host tree branching complexity. This study suggests that an understanding of native epiphytes’ dispersal syndrome, the preservation of forest remnants, and protection of trees—especially large and tall trees exhibiting complex architecture—are crucial for epiphyte conservation in highly urbanized environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, urban areas are becoming more prevalent (Lumsden and Bennett 2005; Sudha and Ravindranath 2000), and urbanization—along with habitat loss—is a major factor contributing to the rapid loss of biodiversity throughout the world (Groombridge and Jenkins 2002). Yet although urban environments tend to have reduced biodiversity compared to natural forest habitats, these areas may contribute to biodiversity conservation (Alvey 2006; Angold et al. 2006; Cornelis and Hermy 2004; Goddard et al. 2010), especially if the urban landscape maintains forest fragments (e.g. secondary forest, urban parks), which may be viable repositories of biodiversity (Alvey 2006; Angold et al. 2006).
Outside of remnant forested patches in urban landscapes, roadside trees may provide opportunities for maintenance of biodiversity, such as by providing corridors for animal movements, nesting or roosting sites, or substrate for epiphyte growth. Indeed, planting and maintenance of trees might be the sole option for the conservation of local biota in urban areas (Yasuda and Koike 2009). Although ecological conditions of roadside trees differ from that of forest trees (e.g. temperature, humidity), a few studies have shown that diverse epiphyte and arthropod communities can colonize urban host trees (Wee 1978; Yasuda and Koike 2009). In particular, promoting epiphyte diversity on urban trees is critical because epiphytes provide microhabitats for fauna (Scheffers et al. 2013; Stuntz et al. 2002); therefore, maximizing epiphyte diversity could have cascading positive effects to animal taxa as well.
Epiphytes are adversely affected by deforestation and urbanization but may also be amenable to conservation in urban landscapes. Epiphyte decline is particularly commonplace in tropical cities such as Singapore, a highly urbanized city state that has lost 62 % of its original 297 native epiphyte species since the 1900s (Turner et al. 1994). Like most cities, the extent and density of urban areas in Singapore is expected to increase with population growth, suggesting even greater pressure on biodiversity in the future.
Despite its focus on economic development and concomitant urbanization, the government of Singapore extensively plants roadside trees (one of the taglines for Singapore is “City in a Garden”; Ministry of National Development 2008). The most widely planted roadside tree in Singapore is Albizia saman (Tan et al. 2009), which is native to South America and was first planted in Singapore in 1876 (Burkill 1935). The wide, umbrella-shaped crown of A. saman provides excellent shade, whereas distinctive characteristics such as flaky bark and leaf-free inner branches allow rain trees to be favourable host trees for epiphyte colonization. Two previous local surveys on urban epiphytes (Wee 1978; Yoon 2011) have shown that A. saman tends to harbour more epiphyte species than other roadside tree species, highlighting it as a potentially valuable roadside tree for the conservation of urban epiphytes.
Very little research has been conducted on epiphyte diversity of roadside trees in Singapore, or in the tropics more broadly (see Barkat 1968; Wee 1978; Yoon 2011). Critically, it is even less clear what factors may affect the distribution of epiphyte diversity in urban settings. Examples of potential factors include the host tree size and morphology (e.g. number of branches), the distance to forest remnants, and the dispersal syndrome of epiphyte species. In this study, we describe the vascular epiphyte diversity on roadside A. saman trees, and investigate the influence of biophysical variables on epiphyte diversity and distribution on the tropical island of Singapore. Understanding the capacity of urban trees to promote urban epiphyte diversity, as well as the factors mitigating it, is important for biodiversity conservation and management in the urban landscape.
Materials and methods
Study design and field surveys
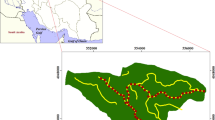
Through collaboration with Singapore’s National Parks Board, we developed a list of all roads having roadside A. saman trees. From that list, 57 roads were randomly selected and visited, or inspected on GoogleTM Street View. A survey site was preliminarily mapped by randomly positioning a starting point on the road, along with a direction for the roadside survey. Each site was classified according to adjacent land use—either urban, grassland, or secondary forest—by determining the land use composition comprising >50 % of the area within octagons of ~200 m in diameter around each site, drawn in GoogleTM Earth (Fig. 1). Land use categorization was based upon physical attributes of the area: “urban” consisted of man-made structures (e.g. buildings), “grassland” was an open area of manicured turf grass, possibly fringed with trees or shrubs, and “secondary forest” included patches of lowland tropical forest or young secondary forest on previously cleared or degraded land, or urban parks dominated by trees (Yee et al. 2011). Thereafter, 13 roadsides were randomly selected for each surrounding habitat type; altogether, 39 roads were selected for study (Fig. 2).
At each site, 30 trees were surveyed, 15 on each side of the road. Systematic observations of vascular epiphytes were conducted on each A. saman tree from base to crown with binoculars. A species list was created for each focal tree. Species identification was accomplished in the field for majority of the epiphytes; for a few individuals (≪10) in the genus Ficus, temporary voucher leaves were collected and the species confirmed with the aid of field guides (Boo et al. 2006; National Parks Board 2013). All epiphytes were recorded and classified based on type (see Benzing 2008): true (possessing specialized physical and physiological adaptations to survive under canopy conditions), facultative (able to inhabit the ground or tree canopy interchangeably), accidental (terrestrial species lacking unique modifications for canopy life but having germinated on the host tree), parasitic (exclusively mistletoes), and hemiepiphyte (germinating on the tree but eventually rooting in the ground). Although ground-based techniques may underestimate the epiphyte species richness in forested environments (Flores-Palacios and García-Franco 2006), detection probability in an urban setting is assumed to be high because roadside A. saman have a single-layer configuration, and the trees tended to be only 15–20 m tall. The survey excluded trees that were known to harbour artificially-planted epiphytes (Yam et al. 2011), those with a stem diameter of less than 30 cm (considered too young to harbour any epiphyte; Wee 1978), or which were recently pruned. The location of every rain tree was recorded using a Garmin® GPSMAP 60CSx GPS. In total, 1170 host trees were surveyed during this study.
Tree characteristic data included DBH (measured with a diameter tape, at 1.3 m above the base), height (estimated using the Suunto® PM-5 clinometer), angle between two primary branches (largest angle of main fork, estimated using a protractor), and number of primary branches (fork structure). The distance of host trees from nearest forest vegetation and nearest distance between host trees on the same road were measured in GoogleTM Earth.
Data analyses
All analyses were conducted using occurrence data. The nonparametric diversity estimator Chao2 (Chao 1987) was used to estimate the species richness of the aggregated tree data set. For each host tree, species richness was taken as the total number of epiphyte species observed. We also assessed diversity at the road (site) level using Simpson’s reciprocal index (1/λ), which was computed based on the species occurrence on all host trees at that site combined (Kersten et al. 2009).
To evaluate the influence of surrounding habitat types and biophysical factors on epiphyte diversity, we conducted the analyses at two scales: (1) individual host tree (i.e. the tree as the unit of replication) and (2) road (i.e. the road as the unit of replication). Tree-level epiphyte richness was modelled by fitting generalized linear mixed models (GLMMs; McCullagh and Nelder 1989), which were fitted using the Poisson distribution with a log link function, with road as a random effect. For road-level analysis, the models were fitted using the Gaussian distribution with an identity link function; the predictors were the average values across all 30 trees, and the response variable was Simpson’s reciprocal index (1/λ). The normality of the Simpson’s reciprocal index was verified by means of Kolmogorov-Smirnov and Shapiro-Wilk tests. The predictors (fixed effects) for both GLMMs were DBH, height, largest angle of main fork, fork structure, distance of host tree from nearest vegetation, nearest distance between host trees, and surrounding habitat type. The Akaike Information Criterion was used for model comparison and step-wise simplification. Spearman’s correlation tests were consequently carried out to illustrate the effects of the statistically significant predictors.
Canonical correspondence analysis (CCA; ter Braak 1986) was used to illustrate the principal relationships between epiphyte species and each biophysical factor. CCA applies an iterative approach involving multiple regression to select the linear combination of biophysical factors that explain variation in the species ordination score of each axis. The vectors in the biplot point towards the direction of maximal value change in biophysical factors with the vector lengths representing maximum rate of change (Gabriel and Odoroff 1990; ter Braak and Verdonschot 1995).
All analyses were done using IBM SPSS Statistics Version 20 except for the diversity estimator test (Chao2) and CCA, which were conducted using PAST Version 2.16 (Hammer et al. 2001).
Results
Overall, a total of 3937 epiphyte occurrences (species presence) were tallied on 1170 roadside A. saman trees. Most of the surveyed trees (87 %) had at least one epiphyte species (mean = 3.4 ± 2.3 species/tree), ranging from 0 to 11 (Fig. 3). The occurrence record contained 26 families, 36 genera, and 51 vascular species (Appendix Table S1); 18 species were accidental epiphytes. The remaining 33 (non-accidental) epiphyte species represent 29 % of Singapore’s 113 extant non-accidental epiphyte species (Chong et al. 2009). Moraceae and Polypodiaceae were the most speciose families with nine species each. The 12 most common species made up 91 % of the total observation frequency (Table 1). Davallia denticulata had the highest relative occurrence, followed by Asplenium nidus, and Pyrrosia piloselloides. There were twice as many angiosperm species (71 %) observed as pteridophyte species (29 %). Most roadside epiphytes were native (59 %) whereas exotics and weeds of uncertain origin (WUO) accounted for 33 and 8 % of the epiphyte species respectively (Appendix Table S1). There was also an equal number of true and accidental epiphytes species observed (18 species), nine facultative epiphytes, five hemiepiphytes, and one parasitic epiphyte. Predominantly arboreal epiphytes (true epiphytes, facultative epiphytes, hemiepiphytes, and parasitic epiphytes) had higher species richness (71 %) and occurrence (96 %) as compared to accidental epiphytes (29; 4 %).
The overall species richness was 51 (RichnessChao2 = 48.7 ± 2.6) and with urban = 35 species (RichnessChao2 = 33.2 ± 5.6), grassland = 31 (RichnessChao2 = 33.3 ± 3.3) and secondary forest = 41 (RichnessChao2 = 40.4 ± 8.3). The similar estimates of species richness generated by Chao2 diversity estimator suggested that our sample size (n = 1170) was sufficient to capture the vast majority of species on urban A. saman trees. Secondary forest roads had the highest epiphyte species occurrence (Occurrencesecondary = 1916; Occurrencegrassland = 1143; Occurrenceurban = 878), and diversity (1/λ secondary = 7.7 ± 1.6; 1/λ grassland = 6.4 ± 1.0; 1/λ urban = 5.3 ± 1.2; ANOVA, F(2,36) = 11.47, P < 0.001). These diversity trends held true when considering only native species: richness was highest in secondary forest roads (Richness = 27 ± 2.4), as compared to grassland (Richness = 22 ± 2.8) and urban (Richness = 19 ± 3.0; Kruskal-Wallis test, H = 7.706, df = 2, P < 0.05). Post-hoc pairwise comparisons between habitat types indicated that the native species occurrence was statistically higher in secondary forest as compared to urban (H = 6.501, df = 1, P < 0.05), but not grassland (H = 3.207, df = 1, P = 0.073). There was also a significant difference between the overall occurrence of native and non-native species (Mann-Whitney test, U = 179, P < 0.001).
The tree-level GLMMs revealed that DBH, fork structure, and distance of host tree from nearest vegetation corresponded to overall epiphyte species richness (Table 2). Surrounding habitat type was not predictive of epiphyte species richness at the tree level. Univariate correlations demonstrated the relationship between species richness and DBH (r = 0.584, P < 0.001), number of branches (r = 0.183, P < 0.001), and distance of host tree from nearest forest vegetation (r = − 0.551, P < 0.001) (Fig. 4). Host trees with DBH <40 cm had no more than five epiphyte species. Of particular note is that there was a linear decline in the maximum number of species found on host trees as a function of distance from forest.
Road-level GLMMs revealed that surrounding habitat type, average fork structure, and average height of host trees were predictive of overall epiphyte species diversity (Table 2). A positive correlation was observed between species diversity and the average height of host trees at a site (r = 0.525, P < 0.01; Fig. 4).
CCA revealed that several species distributions were influenced by biophysical parameters. Dendrophthoe pentandra (a parasite), Ptychosperma marcathurii (an accidental epiphyte), Ficus religiosa, Ficus microcarpa, and Ficus benjamina were more likely to occur at roadsides situated closer to forest (Fig. 5). Pyrrosia lanceolata tended to occur on trees with greater DBH, and Asplenium nidus was found on host trees with greater branching complexity (number of branches). Occurrence of the ferns Drynaria quercifolia, Drynaria sparsisora, and Goniophlebium percussum was positively associated with host tree height (Fig. 5).
Canonical correspondence analysis plot illustrating associations between biophysical factors and occurrence of different epiphyte species. Only species with occurrence >10 were included. All species names were abbreviated for better diagrammatic clarity e.g. A. nid = Asplenium nidus, P. lan = Pyrrosia lanceolata, D. pen = Dendrophthoe pentandra, P. mar = Ptychosperma marcathurii, F. rel = Ficus religiosa, F. mic = Ficus microcarpa, F. ben = Ficus benjamina, D. que = Drynaria quercifolia, D. spa = Drynaria sparsisora, G. per = Goniophlebium percussum
Discussion
Roadside trees as viable epiphyte repositories
Singapore is one of the world’s most modified countries (Sodhi et al. 2004), having lost more than 99 % of its original forest cover (Corlett 1992). So in this regard, Singapore is an extreme example of habitat loss requiring novel urban solutions for biodiversity conservation. Addressing this need, Singapore also has an extensive network of roadside trees, having planted 660,000 trees in public areas (one tree to every four persons) by the 1980s (Yuen 1996). So in this way, Singapore can be a model case study. This study found that most A. saman roadside trees had epiphyte colonization. Furthermore, epiphytes found on roadside A. saman accounted for one-third of Singapore’s extant, and primarily native, epiphyte species. Therefore, although roadside tree communities are likely to be depauperate in comparison with forested systems, our results suggest that roadside trees may indeed serve as a foundation to conserve biodiversity even in highly urbanized landscapes. It is imperative for potential roadside trees to possess desirable structural characteristics; this consideration may enhance the epiphyte diversity in non-forested environments.
Tree architecture
Tree architecture—diameter, branching complexity, and height—was associated with epiphyte diversity. In general, DBH is positively associated with tree age (Cummings et al. 2006; Etisa 2010). Trees with larger DBH will have greater surface area than smaller, younger ones, allowing for greater diaspore interception and epiphyte colonization (Benzing 2004; Flores-Palacios and García-Franco 2006; Zotz and Vollrath 2003). Thus, the likelihood of trees accumulating more species will increase over time (Cummings et al. 2006; Etisa 2010; Zotz 1997). Host trees may also have complex multiple branches of different sizes, with variable nooks and crevices. It is important to note that branch multiplicity is not necessarily associated with age and may be dependent on the tree species, architectural modifications such as natural or artificial pruning, or by virtue of the fact that the trees are growing in open conditions compared with forest counterparts. These structural complexities tend to form bases which provide mechanical support, facilitate accumulation of water, dead leaves, and dust (Mojiol et al. 2009; ter Steege and Cornelissen 1989; Wee 1978), and reduce desiccation (Kersten et al. 2009). These characteristics may also provide various microhabitats for epiphytes (Annaselvam and Parthasarathy 2001; Sillett 1999) and encourage epiphyte colonization and growth, particularly for large-growing and heavy species which require large spaces for sturdy support (e.g. Asplenium nidus). Several previous studies have shown a positive relationship between tree height and epiphyte diversity (Flores-Palacios and García-Franco 2006; Laube and Zotz 2006; Malizia 2003; Zimmerman and Olmsted 1992). However, unlike trunk diameter which increases indeterminately, tree heights does not; maximum height is mainly constrained by water transport mechanisms within the xylem or habitat stress (Koch et al. 2004; Thomas 1996). Nevertheless, this positive relationship may reflect the vertically stratified epiphyte distribution (ter Steege and Cornelissen 1989; Zotz 2007), resulting from greater microclimate and microhabitat heterogeneity on taller trees (Bennett 1986; Hsu et al. 2002; Nadkarni 1994). For example, while complex branching may provide adequate moisture conditions, tall trees may provide more exposed crowns (Flores-Palacios and García-Franco 2006) which offer higher light (Benzing 2004) and xeric conditions (Benzing 2008). These conditions might provide opportunities for drought tolerant species, such as the CAM-plants Drynaria quercifolia, Drynaria sparsisora, and Goniophlebium percussum, to colonize niches which are more exposed (Hsu et al. 2002). Overall, our results suggest that large, tall, and structurally complex trees in urban landscapes are more suitable repositories for biodiversity than small, short, and structurally simple trees. In extension, such trees will be expected to serve as sources of diaspores for adjoining host trees, thus enhancing connectivity.
Conservation of forest fragments
The surrounding land use influenced host tree epiphyte richness and diversity; this corroborates previous studies that have investigated the land use–epiphyte diversity relationship in natural systems (Belinchón et al. 2007; Etisa 2010; Flores-Palacios and García-Franco 2008; Hietz et al. 2006; Hietz-Seifert et al. 1995). Importantly, sites near secondary forest exhibited the highest species richness, and the largest proportion of epiphytes being of native origin. This trend indicates that nearby secondary forest patches may be effective diaspore sources for the colonization of native epiphytes on roadside host trees. In contrast, sites without any nearby vegetation (urban) had both the lowest epiphyte diversity and the highest proportion of non-native species occurrence. For example, the non-native epiphytes Bergera koenigii, Cleome rutidosperma, Ficus celebensis, and Mimosa pudica were found exclusively on roadsides in the “urban” habitat. These species are planted in local gardens and greeneries (Boo et al. 2006) and can only be found under direct human care (Chong et al. 2009). Thus, the results suggest that even small patches of native vegetation may be important repositories of diaspores; yet the maintenance of larger, secondary (or primary) forest patches across an urban landscape may be crucial to maximize the potential for native epiphyte communities to persist in highly urbanized ecosystems.
Further evidence of the crucial nature of remnant vegetation on roadside epiphyte diversity is that species richness declined as a function of distance from the nearest forested patch (see also Hietz-Seifert et al. 1995). Notably, zoochoric epiphytes appeared to be strongly distance-dependent; Dendrophthoe pentandra, Ficus religiosa, Ficus microcarpa, Ficus benjamina, and Ptychosperma marcathurii were more likely to be found on roadsides nearer to forested areas. This suggests that the positive influence of forest patches on epiphyte diversity in urban areas is spatially limited (Hietz-Seifert et al. 1995) and may extend beyond dispersion (e.g. ambient temperature and humidity). This notion highlights the need for urban planning to consider the inter-patch distance if urban roadside biodiversity is to be maintained. Further, the potential for large, tall, and complex trees (discussed above) to serve as corridors between remnant forest patches in highly urbanized landscapes should be addressed with future research.
Dispersal syndrome and circa situm conservation
The negative correlation between epiphyte diversity and distance to nearest forest vegetation suggests varying levels of dispersal limitation among epiphyte species. The dispersal syndrome of epiphytes may therefore be a contributing factor in determining epiphyte occurrence across the urban landscape. Although the angiosperm epiphyte richness was twice of pteridophytes’, ferns exhibited greater occurrence and comprised the top five most common species overall. This may be explained by the extensive and frequent long distance wind dispersal of dust-like spores of pteridophytes (Caulton et al. 2000). Unlike ferns, however, the wind-dispersed orchids showed low occurrence and diversity. In the case of orchids, the specialized habitat requirements may explain their low occurrences. For example, orchids typically require specific pollinators for cross-pollination (Pemberton 2010; Vereecken et al. 2010) and fungal symbionts for both germination and growth (Hartley and Smith 1983); the availability of these conditions may vary widely in the urban environment. In contrast, the lower occurrence among animal-dispersed taxa may be an indication of stronger constraints to zoochorous species distributions in urban landscapes. One exception may be the family Moraceae, which was the most diverse group of vascular epiphytes in this study. Previous work has highlighted the success of some fig species in urban areas (Corlett 2006), mainly owing to dispersal by cosmopolitan and disturbance-tolerant fauna such as the Asian glossy starling (Aplonis panayensis) and the plantain squirrel (Callosciurus notatus; Peh and Chong 2003), complemented by fleshy stem tubers for water-storage and utilization of nitrates as osmotic compounds to counter the effects of water stress (Schmidt and Tracey 2006). These advantageous life history traits may allow Ficus spp. to establish and survive in water-limited sites such as tree canopies and even hard structures such as pavement and buildings (Mun 2004; Wee 1992). The introduction of poorly-dispersed native epiphyte families/species with suitable traits (e.g. Orchidaceae) on roadside habitats may be a viable option to increase epiphyte diversity in urban habitats, primarily roadsides.
Circa situm conservation (“farmer-based conservation”) is a term that has been used to discern the contrasting circumstances of conservation within modified agricultural landscapes (e.g. home gardens, agroforestry systems) outside natural habitats but within a species’ native geographical range (Boshier et al. 2004). As illustrated in this study, this concept could be applied to the urban context. For example, Asplenium nidus is known to serve as habitats for several species of arboreal frogs (Scheffers et al. 2013). Also, the success of some fig species (e.g. Ficus microcarpa) in urban areas (Corlett 2006) may be ascribed to their mutualistic pollinators, fig wasps (Cook and Rasplus 2003), and dispersers such as the plantain squirrel (Callosciurus notatus; Peh and Chong 2003). Thus, the successful colonization of these native epiphytes in urban environments may not only improve their gene flow, it may also help maintain populations of epiphyte-dependent fauna, especially pollinators and dispersers.
Conclusions
This study supports three conclusions for the conservation of epiphyte diversity in highly urbanized systems. First is that the remnant forest vegetation is crucial to serve as a source for (re-)colonization of epiphytes on planted roadside trees. In Singapore, for example, forest patches include both mature and degraded secondary forest. Indeed whereas this study could not evaluate the relative effectiveness of mature versus degraded secondary forest as a diaspore source, it is clear based on our analysis that some form of forest is better than none. We would expect that mature forest would be a far superior source than degraded secondary forest, and future research could address such a hypothesis. Urban planning that incorporates biodiversity should consider maintaining large patches of native vegetation spaced at appropriate intervals that would increase the likelihood of roadside colonization by epiphytes and potentially, other species.
Second is that several tree architecture traits (DBH, height, branch complexity) corresponded to higher epiphyte diversity. It is thus apparent that protecting old, tall, and structurally complex trees is an essential component of urban biodiversity conservation. Indeed there may be a tension between roadside maintenance requirements (e.g. roadside safety) and biodiversity conservation. However, when possible, decisions should favour retention of old, complex trees rather than removal and replanting. Third is that the dispersal syndrome of native epiphytes may influence the ability of epiphytes to colonize and persist in the urban setting. Seed dispersal in particular appeared to be a major filter in the dispersion of epiphytes in Singapore. Intervention action to increase epiphyte diversity should recognize dispersal limitations and focus on enrichment of species that may not easily disperse outside of forests. It is also recommended to consider other life history factors (e.g. growth rate, reproductive age) in the planning stages prior to host tree planting or direct epiphyte transplantation.
Finally, expectations for conserving epiphyte diversity on roadsides should be realistic. More than 60 % of Singapore’s epiphyte flora has been extirpated, and only about 30 % of the remaining species were encountered on roadside trees in this study. It is likely that other tropical urban environments have similar characteristics. Identifying which epiphyte species are most amenable to roadside planting, and which could maintain reproductive populations in highly urbanized environments, would be an important contribution. With proper mitigation measures and planning, urban landscapes can be transformed into valuable species storehouses for native vascular epiphytes and perhaps their associated urban fauna as well. However, it is most likely that the urban environment will retain only a fraction of the original species composition found in native forest.
References
Alvey AA (2006) Promoting and preserving biodiversity in the urban forest. Urban For Urban Gree 5:195–201
Angold PG, Sadler JP, Hill MO, Pullin A, Rushton S, Austin K, Small E, Wood B, Wadsworth R, Sanderson R, Thompson K (2006) Biodiversity in urban habitat patches. Sci Total Environ 360:196–204
Annaselvam J, Parthasarathy N (2001) Diversity and distribution of herbaceous vascular epiphytes in a tropical evergreen forest at Varagalaiar, Western Ghats, India. Biodivers Conserv 10:317–329
Barkat A (1968) Autecology of epiphytes on Fagraea fragrans and Swietenia macrophylla in Singapore. Dissertation, University of Singapore
Belinchón R, Martínez I, Escudero A, Aragón G, Valladares F (2007) Edge effects on epiphytic communities in a Mediterranean Quercus pyrenaica forest. J Veg Sci 18:81–90
Bennett BC (1986) Patchiness, diversity, and abundance relationships of vascular epiphytes. Selbyana 9:70–75
Benzing DH (2004) Vascular epiphytes. Elsevier Academic Press, Sydney
Benzing DH (2008) Vascular epiphytes: general biology and related biota. Cambridge University Press, Cambridge
Boo CM, Omar-Hor K, Ou-Yang CL (2006) 1001 Garden plants in Singapore, 2nd edn. National Parks Board, Singapore
Boshier DH, Gordon JE, Barrance AJ (2004) Prospects for circa situm tree conservation in Mesoamerican dry forest agro-ecosystems. In: Frankie GW, Mata A, Vinson SB (eds) Biodiversity conservation in Costa Rica: learning the lessons in a seasonal dry forest. University of California Press, Berkeley
Burkill IH (1935) A dictionary of the economic products of the Malay Peninsula. Crown Agents for the Colonies, London
Caulton E, Keddie S, Carmichael R, Sales J (2000) A ten year study of the incidence of spores of bracken (Pteridium aquilinum) in an urban rooftop airstream in south east Scotland. Aerobiologia 16:29–33
Chao A (1987) Estimating the population size for capture-recapture data with unequal catchability. Biometrics 43:783–791
Chong KY, Tan HTW, Corlett RT (2009) A checklist of the total vascular plant flora of Singapore: native, naturalised and cultivated species. Raffles Museum of Biodiversity Research, Singapore
Cook JM, Rasplus J-Y (2003) Mutualists with attitude: coevolving fig wasps and figs. Trends Ecol Evol 18:241–248
Corlett RT (1992) The ecological transformation of Singapore, 1819–1990. J Biogeogr 19:411–420
Corlett RT (2006) Figs (Ficus, Moraceae) in urban Hong Kong, South China. Biotropica 38:116–121
Cornelis J, Hermy M (2004) Biodiversity relationships in urban and suburban parks in Flanders. Landsc Urban Plan 69:385–401
Cummings J, Martin M, Rogers A (2006) Quantifying the abundance of four large epiphytic fern species in remnant complex notophyll vine forest on the Atherton Tableland, North Queensland, Australia. Cunninghamia 9:521–527
Etisa AT (2010) Diversity of vascular epiphytes along disturbance gradient in Yayu Forest, southwest Oromia, Ethiopia. Dissertation, Addis Ababa University
Flores-Palacios A, García-Franco JG (2006) The relationship between tree size and epiphyte species richness: testing four different hypotheses. J Biogeogr 33:323–330
Flores-Palacios A, García-Franco JG (2008) Habitat isolation changes the beta diversity of the vascular epiphyte community in lower montane forest, Veracruz, Mexico. Biodivers Conserv 17:191–207
Gabriel KR, Odoroff CL (1990) Biplots in biomedical research. Stat Med 9:469–485
Goddard MA, Dougill AJ, Benton TG (2010) Scaling up from gardens: biodiversity conservation in urban environments. Trends Ecol Evol 25:90–98
Groombridge B, Jenkins MD (2002) World atlas of biodiversity: earth’s living resources in the 21st Century. University of California Press, Berkeley
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontolological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Hartley JL, Smith SE (1983) Mycorrhizal symbiosis. Academic Press, New York
Hietz P, Buchberger G, Winkler M (2006) Effect of forest disturbance on abundance and distribution of epiphytic bromeliads and orchids. Ecotropica 12:103–112
Hietz-Seifert U, Hietz P, Guevara S (1995) Epiphyte vegetation and diversity on remnant trees after forest clearance in southern Veracruz, Mexico. Biol Conserv 75:103–111
Hsu C, Horng F, Kuo C (2002) Epiphyte biomass and nutrient capital of a moist subtropical forest in north-eastern Taiwan. J Trop Ecol 18:659–670
Kersten RA, Borgo M, Silva SM (2009) Diversity and distribution of vascular epiphytes in an insular Brazilian coastal forest. Rev Biol Trop 57:749–759
Koch GW, Sillett SC, Jennings GM, Davis SD (2004) The limits to tree height. Nature 428:851–854
Laube S, Zotz G (2006) Neither host-specific nor random: vascular epiphytes on three tree species in a Panamanian lowland forest. Ann Bot London 97:1103–1114
Lumsden LF, Bennett AF (2005) Scattered trees in rural landscapes: foraging habitat for insectivorous bats in south-eastern Australia. Biol Conserv 122:205–222
Malizia A (2003) Host tree preference of vascular epiphytes and climbers in subtropical montane cloud forest of northwest Argentina. Selbyana 24:196–205
McCullagh P, Nelder JA (1989) Generalized linear models: monographs on statistics and applied probability, 2nd edn. Chapman and Hall, New York
Ministry of National Development (2008) An endearing home, a distinctive global city. Ministry of National Development, Singapore
Mojiol AR, Jitinu AMA, Adella A, Ganang GM, Nasly N (2009) Vascular epiphytes diversity at Pusat Sejadi, Kawang forest reserve, Sabah, Malaysia. J Sustain Dev 2:121–127
Mun MC (2004) The ecology of pavement plants. Dissertation, National University of Singapore
Nadkarni NM (1994) Diversity of species and interactions in the upper tree canopy of forest ecosystems. Am Zool 34:70–78
National Parks Board (2013) NParks Flora & Fauna Web. National Parks Board, Singapore. https://florafaunaweb.nparks.gov.sg/
Peh KSH, Chong FL (2003) Seed dispersal agents of two Ficus species in a disturbed tropical forest. Ornithol Sci 2:119–125
Pemberton RW (2010) Biotic resource needs of specialist orchid pollinators. Bot Rev 76:275–292
Scheffers BR, Phillips B, Laurance WF, Sodhi NS, Diesmos A, Williams SE (2013) Increasing arboreality with altitude: a novel biogeographic dimension. P R Soc B 280:1471–2954
Schmidt S, Tracey DP (2006) Adaptations of strangler figs to life in the rainforest canopy. Funct Plant Biol 33:465–475
Sillett SC (1999) Tree crown structure and vascular epiphytes distribution in Sequoia sempervirens rainforest canopies. Selbyana 20:76–79
Sodhi NS, Koh LP, Brook BW, Ng PKL (2004) Southeast Asian biodiversity: an impending disaster. Trends Ecol Evol 19:654–660
Stuntz S, Ziegler C, Simon U, Zotz G (2002) Diversity and structure of the arthropod fauna within three canopy epiphyte species in central Panama. J Trop Ecol 18:161–176
Sudha P, Ravindranath NH (2000) A study of Bangalore urban forest. Landsc Urban Plan 47:47–63
Tan PY, Yeo B, Yip WX, Lua HS (2009) Carbon storage and sequestration by urban trees in Singapore. Centre for Urban Greenery and Ecology, Singapore
ter Braak CJE (1986) Canonical correspondence analysis, a new eigenvector technique for multivariate direct gradient analysis. Ecology 67:1167–1179
ter Braak CJE, Verdonschot PFM (1995) Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat Sci 57:255–289
ter Steege H, Cornelissen JHC (1989) Distribution and ecology on vascular epiphytes in lowland rain forest of Guyana. Biotropica 21:331–339
Thomas SC (1996) Asymptotic height as a predictor of growth and allometric characteristics in Malaysia rain forest trees. Am J Bot 83:556–566
Turner IM, Tan HTW, Wee YC, Ali BI, Chew PT, Corlett RT (1994) A study of plant species extinction in Singapore: lessons for the conservation of tropical biodiversity. Conserv Biol 8:704–712
Vereecken NJ, Dafni A, Cozzolino S (2010) Pollination syndromes in Mediterranean orchids—implications for speciation, taxonomy and conservation. Bot Rev 76:220–240
Wee YC (1978) Vascular epiphytes of Singapore’s wayside trees. Gard Bull Singap 312:114–117
Wee YC (1992) The occurrence of Ficus spp. on high-rise buildings in Singapore. Int Biodeter Biodegr 29:53–59
Yam TW, Tay F, Ang P, Soh W (2011) Conservation and reintroduction of native orchids of Singapore—the next phase. Eur J Environ Sci 1:38–47
Yasuda M, Koike F (2009) The contribution of the bark of isolated trees as habitat for ants in an urban landscape. Landsc Urban Plan 92:276–281
Yee ATK, Corlett RT, Liew SC, Tan HTW (2011) The vegetation of Singapore—an updated map. Gard Bull Singap 63:205–212
Yoon HL (2011) The ecology of vascular epiphytes on roadside trees in Singapore. Dissertation, National University of Singapore
Yuen B (1996) Creating the garden city: the Singapore experience. Urban Stud 33:955–970
Zimmerman JK, Olmsted IC (1992) Host tree utilization by vascular epiphytes in a seasonally inundated forest (Tintal) in Mexico. Biotropica 24:402–407
Zotz G (1997) Substrate use of three epiphytic bromeliads. Ecography 20:264–270
Zotz G (2007) Johansson revisited: the spatial structure of epiphyte assemblages. J Veg Sci 18:123–130
Zotz G, Vollrath B (2003) The epiphyte vegetation of the palm Socratea exorrhiza—correlations with tree size, tree age and bryophyte cover. J Top Ecol 19:81–90
Acknowledgments
We are thankful to M. Nadheera for her help on data collection. We further thank L.S. Wijedasa and T.W. Yam for providing supplementary information on A. saman and native orchids. We thank four anonymous reviewers for their helpful comments on early versions of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Pradeep Kumar Divakar.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Izuddin, M., Webb, E.L. The influence of tree architecture, forest remnants, and dispersal syndrome on roadside epiphyte diversity in a highly urbanized tropical environment. Biodivers Conserv 24, 2063–2077 (2015). https://doi.org/10.1007/s10531-015-0932-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-015-0932-6